Evaluation of Formate Salt PCM’s for Latent Heat Thermal Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Latent Heat and Thermal Stability of PCMs
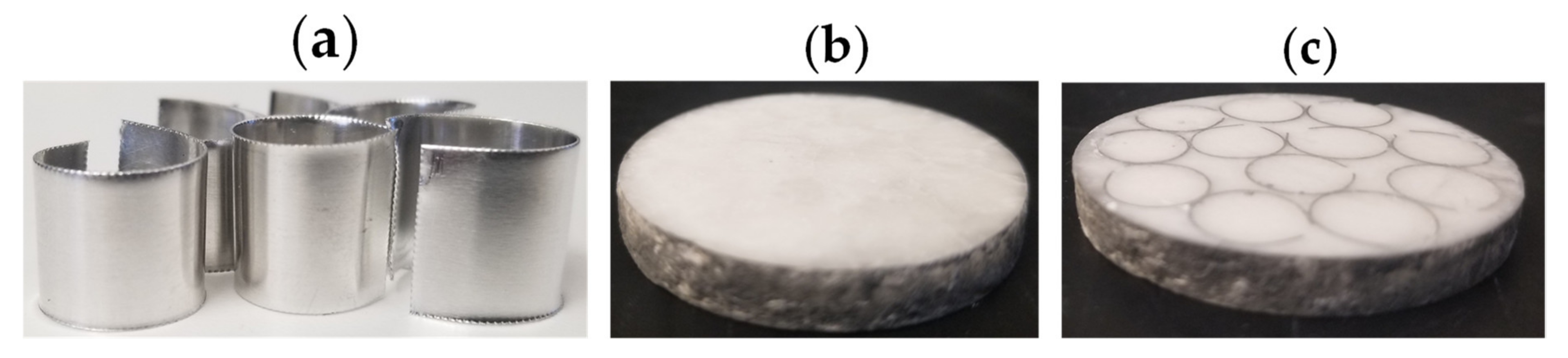
2.2. Thermal Conductivity/Thermal Enhancement
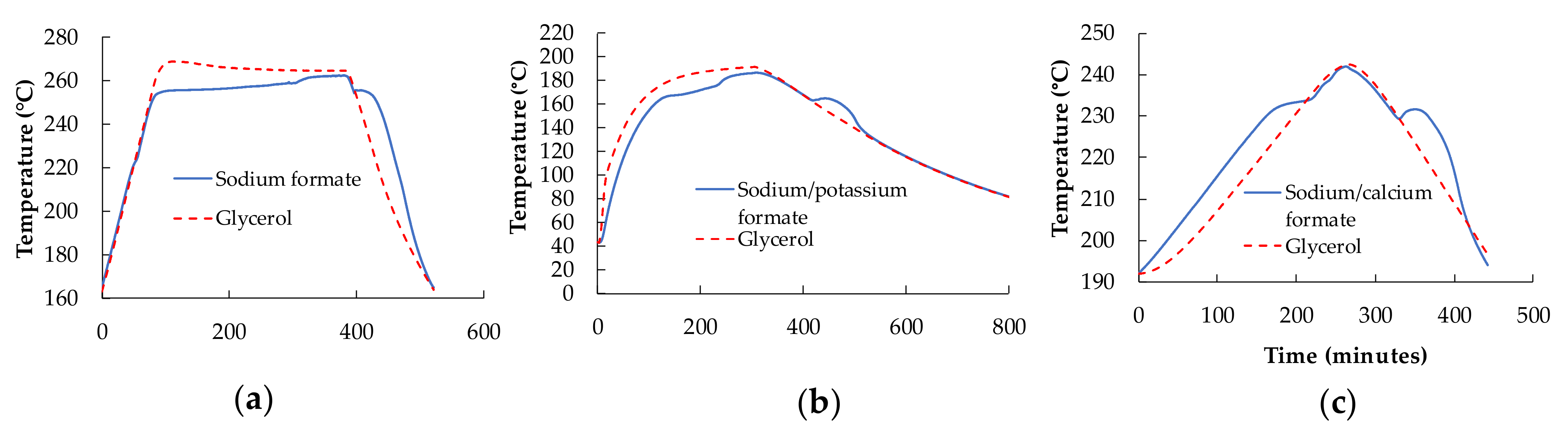
2.3. T-History Method
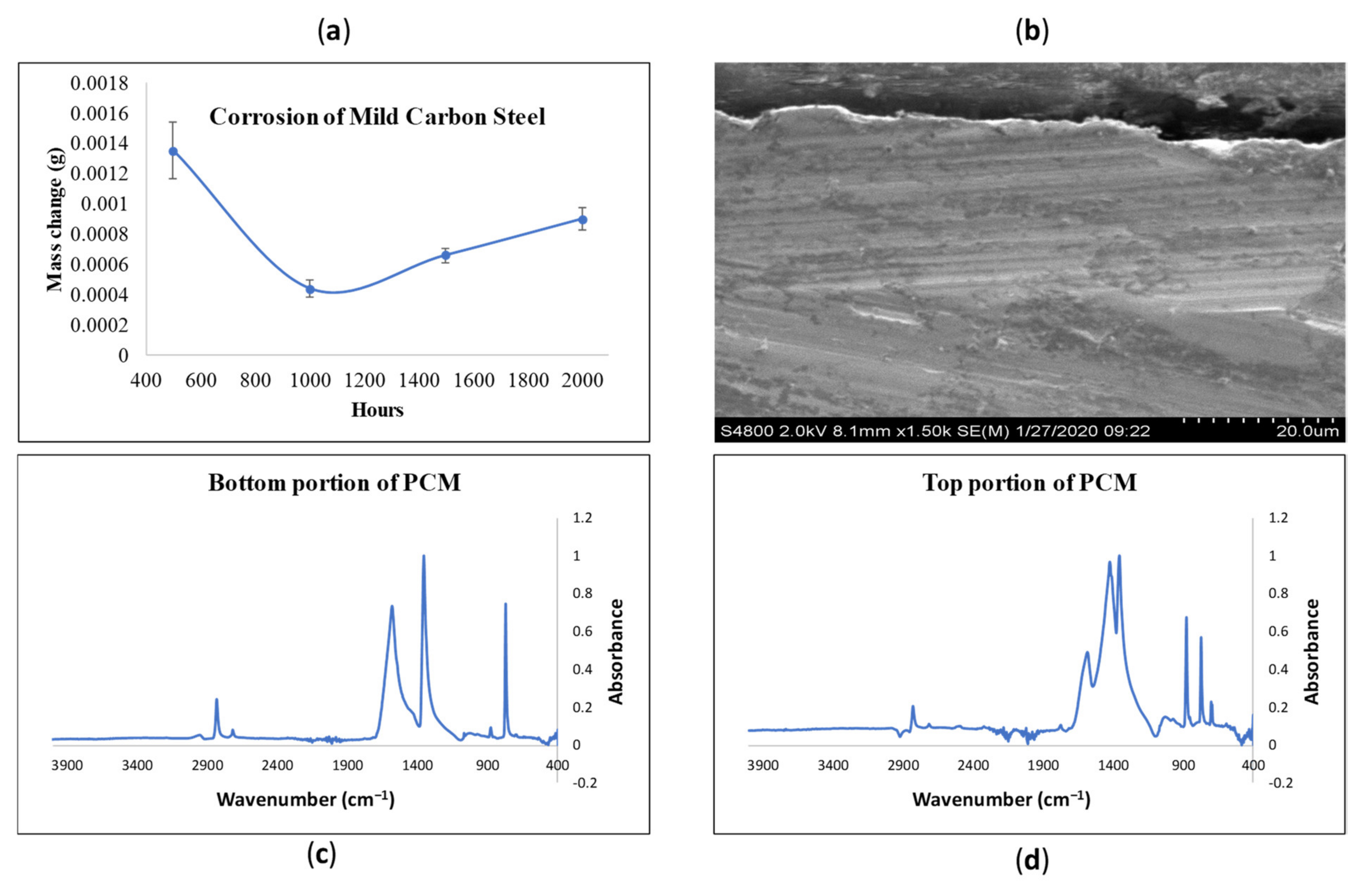
2.4. Corrosion Studies
3. Results and Discussion
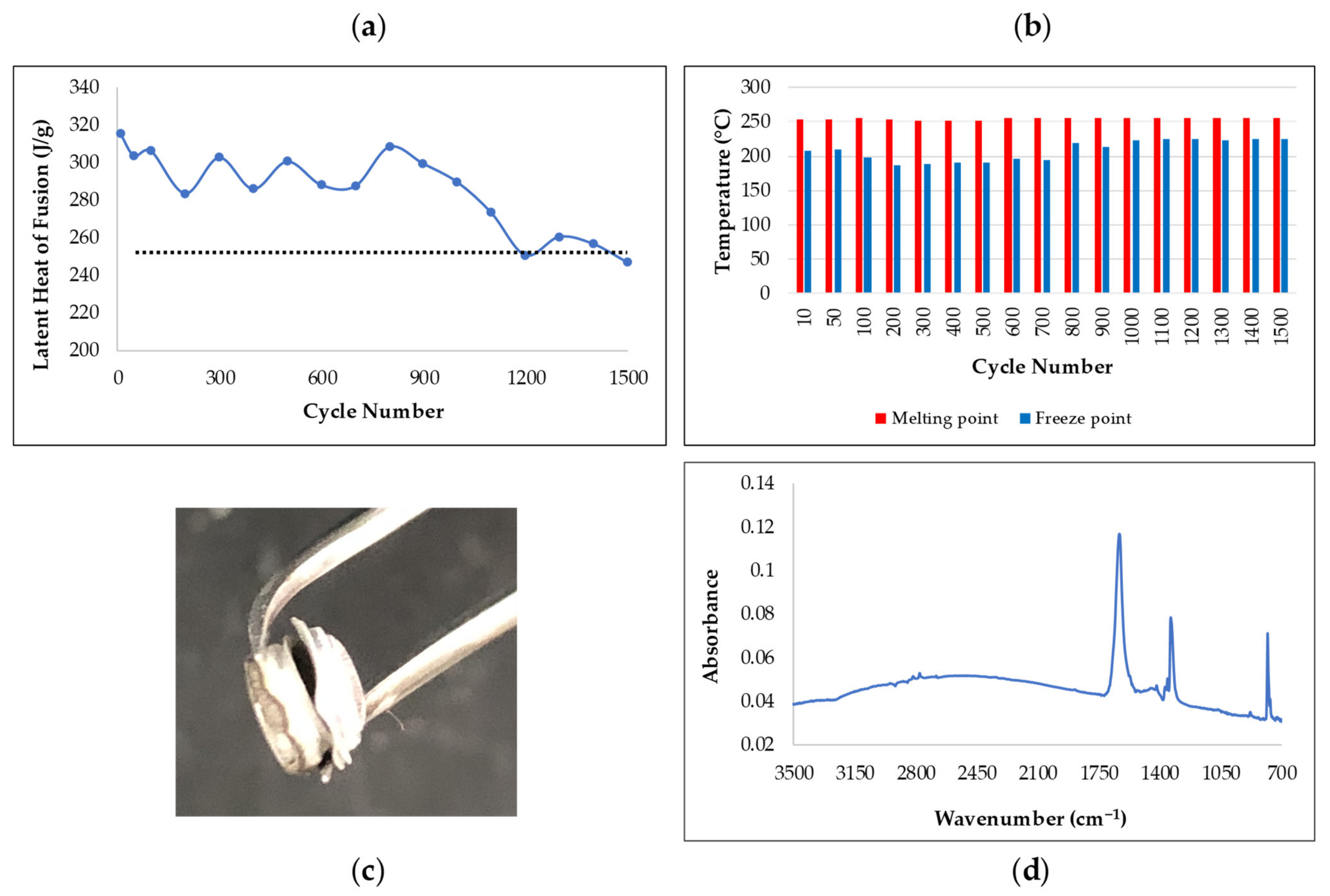
3.1. Latent Heat and Thermal Stability of PCMs
3.2. Supercooling Measurement Using T-History Method
3.3. Thermal Conductivity Enhancement
3.4. Density Measurements
3.5. Corrosion Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McMillan, C.A.; Ruth, M. Using Facility-Level Emissions Data to Estimate the Technical Potential of Alternative Thermal Sources to Meet Industrial Heat Demand. Appl. Energy 2019, 239, 1077–1090. [Google Scholar] [CrossRef]
- Turchi, C.S.; Kurup, P.; Zhu, G. Revisiting Parabolic Trough Concentrators for Industrial Process Heat in the United States. In Proceedings of the ASME 2016 Power Conference collocated with the ASME 2016 10th International Conference on Energy Sustainability and the ASME 2016 14th International Conference on Fuel Cell Science, Engineering and Technology, Charlotte, NC, USA, 26–30 June 2016. [Google Scholar]
- Jin, X.; Hu, H.; Shi, X.; Zhou, X.; Zhang, X. Comparison of Two Numerical Heat Transfer Models for Phase Change Material Board. Appl. Therm. Eng. 2018, 128, 1331–1339. [Google Scholar] [CrossRef]
- Sharan, P.; Turchi, C.; Kurup, P. Optimal Design of Phase Change Material Storage for Steam Production Using Annual Simulation. Sol. Energy 2019, 185, 494–507. [Google Scholar] [CrossRef]
- Fox, D.B.; Sutter, D.; Tester, J.W. The Thermal Spectrum of Low-Temperature Energy Use in the United States; Cornell University: Ithaca, NY, USA, 2011. [Google Scholar]
- Pereira da Cunha, J.; Eames, P. Thermal Energy Storage for Low and Medium Temperature Applications Using Phase Change Materials—A Review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Li, P.; Chan, C. Application of Phase Change Materials for Thermal Energy Storage in Concentrated Solar Thermal Power Plants: A Review to Recent Developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase Change Materials for Thermal Energy Storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Nakaso, K.; Teshima, H.; Yoshimura, A.; Nogami, S.; Hamada, Y.; Fukai, J. Extension of Heat Transfer Area Using Carbon Fiber Cloths in Latent Heat Thermal Energy Storage Tanks. Chem. Eng. Process. Process Intensif. 2008, 47, 879–885. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R.L. Thermal Cycling Test of Few Selected Inorganic and Organic Phase Change Materials. Renew. Energy 2008, 33, 2606–2614. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A Review on Phase Change Energy Storage: Materials and Applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Liu, Z.; Sun, W.; Liu, S. Formation of Sodium Oxalate by Thermal Decomposition of Sodium Formate. J. Beijing Uni. Chem. Tech. 2007, 34, 566–569. [Google Scholar]
- Yinping, Z.; Yi, J.; Yi, J. A Simple Method, the History Method, of Determining the Heat of Fusion, Specific Heat and Thermal Conductivity of Phase-Change Materials. Meas. Sci. Technol. 1999, 10, 201–205. [Google Scholar] [CrossRef]
- Gadd, H.; Werner, S. Achieving Low Return Temperatures from District Heating Substations. Appl. Energy 2014, 136, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Gomez, J.C. High-Temperature Phase Change Materials (PCM) Candidates for Thermal Energy Storage (TES) Applications; NREL/TP-5500-51446; National Renewable Energy Laboratory: Denver W Pkwy, CO, USA, 2011. [Google Scholar] [CrossRef] [Green Version]
- Acree, W.E.; Chickos, J.S. Evaluated Infrared Reference Spactra. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [Google Scholar] [CrossRef]
- Meisel, T.; Halmos, Z.; Seybold, K.; Pungor, E. The Thermal Decomposition of Alkali Metal Formates. J. Therm. Anal. 1975, 7, 73–80. [Google Scholar] [CrossRef]
- Beaupere, N.; Soupremanien, U.; Zalewski, L. Nucleation Triggering Methods in Supercooled Phase Change Materials (PCM), a Review. Thermochim. Acta 2018, 670, 184–201. [Google Scholar] [CrossRef]
- Cui, W.; Yuan, Y.; Sun, L.; Cao, X.; Yang, X. Experimental Studies on the Supercooling and Melting/Freezing Characteristics of Nano-Copper/Sodium Acetate Trihydrate Composite Phase Change Materials. Renew. Energy 2016, 99, 1029–1037. [Google Scholar] [CrossRef]

| Sodium Formate | Sodium/Calcium Formate | Sodium/Potassium Formate | |
|---|---|---|---|
| Average Hf (J/g) | 252.3 ± 0.7 | 171.1 ± 0.7 | 134.0 ± 1.1 |
| Average melting point (°C) | 256.5 ± 2.8 | 229.4 ± 0.1 | 155.3 ± 0.3 |
| Temperature (°C) | Thermal Conductivity (W/m·K) without Rings | Thermal Conductivity (W/m·K) with Rings |
|---|---|---|
| 32 | 0.42 | 3.5 |
| 57 | 0.44 | 3.7 |
| 82 | 0.46 | 3.7 |
| 107 | 0.47 | 3.7 |
| PCM | Liquid Density (g/mL) | Solid Density (g/mL) |
|---|---|---|
| Sodium/potassium formate | 1.67 | 1.72 |
| Sodium/calcium formate | 1.61 | 1.76 |
| Sodium formate | 1.50 | 1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gage, S.; Sharan, P.; Turchi, C.; Netter, J. Evaluation of Formate Salt PCM’s for Latent Heat Thermal Energy Storage. Energies 2021, 14, 765. https://doi.org/10.3390/en14030765
Gage S, Sharan P, Turchi C, Netter J. Evaluation of Formate Salt PCM’s for Latent Heat Thermal Energy Storage. Energies. 2021; 14(3):765. https://doi.org/10.3390/en14030765
Chicago/Turabian StyleGage, Samuel, Prashant Sharan, Craig Turchi, and Judy Netter. 2021. "Evaluation of Formate Salt PCM’s for Latent Heat Thermal Energy Storage" Energies 14, no. 3: 765. https://doi.org/10.3390/en14030765
APA StyleGage, S., Sharan, P., Turchi, C., & Netter, J. (2021). Evaluation of Formate Salt PCM’s for Latent Heat Thermal Energy Storage. Energies, 14(3), 765. https://doi.org/10.3390/en14030765






