Improvement of Photoautotrophic Algal Biomass Production after Interrupted CO2 Supply by Urea and KH2PO4 Injection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Strains and Culture Conditions
2.2. Analytical Methods
2.2.1. Analysis of Algal Cell Growth
2.2.2. Determination of pH and Dissolved Inorganic Carbon in the Medium
2.2.3. Lipid Extraction and Lipid Content of Algal Cells
2.3. Outdoor Cultivation of Microalgae Using Flue Gas from Coal-Fired Power Plant
3. Results and Discussion
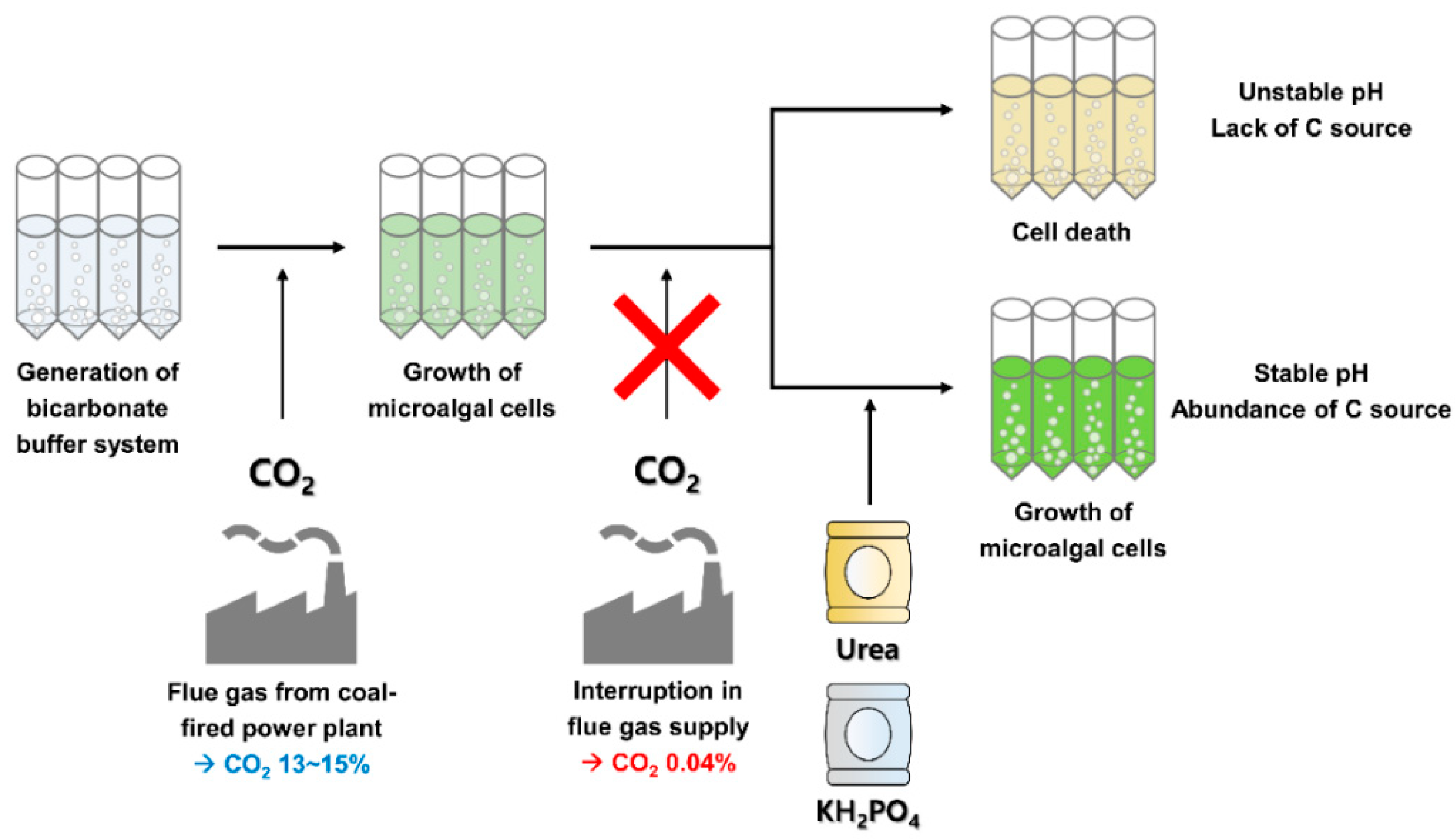
3.1. Introduction of Urea and KH2PO4 to Microalgae Culture System for Preventing Cell Growth Inhibition from Interruption in CO2 Supply
3.2. Effect of Urea and KH2PO4 on Microalgal Biomass Production
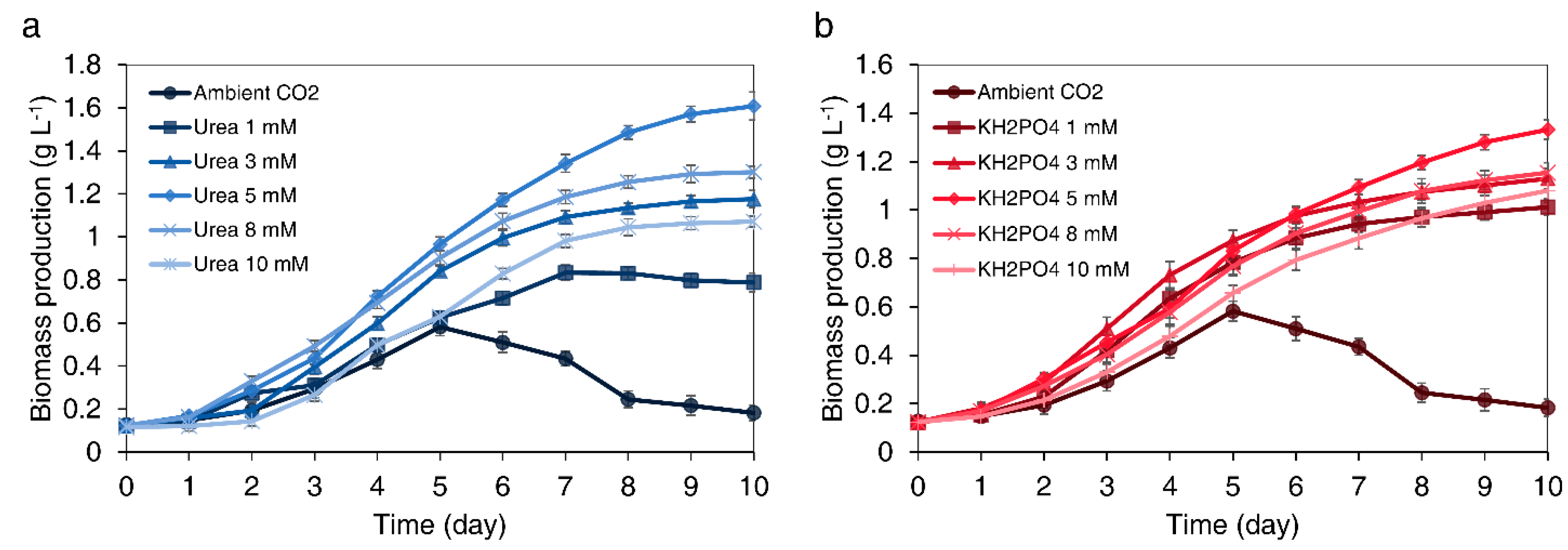
3.2.1. Effect of Urea Concentration on Biomass Production
3.2.2. Effect of KH2PO4 Concentration on Biomass Production
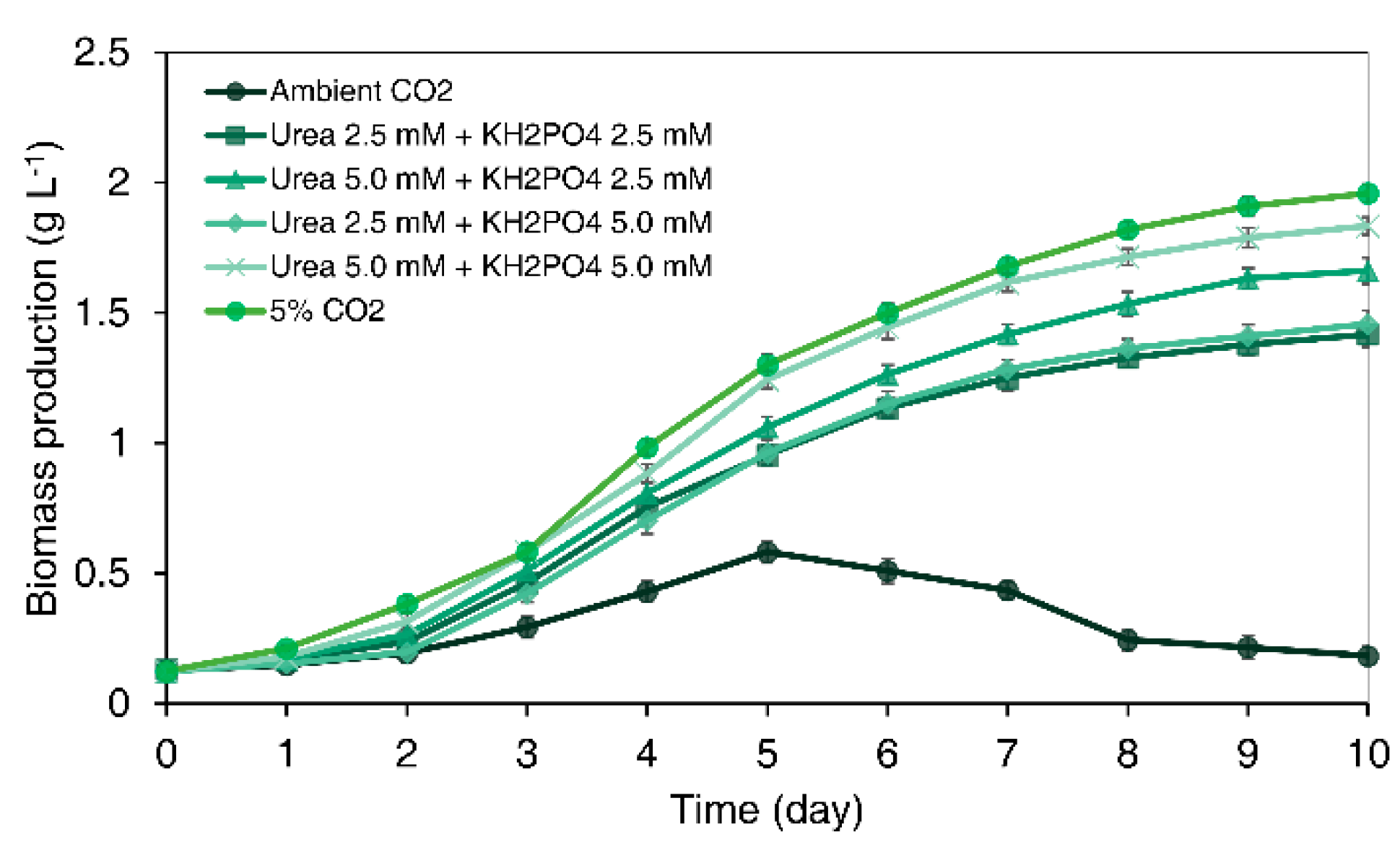
3.3. Synergistic Effect of the Combination of Urea and KH2PO4 on Microalgal Cell Growth
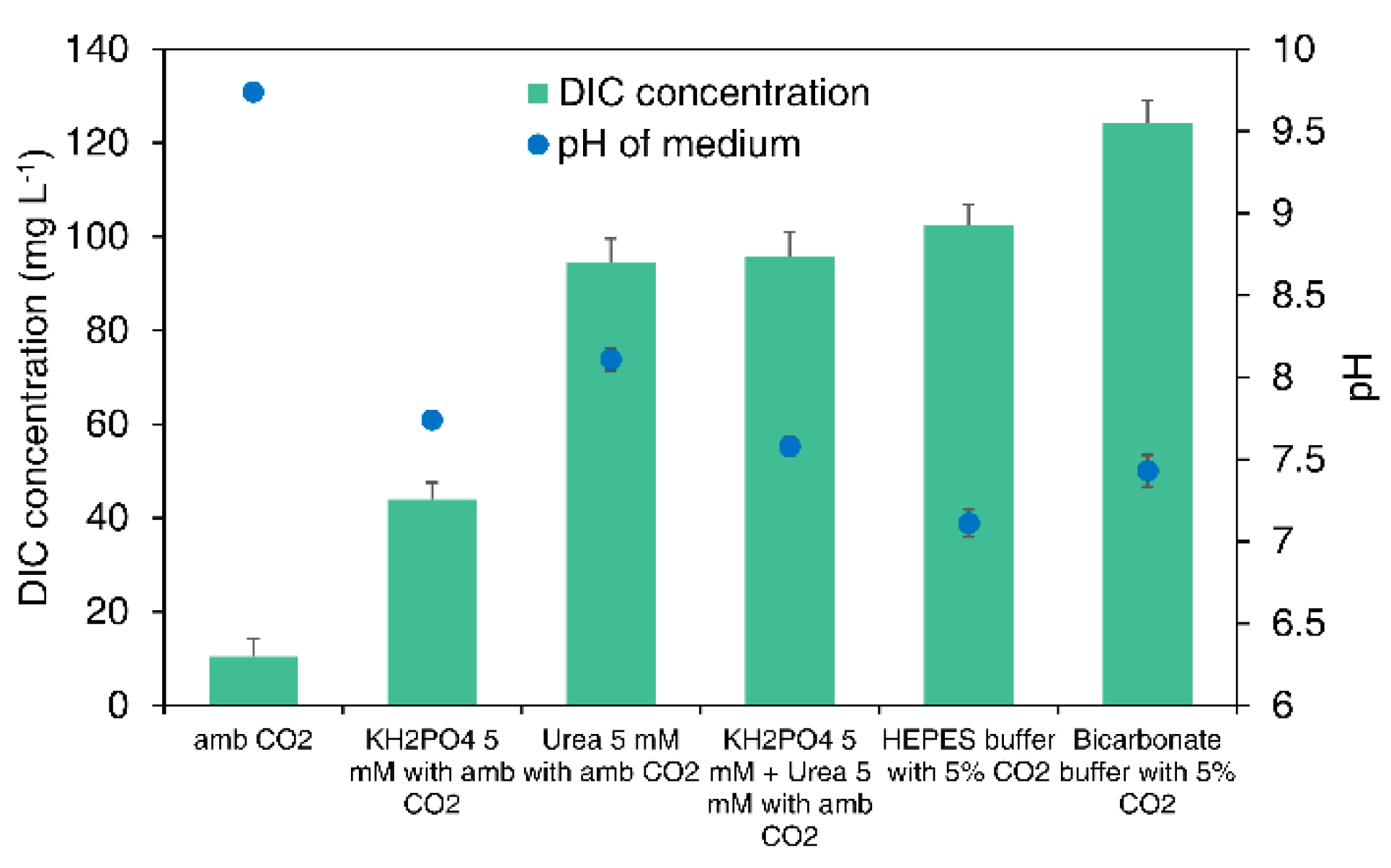
3.4. Dissolved Inorganic Carbon (DIC) Concentration and pH of the Medium with Different Buffer Systems
3.4.1. DIC Concentration of the Medium
3.4.2. pH of the Medium
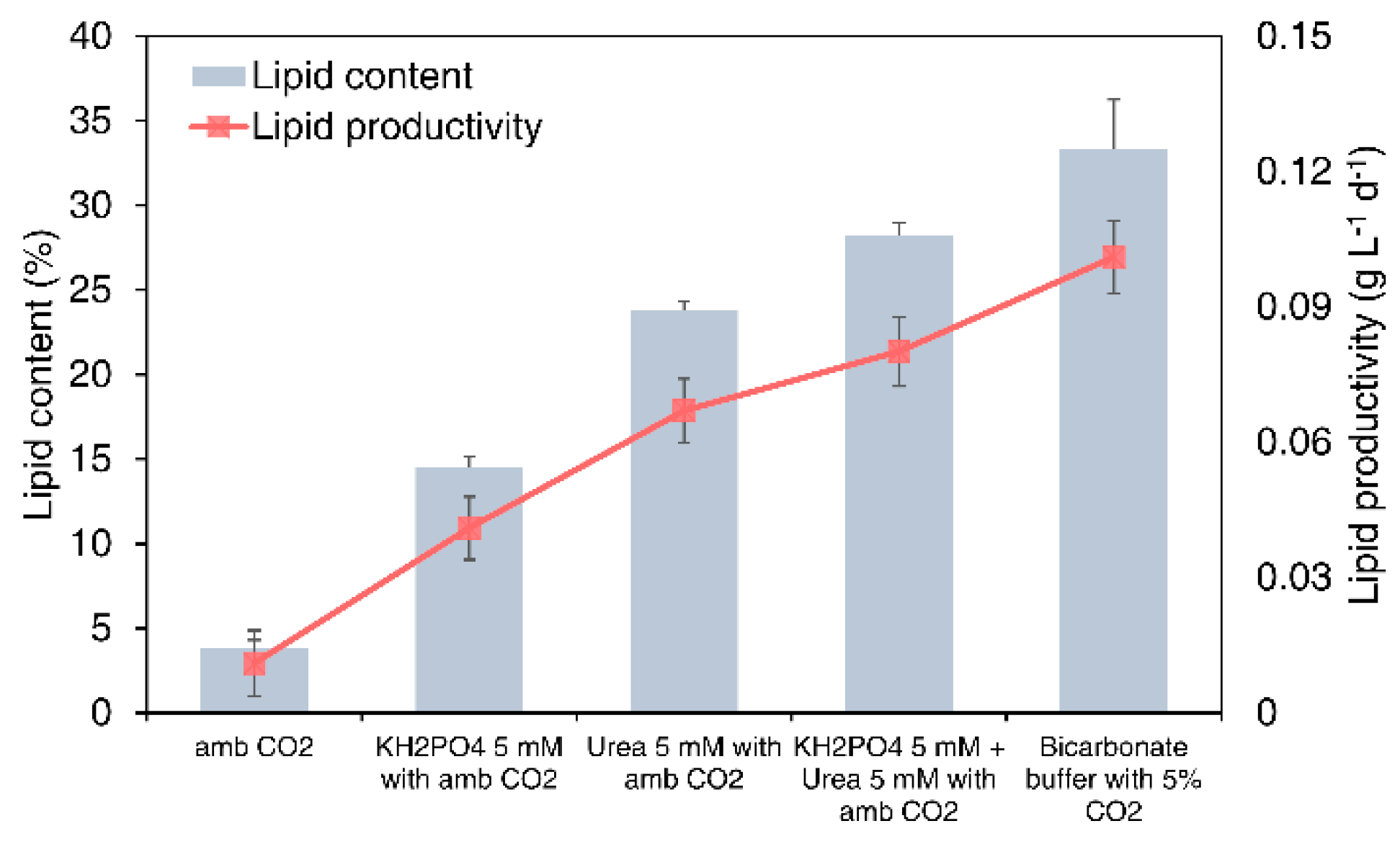
3.5. Comparison of Microalgal Lipid Productivity According to Different Carbon Sources and Buffer Systems
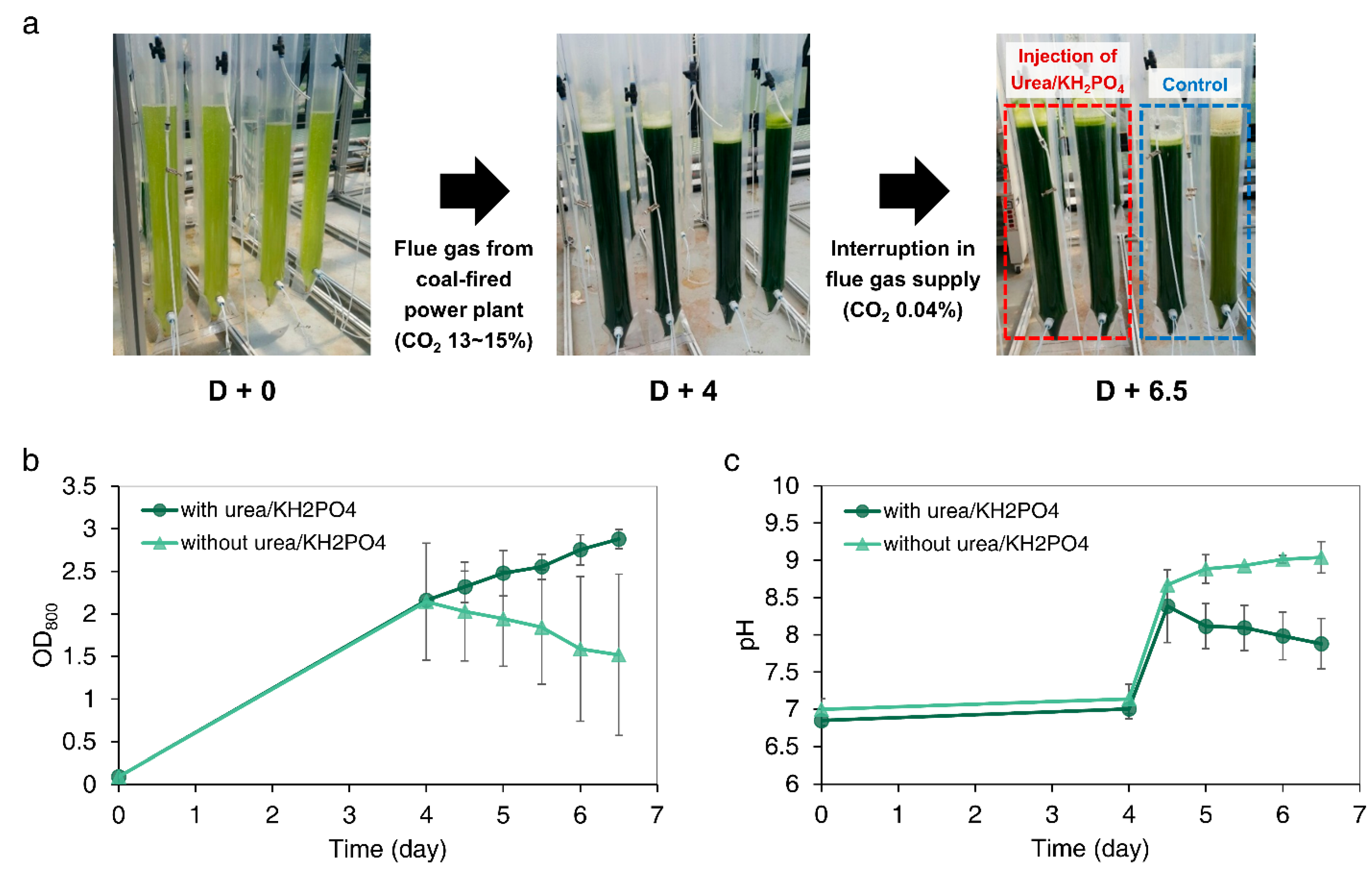
3.6. Outdoor Cultivation of Microalgae after Interruption of Flue Gas Supply from Coal-Fired Power Plant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, S.; Plattner, G.K.; Knutti, R.; Friedlingstein, P. Irreversible Climate Change Due to Carbon Dioxide Emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekwurzel, B.; Boneham, J.; Dalton, M.W.; Heede, R.; Mera, R.J.; Allen, M.R.; Frumhoff, P.C. The Rise in Global Atmospheric CO2, Surface Temperature, and Sea Level from Emissions Traced to Major Carbon Producers. Clim. Chang. 2017, 144, 579–590. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct Human Health Risks of Increased Atmospheric Carbon Dioxide. Natl. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Choi, H.I.; Lee, J.S.; Choi, J.W.; Shin, Y.S.; Sung, Y.J.; Hong, M.E.; Kwak, H.S.; Kim, C.Y.; Sim, S.J. Performance and Potential Appraisal of Various Microalgae as Direct Combustion Fuel. Bioresour. Technol. 2019, 273, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Q.; Qi, Y.; Chen, G.; Song, Y.; Kansha, Y.; Kitamura, Y. Absorption-Microalgae Hybrid CO2 Capture and Biotransformation Strategy—A Review. Int. J. Greenh. Gas Control. 2019, 88, 109–117. [Google Scholar] [CrossRef]
- Hong, M.E.; Yu, B.S.; Patel, A.K.; Choi, H.I.; Song, S.; Sung, Y.J.; Chang, W.S.; Sim, S.J. Enhanced Biomass and Lipid Production of Neochloris oleoabundans Under High Light Conditions by Anisotropic Nature of Light-Splitting CaCO3 Crystal. Bioresour. Technol. 2019, 287, 121483. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Photosynthesis-to-Fuels: From Sunlight to Hydrogen, Isoprene, and Botryococcene Production. Energy Environ. Sci. 2012, 5, 5531–5539. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of Heterotrophic and Photoautotrophic Induction on Astaxanthin Production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Joun, J.M.; Lee, J.; Hong, M.E.; Pham, H.M.; Chang, W.S.; Sim, S.J. Development of Large-Scale and Economic pH Control System for Outdoor Cultivation of Microalgae Haematococcus pluvialis Using Industrial Flue Gas. Bioresour. Technol. 2017, 244, 1235–1244. [Google Scholar] [CrossRef]
- Choi, S.Y.; Wang, J.Y.; Kwak, H.S.; Lee, S.M.; Um, Y.; Kim, Y.; Sim, S.J.; Choi, J.I.; Woo, H.M. Improvement of Squalene Production from CO2 in Synechococcus elongatus PCC 7942 by Metabolic Engineering and Scalable Production in a Photobioreactor. ACS Synth. Biol. 2017, 6, 1289–1295. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, P.C.; Tsai, C.J.; Lee, D.J.; Chang, J.S. Engineering Strategies for Simultaneous Enhancement of C-Phycocyanin Production and CO2 Fixation with Spirulina platensis. Bioresour. Technol. 2013, 145, 307–312. [Google Scholar] [CrossRef]
- Hong, M.E.; Hwang, S.K.; Chang, W.S.; Kim, B.W.; Lee, J.; Sim, S.J. Enhanced Autotrophic Astaxanthin Production from Haematococcus pluvialis Under High Temperature via Heat Stress-Driven Haber–Weiss Reaction. Appl. Microbiol. Biotechnol. 2015, 99, 5203–5215. [Google Scholar] [CrossRef]
- Huntley, M.E.; Redalje, D.G. CO2 Mitigation and Renewable Oil from Photosynthetic Microbes: A New Appraisal. Mitig. Adapt. Strat. Glob. Chang. 2007, 12, 573–608. [Google Scholar] [CrossRef]
- Demirbaş, A. Oily Products from Mosses and Algae via Pyrolysis. Energy Sources A 2006, 28, 933–940. [Google Scholar] [CrossRef]
- Tomei, J.; Helliwell, R. Food Versus Fuel? Going Beyond Biofuels. Land Use Policy 2016, 56, 320–326. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a Raw Material for Biofuels Production. J. Ind. Microbiol. 2009, 36, 269–274. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Moheimani, N.R. Sustainable Biofuels from Algae. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 13–25. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications Along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Hong, M.E.; Chang, W.S.; Sim, S.J. Autotrophic Biodiesel Production from the Thermotolerant Microalga Chlorella sorokiniana by Enhancing the Carbon Availability with Temperature Adjustment. Biotechnol. Bioproc. E 2019, 24, 223–231. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, S.; Ghosh, A.; Oinam, G.; Tiwari, O.N.; Das, P.; Gayen, K.; Mandal, M.K.; Halder, G.N. Production of Biodiesel from Microalgae Through Biological Carbon Capture: A Review. 3 Biotech 2017, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.Y.; Hong, M.E.; Chang, W.S.; Sim, S.J. Enhanced Biodiesel Production in Neochloris oleoabundans by a Semi-Continuous Process in Two Stage Photobioreactors. Bioprocess Biosyst. Eng. 2015, 38, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Cinar, S.O.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef] [PubMed]
- Hempel, F.; Bozarth, A.S.; Lindenkamp, N.; Klingl, A.; Zauner, S.; Linne, U.; Steinbüchel, A.; Maier, U.G. Microalgae as Bioreactors for Bioplastic Production. Microb. Cell Factories 2011, 10, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant Potential of Microalgae in Relation to Their Phenolic and Carotenoid Content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Kang, C.D.; Sim, S.J. Direct Extraction of Astaxanthin from Haematococcus Culture Using Vegetable Oils. Biotechnol. Lett. 2008, 30, 441–444. [Google Scholar] [CrossRef]
- Hong, M.E.; Choi, Y.Y.; Sim, S.J. Effect of Red Cyst Cell Inoculation and Iron(II) Supplementation on Autotrophic Astaxanthin Production by Haematococcus pluvialis Under Outdoor Summer Conditions. J. Biotechnol. 2016, 218, 25–33. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic Pigment Production with Microalgae: Biological Constraints and Opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Florou-Paneri, P. Innovative Microalgae Pigments as Functional Ingredients in Nutrition. Biotechnol. Adv. 2015, 233–243. [Google Scholar]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Bošnjaković, M.; Sinaga, N. The Perspective of Large-Scale Production of Algae Biodiesel. Appl. Sci. 2020, 10, 8181. [Google Scholar] [CrossRef]
- Bekirogullari, M.; Fragkopoulos, I.S.; Pittman, J.K.; Theodoropoulos, C. Production of Lipid-Based Fuels and Chemicals from Microalgae: An Integrated Experimental and Model-Based Optimization Study. Algal Res. 2017, 23, 78–87. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid Productivity as a Key Characteristic for Choosing Algal Species for Biodiesel Production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Bleeke, F.; Milas, M.; Winckelmann, D.; Klöck, G. Optimization of Freshwater Microalgal Biomass Harvest Using Polymeric Flocculants. Int. Aquat. Res. 2015, 7, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Slade, R.; Bauen, A. Micro-Algae Cultivation for Biofuels: Cost, Energy Balance, Environmental Impacts and Future Prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.J.; Patel, A.K.; Yu, B.S.; Choi, H.I.; Kim, J.R.; Jin, E.S.; Sim, S.J. Sedimentation Rate-Based Screening of Oleaginous Microalgae for Utilization as a Direct Combustion Fuel. Bioresour. Technol. 2019, 293, 122045. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-Value Biomass from Microalgae Production Platforms: Strategies and Progress Based on Carbon Metabolism and Energy Conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar] [CrossRef] [Green Version]
- Ramanna, L.; Rawat, I.; Bux, F. Light Enhancement Strategies Improve Microalgal Biomass Productivity. Renew. Sustain. Energy Rev. 2017, 80, 765–773. [Google Scholar] [CrossRef]
- Hong, M.E.; Choi, H.I.; Kwak, H.S.; Hwang, S.W.; Sung, Y.J.; Chang, W.S.; Sim, S.J. Rapid Selection of Astaxanthin-Hyperproducing Haematococcus Mutant via Azide-Based Colorimetric Assay Combined with Oil-Based Astaxanthin Extraction. Bioresour. Technol. 2018, 267, 175–181. [Google Scholar] [CrossRef]
- Pham, H.M.; Kwak, H.S.; Hong, M.E.; Lee, J.W.; Chang, W.S.; Sim, S.J. Development of an X-Shape Airlift Photobioreactor for Increasing Algal Biomass and Biodiesel Production. Bioresour. Technol. 2017, 239, 211–218. [Google Scholar] [CrossRef]
- Praveenkumar, R.; Lee, K.B.; Lee, J.Y.; Oh, Y.K. Breaking Dormancy: An Energy-Efficient Means of Recovering Astaxanthin from Microalgae. Green Chem. 2015, 17, 1226–1234. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef] [Green Version]
- Takht Ravanchi, M.T.; Sahebdelfar, S. Carbon Dioxide Capture and Utilization in Petrochemical Industry: Potentials and Challenges. Appl. Petrochem. Res. 2014, 4, 63–77. [Google Scholar] [CrossRef] [Green Version]
- House, K.Z.; Harvey, C.F.; Aziz, M.J.; Schrag, D.P. The Energy Penalty of Post-Combustion CO2 Capture & Storage and Its Implications for Retrofitting the U.S. Installed Base. Energy Environ. Sci. 2009, 2, 193–205. [Google Scholar]
- Sung, Y.J.; Lee, J.S.; Yoon, H.K.; Ko, H.J.; Sim, S.J. Outdoor Cultivation of Microalgae in a Coal-Fired Power Plant for Conversion of Flue Gas CO2 into Microalgal Direct Combustion Fuels. SMAB 2021, 1, 1–10. [Google Scholar]
- Yoo, J.J.; Choi, S.P.; Kim, J.Y.H.; Chang, W.S.; Sim, S.J. Development of Thin-Film Photo-Bioreactor and Its Application to Outdoor Culture of Microalgae. Bioproc. Biosyst. Eng. 2013, 36, 729–736. [Google Scholar] [CrossRef]
- Sayre, R. Microalgae: The Potential for Carbon Capture. J. Biosci. 2010, 60, 722–727. [Google Scholar] [CrossRef]
- Yadav, G.; Dubey, B.K.; Sen, R. A Comparative Life Cycle Assessment of Microalgae Production by CO2 Sequestration from Flue Gas in Outdoor Raceway Ponds Under Batch and Semi-Continuous Regime. J. Clean. Prod. 2020, 258. [Google Scholar] [CrossRef]
- Hong, M.E.; Chang, W.S.; Patel, A.K.; Oh, M.S.; Lee, J.J.; Sim, S.J.; Carbon, M.-B. Microalgal-Based Carbon Sequestration by Converting LNG-Fired Waste CO2 into Red Gold Astaxanthin: The Potential Applicability. Energies 2019, 12, 1718. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Jiang, J.; Fa, Y. Overcoming the Biological Contamination in Microalgae and Cyanobacteria Mass Cultivations for Photosynthetic Biofuel Production. Molecules 2020, 25, 5220. [Google Scholar] [CrossRef]
- Ma, M.; Yuan, D.; He, Y.; Park, M.S.; Gong, Y.; Hu, Q. Effective Control of Poterioochromonas malhamensis in Pilot-Scale Culture of Chlorella sorokiniana GT-1 by Maintaining CO2-Mediated Low Culture pH. GT. Algal Res. 2017, 26, 436–444. [Google Scholar] [CrossRef]
- Aishvarya, V.; Pradhan, N.; Nayak, R.R.; Sukla, L.B.; Mishra, B.K. Enhanced Inorganic Carbon Uptake by Chlorella sp. IMMTCC-2 under Autotrophic Conditions for Lipid Production and CO2 Sequestration. J. Appl. Phycol. 2012, 24, 1455–1463. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, S.J.; Hong, M.E.; Chang, W.S.; Sim, S.J. Repeated-Batch Production of omega-3 Enriched Biomass of Chlorella sorokiniana via Calcium-Induced Homeoviscous Adaptation. Bioresour. Technol. 2020, 303, 122944. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Hong, M.E.; Jin, E.S.; Woo, H.M.; Sim, S.J. Improvement in Modular Scalability of Polymeric Thin-Film Photobioreactor for Autotrophic Culturing of Haematococcus pluvialis Using Industrial Flue Gas. Bioresour. Technol. 2018, 249, 519–526. [Google Scholar] [CrossRef]
- Dhup, S.; Kannan, D.C.; Dhawan, V. Understanding Urea Assimilation and Its Effect on Lipid Production and Fatty Acid Composition of Scenedesmus sp. SOJ Biochem. 2016, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Giordano, C.; Erpen, C.; Yao, W.; Antonietti, M. Synthesis of Mo and W Carbide and Nitride Nanoparticles via a Simple “Urea Glass” Route. Nano Lett. 2008, 8, 4659–4663. [Google Scholar] [CrossRef]
- Radkova, M.; Stoyneva-Gärtner, M.S.; Dincheva, I.; Stoykova, P.; Uzunov, B.; Dimitrova, P.; Borisova, C.; Gärtner, G. Chlorella vulgaris H1993 and Desmodesmus communis H522 for Low-Cost Production of High-Value Microalgal Products. Biotechnol. Biotechnol. Equip. 2019, 33, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.C.D.; Kalita, M.C. Scenedesmus dimorphus and Scenedesmus quadricauda: Two Potent Indigenous Microalgae Strains for Biomass Production and CO2 Mitigation—A Study on Their Growth Behavior and Lipid Productivity under Different Concentration of Urea as Nitrogen Source. J. Algal Biomass Utln 2011, 2, 42–49. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.S.; Sung, Y.J.; Hong, M.E.; Sim, S.J. Improvement of Photoautotrophic Algal Biomass Production after Interrupted CO2 Supply by Urea and KH2PO4 Injection. Energies 2021, 14, 778. https://doi.org/10.3390/en14030778
Yu BS, Sung YJ, Hong ME, Sim SJ. Improvement of Photoautotrophic Algal Biomass Production after Interrupted CO2 Supply by Urea and KH2PO4 Injection. Energies. 2021; 14(3):778. https://doi.org/10.3390/en14030778
Chicago/Turabian StyleYu, Byung Sun, Young Joon Sung, Min Eui Hong, and Sang Jun Sim. 2021. "Improvement of Photoautotrophic Algal Biomass Production after Interrupted CO2 Supply by Urea and KH2PO4 Injection" Energies 14, no. 3: 778. https://doi.org/10.3390/en14030778
APA StyleYu, B. S., Sung, Y. J., Hong, M. E., & Sim, S. J. (2021). Improvement of Photoautotrophic Algal Biomass Production after Interrupted CO2 Supply by Urea and KH2PO4 Injection. Energies, 14(3), 778. https://doi.org/10.3390/en14030778






