Indirect Drying and Coking Characteristics of Coking Coal with Soda Residue Additive
Abstract
1. Introduction
- Based on the research on drying devices, an indirect, self-made coking coal drying system was set up.
- Based on the indirect drying method, the drying characteristics, temperature, and humidity transfer characteristics of raw coking coal were studied.
- Based on the effects of the drying temperature and alkali slag additives on the heating rate, the optimum drying temperature was determined.
- The influence of additives on the temperature and humidity transfer characteristics of coking coal during the drying process was studied.
- The samples of dried coking coal added with additives were tested for coking, and the influencing characteristics of the additives on coke strength, thermal reactivity, and specific surface area were studied to find out the optimal additive proportion.
- An economic benefit analysis was conducted.
2. Materials and Methods
2.1. Materials
2.2. Experimental Equipment and Instruments
2.2.1. Indirect Drying Equipment
2.2.2. Experimental Instruments
2.3. Methods
2.3.1. Preparation of Sample
2.3.2. Parametric Measurement
Drying Rate
Temperature and Humidity Distribution and Transfer
Coking Characteristics
- (1)
- Cold mechanical strength
- (2)
- Coke thermal performance
- (3)
- Specific surface area
3. Results
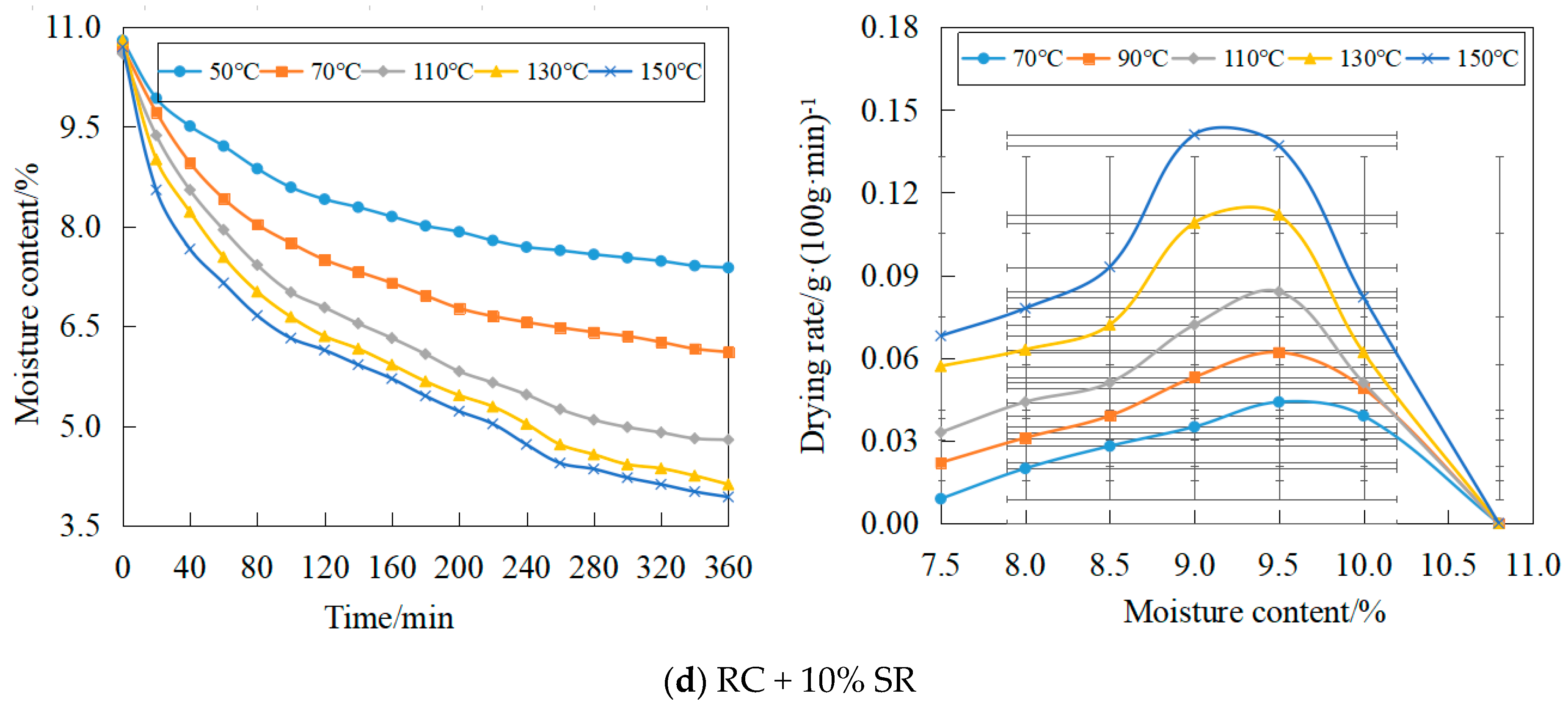
3.1. Effects of Soda Residue on Drying Rate
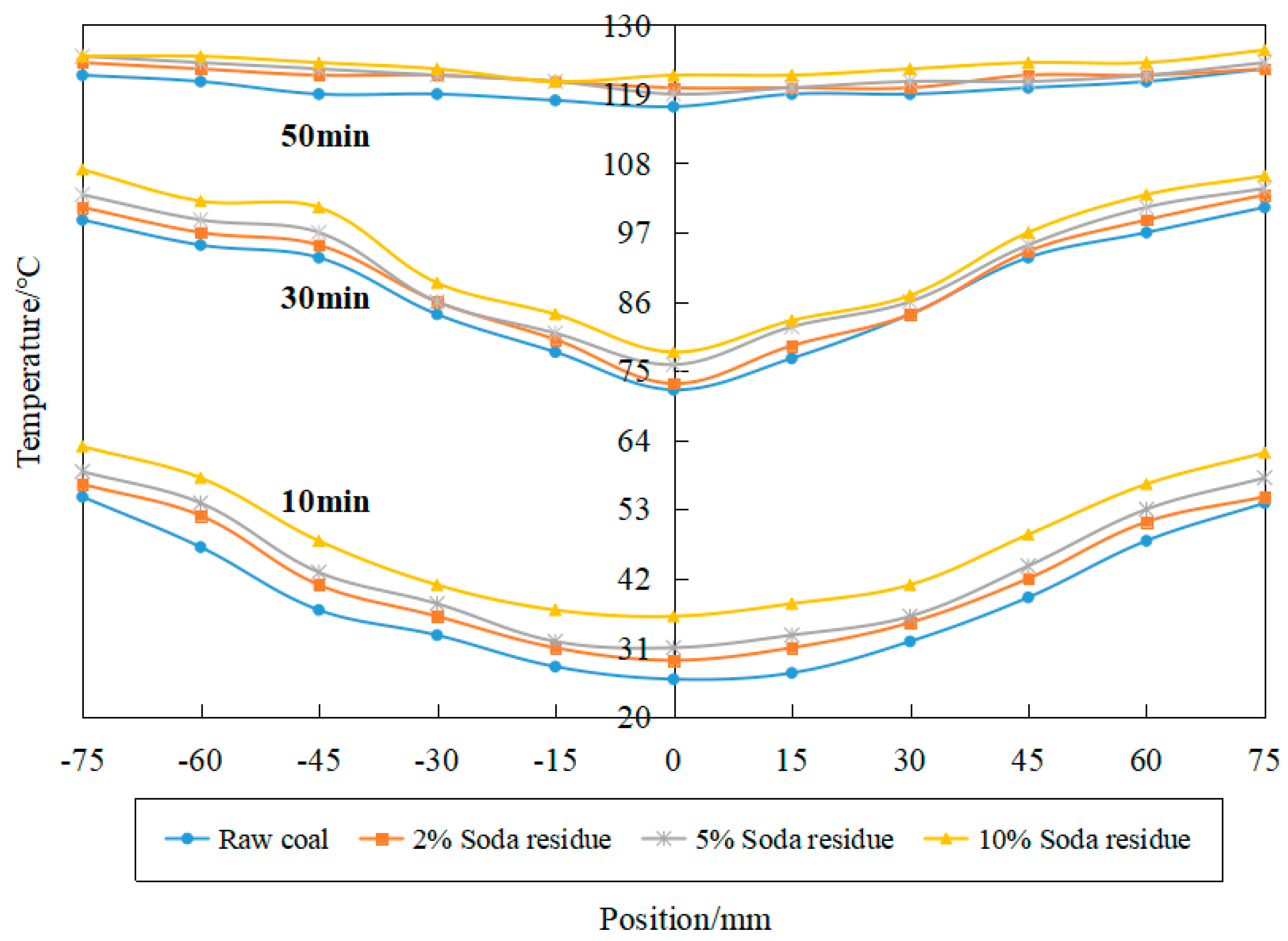
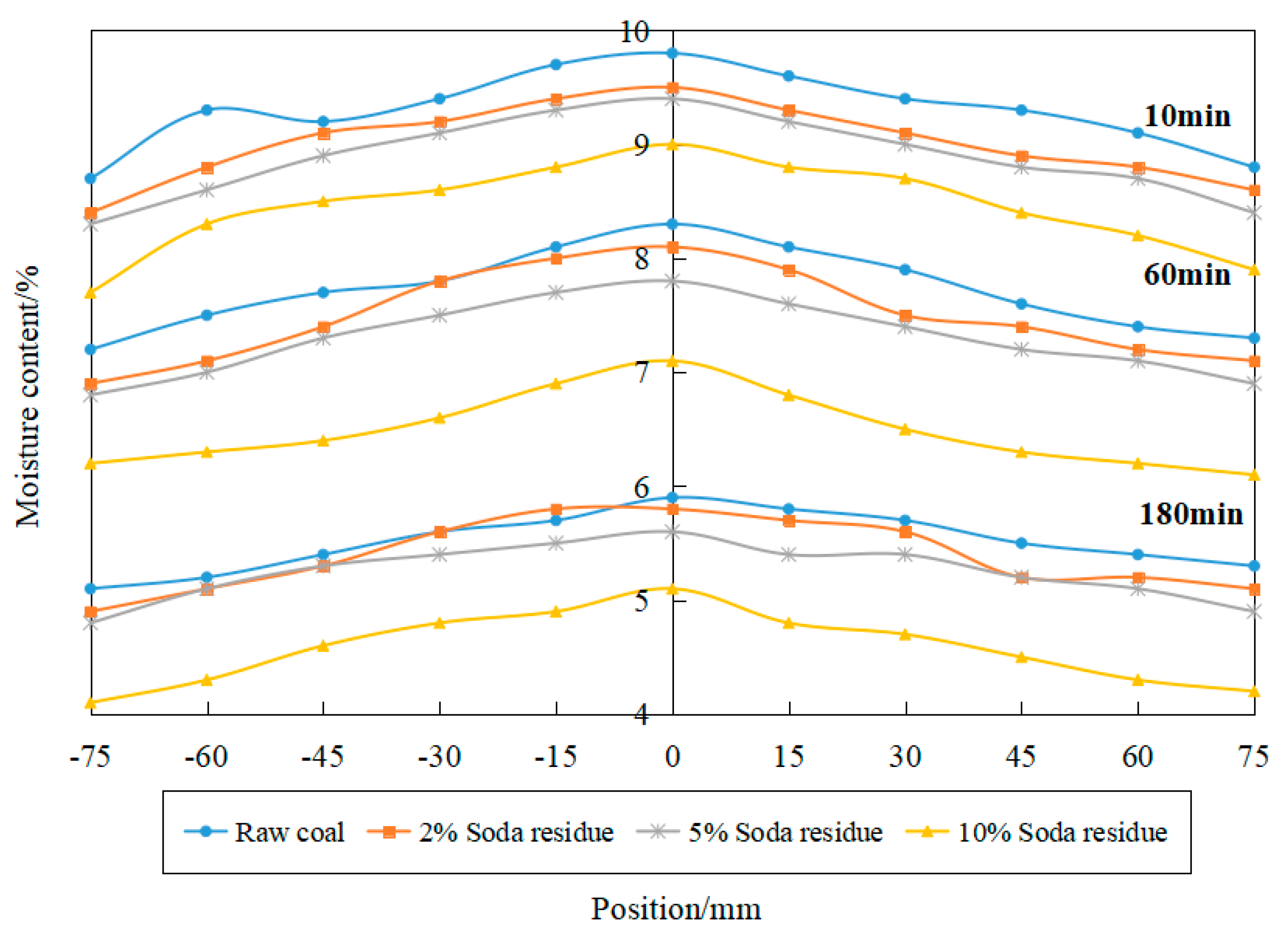
3.2. Effect of Soda Residue on Temperature and Moisture Gradient during Drying
3.3. Effect of Soda Residue on Coking Properties
3.3.1. Mechanical Strength of Coke in Cold State
Falling Strength
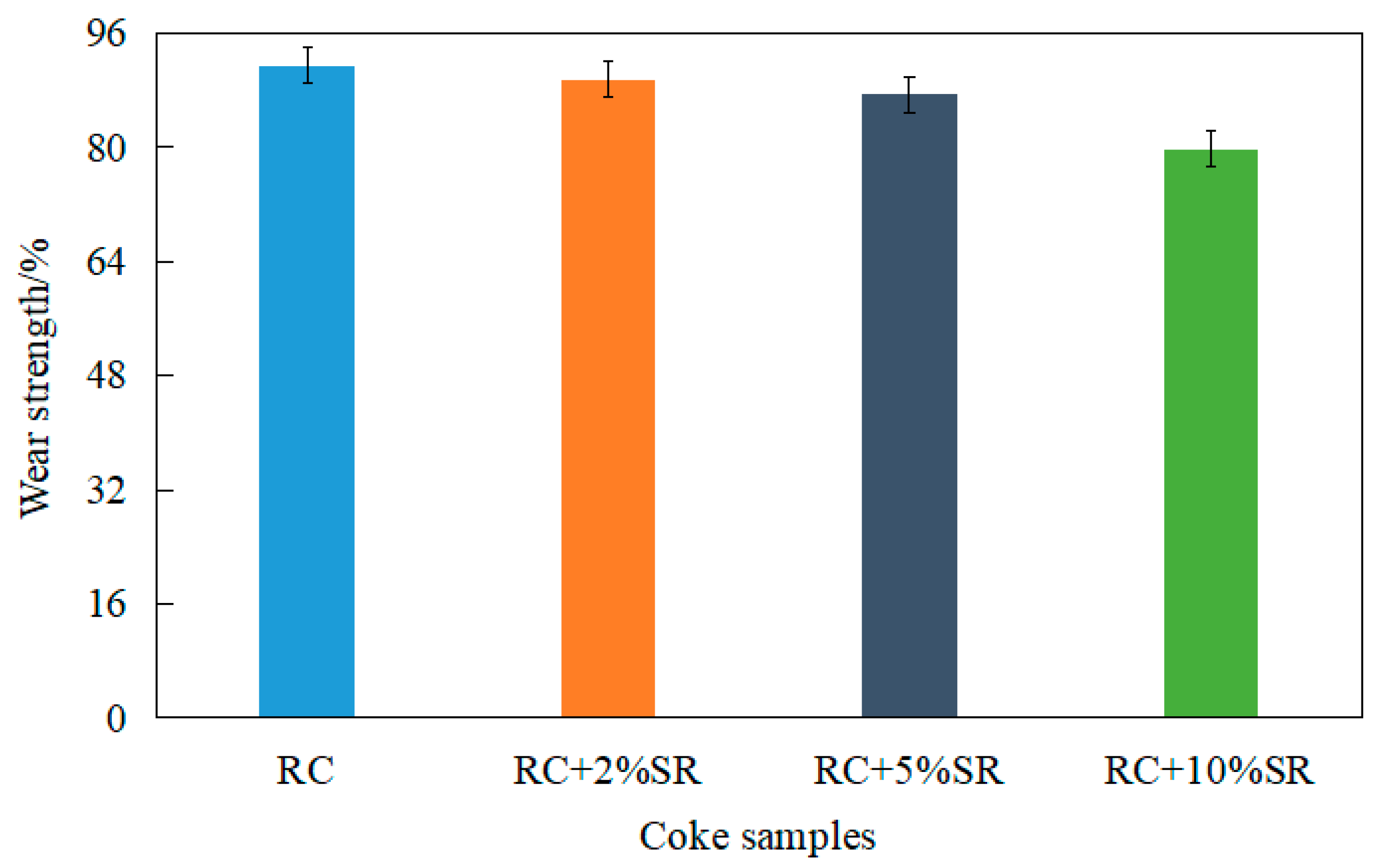
Coke Wear Strength
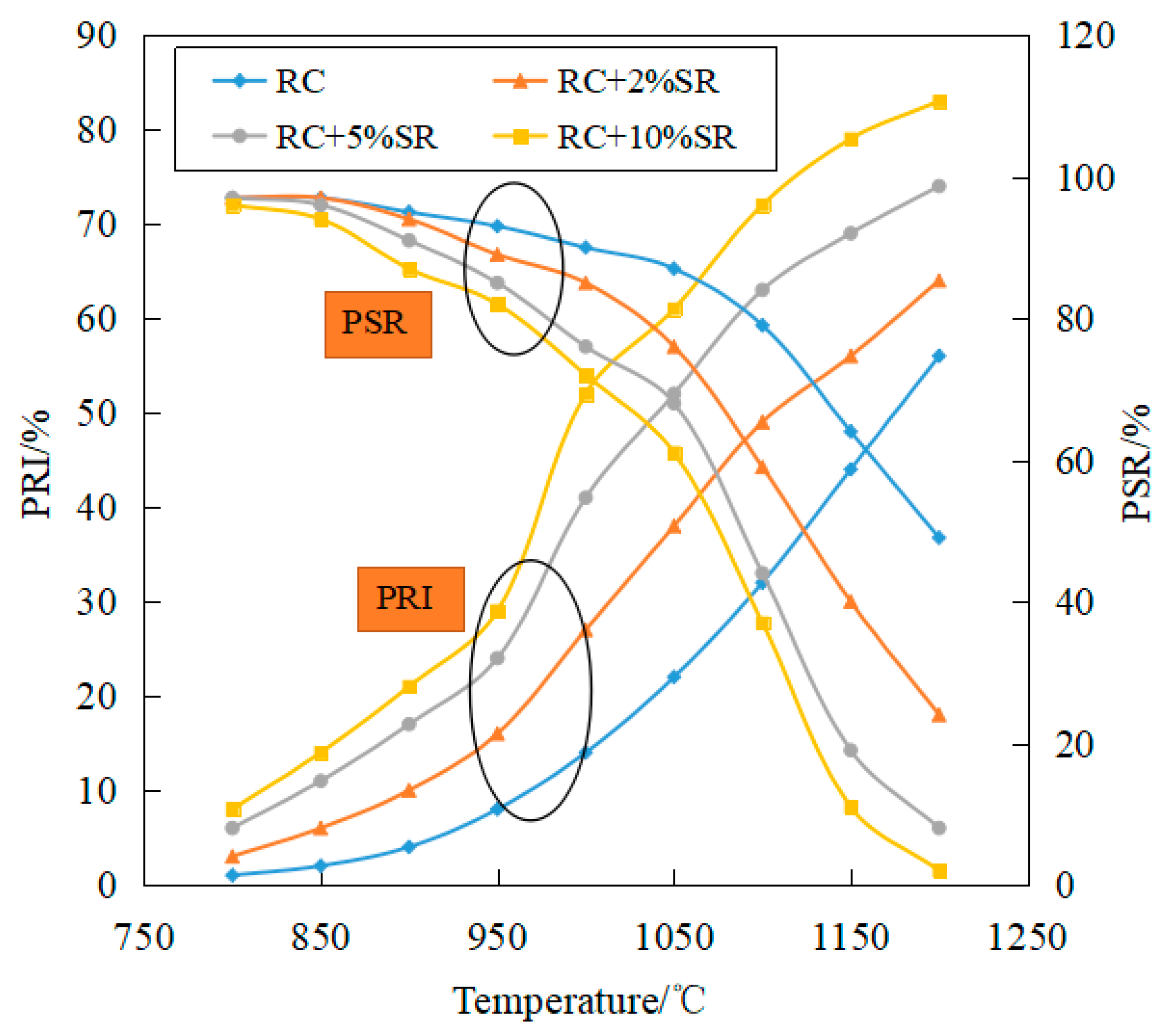
3.3.2. Thermal Performance Test
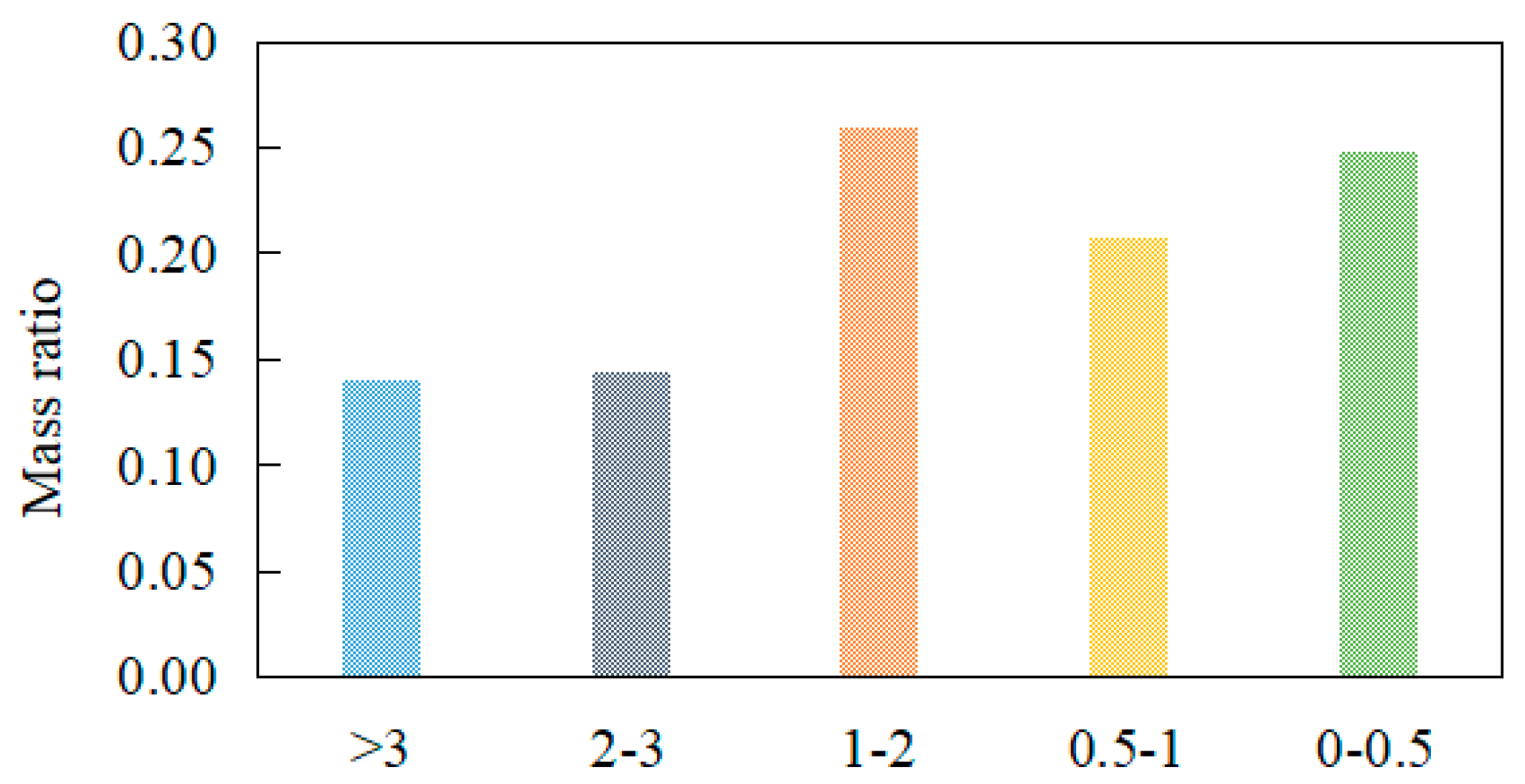
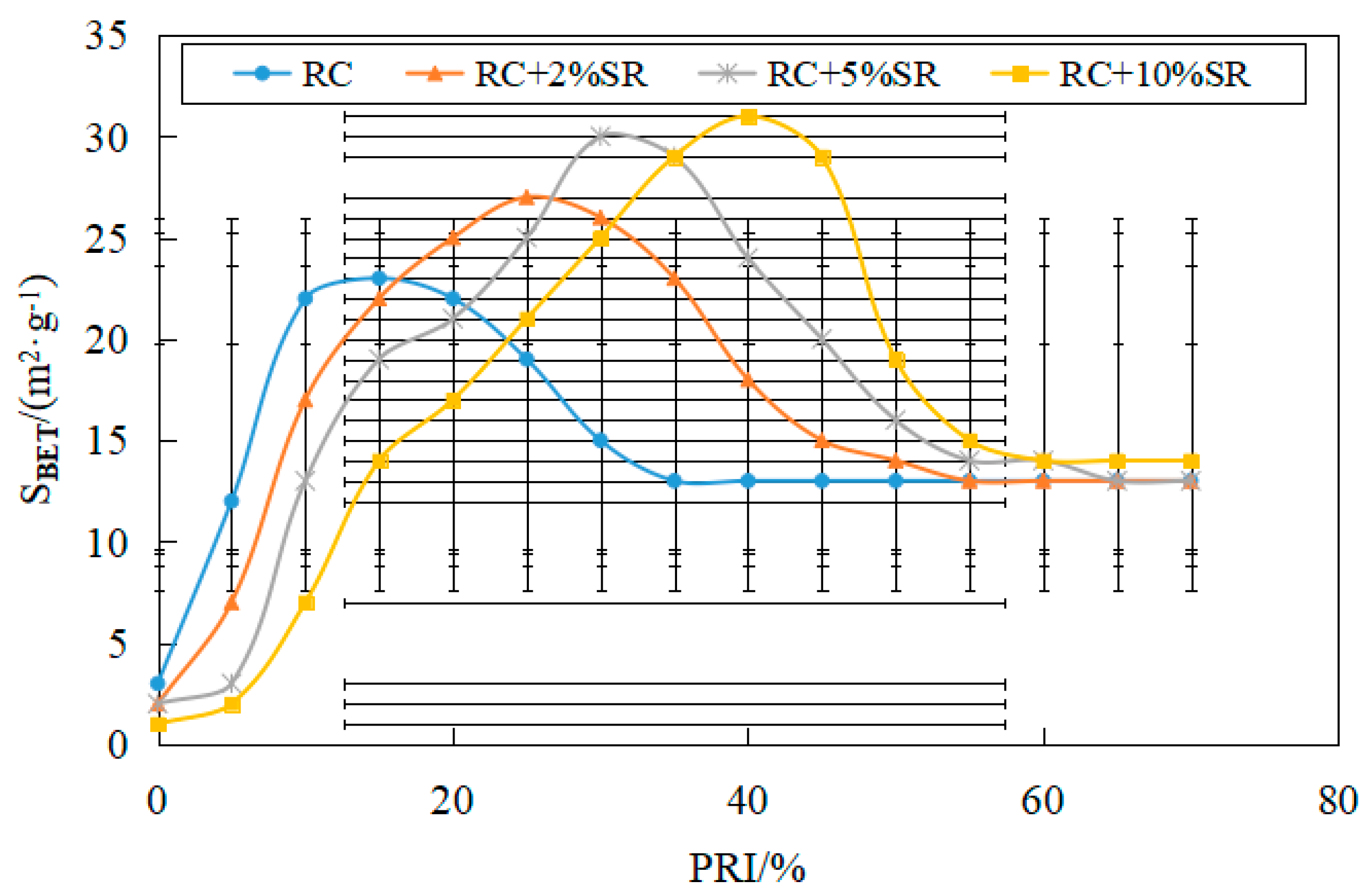
3.3.3. Relationship between Coke Reactivity and Specific Surface Area
3.4. Economic Benefit Analysis
4. Discussion
5. Conclusions
- The soda residue has the most significant influence on coking coal drying rate at 110 °C. Adding 2%, 5%, and 10% soda residue, the average drying rate of coke coal increases by 11.50%, 25.3%, and 37.3%, respectively.
- The addition of additives can improve the drying temperature of a coal sample and increase its moisture removal rate, but the additive has little influence on the transverse temperature and humidity transfer gradient of coking coal from edge to center.
- The addition of soda residue reduces the coke falling strength. The effect of adding 10% soda residue was the most obvious: the loss quantity was as high as 8.82 g. When the proportion of soda residue was not more than 5%, the loss quantity decreased by less than 10%; this range meets industrial requirements.
- The reactivity of coke increases with an increase in soda residue. With the increase in the reactivity, the specific surface area of coke increased first and then decreased; the maximum specific surface area corresponding to PRI increased with an increase in soda residue quantity. The addition of soda residue can increase the active point of the carbonization reaction and improve the reactivity of coke.
- Adding 2–5% soda residue, the coking coal sample mixed with soda residue has an obvious, energy saving advantage in the drying process. The total cost input is only 77–86% that of normal drying.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.Q. Coking Process; Chemical Industry Press: Beijing, China, 2015. [Google Scholar]
- Wang, W. Study on drying and preheating of coking coal. Chem. Enter. Manag. 2020, 3, 156–157. (In Chinese) [Google Scholar]
- Pang, K.L.; Wang, C.; Zhu, Q.M.; Wu, J.; Liu, D.J. Development and application of coal humidification technology in coking process. Angang Technol. 2017, 1, 6–11. (In Chinese) [Google Scholar]
- Guo, Y.L.; Hu, J.G.; Zhou, W.T. Development and application status of CMC in China. Mod. Chem. Ind. 2016, 36, 8–12. (In Chinese) [Google Scholar]
- Rao, Z.; Zhao, Y.; Huang, C.; Duan, C.; He, J. Recent developments in drying and dewatering for low rank coals. Prog. Energ. Combust. Sci. 2015, 46, 1–11. [Google Scholar] [CrossRef]
- Li, Z.-K.; Yan, H.-L.; Yan, J.-C.; Wang, Z.-C.; Lei, Z.-P.; Ren, S.-B.; Shui, H.-F. Drying and depolymerization technologies of Zhaotong lignite: A review. Fuel Process. Technol. 2019, 186, 88–98. [Google Scholar] [CrossRef]
- Hu, E.; Zhu, C.; Rogers, K.; Han, X.; Wang, J.; Zhao, J.; Fu, X. Coal pyrolysis and its mechanism in indirectly heated fixed-bed with metallic heating plate enhancement. Fuel 2016, 185, 656–662. [Google Scholar] [CrossRef]
- Dou, Y.; Song, Z.X.; Zhao, X.; Liu, H. Actuality and Prospect of Coal-drying Technologies. Chem. Mach. 2017, 44, 239–244. [Google Scholar]
- Chen, L.; Liao, H.-T.; Huang, X.-W.; Yang, L.-J.; Du, X.-Z.; Yang, Y.-P. Thermo-flow characteristics of indirect dry cooling system with elliptically arranged heat exchanger bundles around a traditional circular cooling tower. Appl. Therm. Eng. 2017, 121, 419–430. [Google Scholar] [CrossRef]
- Essalhi, H.; Benchrifa, M.; Tadili, R.; Bargach, M.N. Experimental and theoretical analysis of drying grapes under an indirect solar dryer and in open sun. Innov. Food Sci. Emerg. Technol. 2018, 49, 58–64. [Google Scholar] [CrossRef]
- Richards, A.P.; Haycock, D.; Frandsen, J.; Fletcher, T.H. A review of coal heating value correlations with application to coal char, tar, and other fuels. Fuel 2021, 283, 118942. [Google Scholar] [CrossRef]
- Kim, H.-S.; Matsushita, Y.; Oomori, M.; Harada, T.; Miyawaki, J.; Yoon, S.-H.; Mochida, I. Fluidized bed drying of Loy Yang brown coal with variation of temperature, relative humidity, fluidization velocity and formulation of its drying rate. Fuel 2013, 105, 415–424. [Google Scholar] [CrossRef]
- Li, H.; Zheng, C.; Lu, J.; Tian, L.; Lu, Y.; Ye, Q.; Luo, W.; Zhu, X. Drying kinetics of coal under microwave irradiation based on a coupled electromagnetic, heat transfer and multiphase porous media model. Fuel 2019, 256, 115966. [Google Scholar] [CrossRef]
- Song, Z.; Jing, C.; Yao, L.; Zhao, X.; Sun, J.; Wang, W.; Mao, Y.; Ma, C. Coal slime hot air/microwave combined drying characteristics and energy analysis. Fuel Process. Technol. 2017, 156, 491–499. [Google Scholar] [CrossRef]
- Zhou, F.; Cheng, J.; Liu, J.; Wang, Z.; Zhou, J.; Cen, K. Improving the permittivity of Indonesian lignite with NaCl for the microwave dewatering enhancement of lignite with reduced fractal dimensions. Fuel 2015, 162, 8–15. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, F.; Wang, X.; Liu, J.; Wang, Z.; Zhou, J.; Cen, K. Optimization of microwave dewatering of an Indonesian lignite. Fuel Process. Technol. 2016, 144, 71–78. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, S.; Suo, G.; Qiu, G.; Yin, C.; Li, Y.; Hu, M.; Xiao, X.; Wen, L. Effects of Fe2O3 addition on the thermoplasticity and structure of coking coal matrix during thermoplastic stage of pyrolysis. Fuel 2020, 260, 116305. [Google Scholar] [CrossRef]
- Wang, G.; Ren, S.; Zhang, J.; Ning, X.; Liang, W.; Zhang, N.; Wang, C. Influence mechanism of alkali metals on CO2 gasification properties of metallurgical coke. Chem. Eng. J. 2020, 387, 124093. [Google Scholar] [CrossRef]
- Gornostayev, S.; Härkki, J.; Kerkkonen, O. Transformations of pyrite during formation of metallurgical coke. Fuel 2009, 88, 2032–2036. [Google Scholar] [CrossRef]
- Liu, P.F.; Zhang, S.; Wang, J.P.; Li, P.; Liang, Y.H. Improving coke reactivity by adding soda residue. Coal Convers. 2015, 38, 65–69. (In Chinese) [Google Scholar]
- Wang, K. Research on Sulfur Transformation and Effect Behavior of Additives in Coking Processing of High Sulfur Coal. Doctoral Thesis, Chongqing University, Chongqing, China, 2012. (In Chinese). [Google Scholar]
- Zhao, X.; Liu, C.; Wang, L.; Zuo, L.; Zhu, Q.; Ma, W. Physical and mechanical properties and micro characteristics of fly ash-based geopolymers incorporating soda residue. Cem. Concr. Compos. 2019, 98, 125–136. [Google Scholar] [CrossRef]
- Yan, M.; Song, X.N.; Tian, J.; Lv, X.B.; Zhang, Z.; Yu, X.Y.; Zhang, S.T. Construction of a New Type of Coal Moisture Control Device Based on the Characteristic of Indirect Drying Process of Coking Coal. Energies 2020, 13, 4162. [Google Scholar] [CrossRef]
- Zhu, J.-F.; Liu, J.-Z.; Wu, J.-H.; Cheng, J.; Zhou, J.-H.; Cen, K.-F. Thin-layer drying characteristics and modeling of Ximeng lignite under microwave irradiation. Fuel Process. Technol. 2015, 130, 62–70. [Google Scholar] [CrossRef]
- Tang, J.; Feng, L.; Li, Y.; Liu, J.; Liu, X. Fractal and pore structure analysis of Shengli lignite during drying process. Powder Technol. 2016, 303, 251–259. [Google Scholar] [CrossRef]
- Xu, G.; Huang, J.; Hu, G.; Yang, N.; Zhu, J.; Chang, P. Experimental study on effective microwave heating/fracturing of coal with various dielectric property and water saturation. Fuel Process. Technol. 2020, 202, 106378. [Google Scholar] [CrossRef]
- Karthikeyan, M. Minimization of Moisture Readsorption in Dried Coal Samples. Dry. Technol. 2008, 26, 948–955. [Google Scholar] [CrossRef]
- Demirbas, A. Gaseous products from biomass by pyrolysis and gasification: Effects of catalyst on hydrogen yield. Energy Convers. Manag. 2002, 43, 897–909. [Google Scholar] [CrossRef]
- Yu, X.; Yu, D.; Liu, F.; Wu, J.; Xu, M. High-temperature pyrolysis of petroleum coke and its correlation to in-situ char-CO2 gasification reactivity. Proc. Combust. Inst. 2020. In press. [Google Scholar] [CrossRef]
- Li, K.; Khanna, R.; Zhang, J.; Liu, Z.; Sahajwalla, V.; Yang, T.; Kong, D. The evolution of structural order, microstructure and mineral matter of metallurgical coke in a blast furnace: A review. Fuel 2014, 133, 194–215. [Google Scholar] [CrossRef]
- Grigore, M.; Sakurovs, R.; French, D.; Sahajwalla, V. Mineral matter in coals and their reactions during coking. Int. J. Coal Geol. 2008, 76, 301–308. [Google Scholar] [CrossRef]
- Dastidar, M.G.; Bhattacharyya, A.; Sarkar, B.K.; Dey, R.; Mitra, M.K.; Schenk, J. The effect of alkali on the reaction kinetics and strength of blast furnace coke. Fuel 2020, 268, 1–10. [Google Scholar] [CrossRef]
- Xiong, Z.; Syed-Hassan, S.S.A.; Hu, X.; Guo, J.; Qiu, J.; Zhao, X.; Su, S.; Hu, S.; Wang, Y.; Xiang, J. Pyrolysis of the aromatic-poor and aromatic-rich fractions of bio-oil: Characterization of coke structure and elucidation of coke formation mechanism. Appl. Energy 2019, 239, 981–990. [Google Scholar] [CrossRef]
- Andreoli, S.; Eser, S. Relating reactivity to structure in cokes and carbon materials: Temperature-programmed oxidation and microscopy techniques. Carbon 2020, 168, 362–371. [Google Scholar] [CrossRef]
- Orikasa, H.; Kyotani, T.; Shimoyama, I.; Anraku, D. Preparation of Highly Reactive and High Strength Coke by the Addition of Ca-ion Exchanged Brown Coal. Tetsu Hagane-J. Iron Steel Inst. Jan. 2010, 96, 272–279. [Google Scholar] [CrossRef][Green Version]
- Liu, M.; He, Q.; Bai, J.; Yu, J.; Kong, L.; Bai, Z.; Li, H.; He, C.; Cao, X.; Ge, Z.; et al. Char reactivity and kinetics based on the dynamic char structure during gasification by CO2. Fuel Process. Technol. 2021, 211, 106583. [Google Scholar] [CrossRef]
- Everson, R.C.; Neomagus, H.W.J.P.; van der Merwe, G.W.; Koekemoer, A.; Bunt, J.R. The properties of large coal particles and reaction kinetics of corresponding chars. Fuel 2015, 140, 17–26. [Google Scholar] [CrossRef]
- Coetzee, S.; Neomagus, H.W.J.P.; Bunt, J.R.; Everson, R.C. Improved reactivity of large coal particles by K2CO3 addition during steam gasification. Fuel Process. Technol. 2013, 114, 75–80. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, B.B.; Wang, F.X.; Liang, Y.H. Effect of alkaline metal on thermal properties of coke. Coke Convers. 2008, 31, 43–47. [Google Scholar]
- Bunt, J.R.; Neomagus, H.W.J.P.; Botha, A.A.; Waanders, F.B. Reactivity study of fine discard coal agglomerates. J. Anal. Appl. Pyrolysis 2015, 113, 723–728. [Google Scholar] [CrossRef]








| Samples | Proximate Analysis (wt%, d) | Ultimate Analysis (wt%, daf) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mar | Ad | Vdaf | Fad | C | H | N | S | |
| Xingtai | 10.80 | 7.88 | 36.19 | 19.66 | 86.33 | 5.49 | 1.52 | 0.38 |
| CaCO3 | Na2CO3 | Al2O3 | KCl | NaCl | Mg(OH)2 | SiO2 | Insoluble |
|---|---|---|---|---|---|---|---|
| 53.22 | 14.78 | 3.24 | 0.33 | 6.97 | 5.12 | 8.96 | 7.38 |
| Coke Samples | Mass before Drop (g) | Mass after Drop (g) | Drop Strength (%) | Loss Quantity (g) |
|---|---|---|---|---|
| RC | 400.26 | 395.74 | 98.87 | 4.52 |
| RC + 2% SR | 400.78 | 396.11 | 98.83 | 4.67 |
| RC + 5% SR | 403.82 | 398.83 | 98.76 | 4.99 |
| RC + 10% SR | 402.32 | 393.50 | 97.80 | 8.82 |
| L (cm) | Sample | W0 (%) | Wt (%) | T (min) | Q (KW·h) | H (KJ·kg−1) | C (USD·kg−1) |
|---|---|---|---|---|---|---|---|
| 15 | RC | 11 | 6 | 220 | 2.93 | 5274 | 0.2212 |
| 2%SR + RC | 197 | 2.53 | 4554 | 0.1912 | |||
| 5%SR + RC | 175 | 2.27 | 4086 | 0.1712 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, S. Indirect Drying and Coking Characteristics of Coking Coal with Soda Residue Additive. Energies 2021, 14, 575. https://doi.org/10.3390/en14030575
Zhang Z, Zhang S. Indirect Drying and Coking Characteristics of Coking Coal with Soda Residue Additive. Energies. 2021; 14(3):575. https://doi.org/10.3390/en14030575
Chicago/Turabian StyleZhang, Ze, and Shuting Zhang. 2021. "Indirect Drying and Coking Characteristics of Coking Coal with Soda Residue Additive" Energies 14, no. 3: 575. https://doi.org/10.3390/en14030575
APA StyleZhang, Z., & Zhang, S. (2021). Indirect Drying and Coking Characteristics of Coking Coal with Soda Residue Additive. Energies, 14(3), 575. https://doi.org/10.3390/en14030575





