Applicability of Waste Engine Oil for the Direct Production of Electricity
Abstract
:1. Introduction
- Restoring oil properties through the application of cleaning processes such as filtration, centrifugation, and evaporation under vacuum in order to use them later, in accordance with their original purpose, or as a lubricant of a lower quality class;
- Reprocessing oils, involving the removal of mechanical impurities and water from waste oils to obtain a fuel component of a quality compliant with the substitute fuel specification;
- Deep regeneration (re-refining), including appropriate physicochemical processing and obtaining waste petrochemical raw materials from oils that can be used for the production of new lubricating oils or light heating oils;
- Recycling by applying waste oil as a resource in a refining process, or in the production of base oil in an installation working in the area of refining; and
- Re-using oil directly as a fuel.
2. Materials and Methods
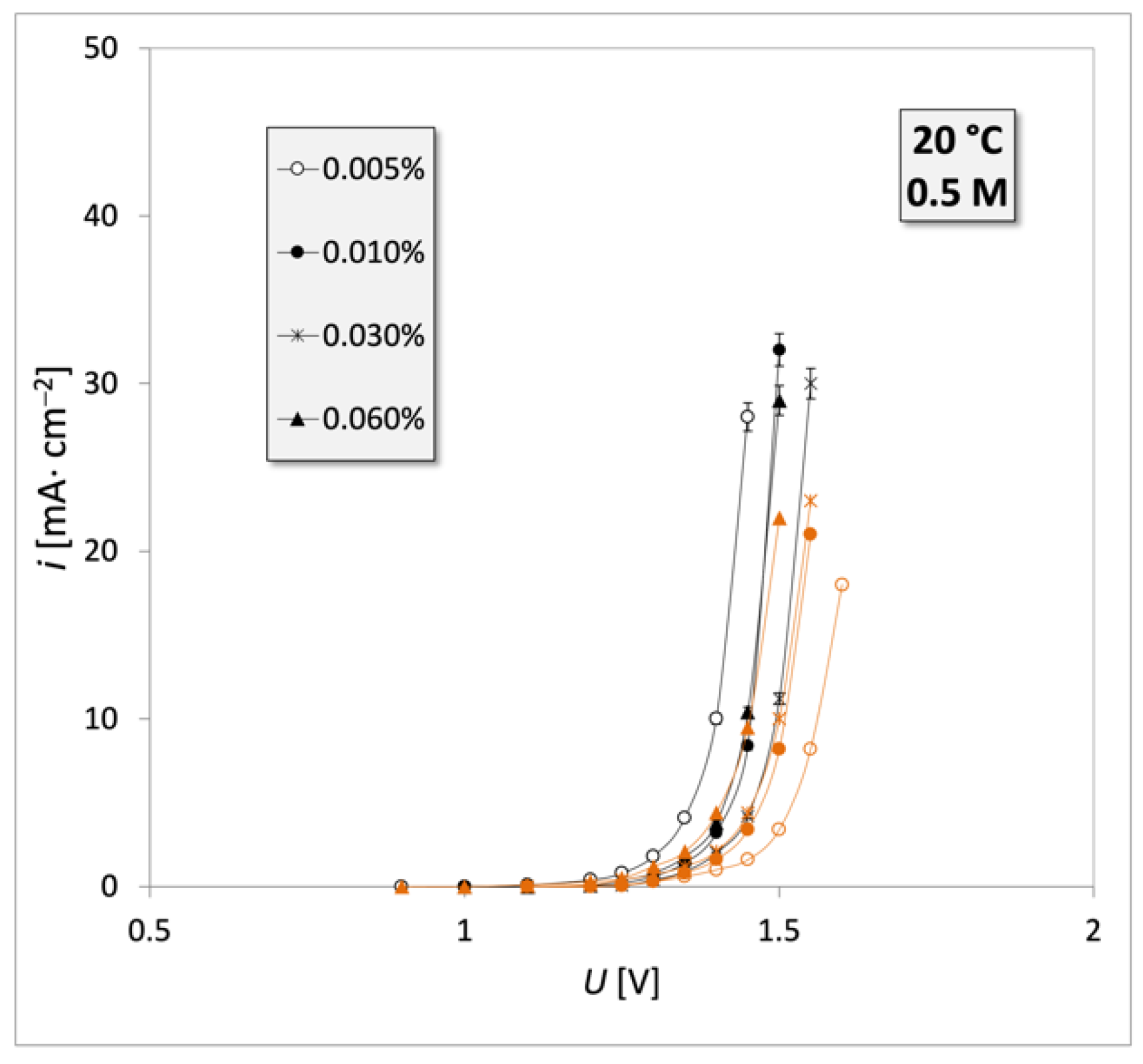
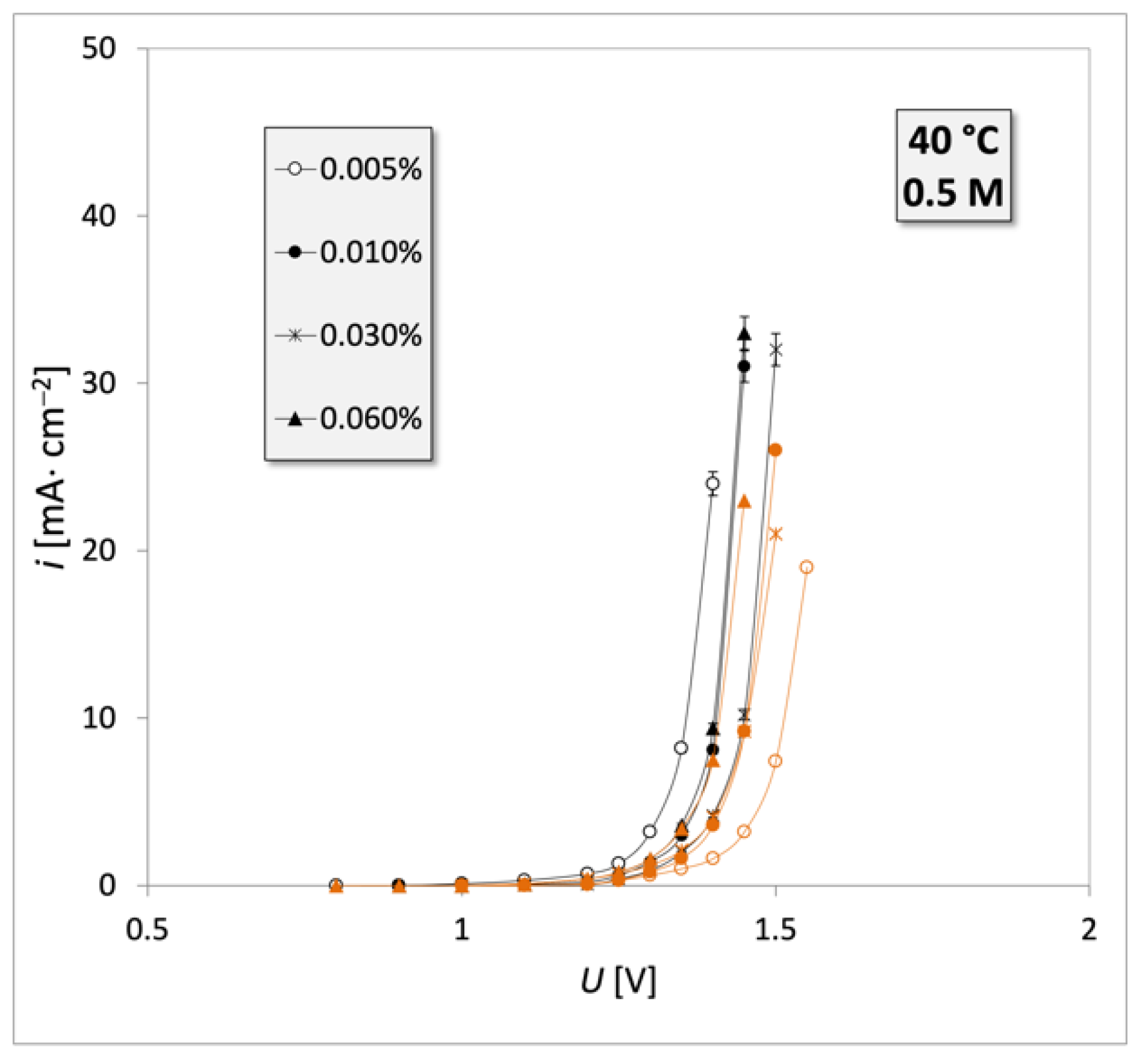
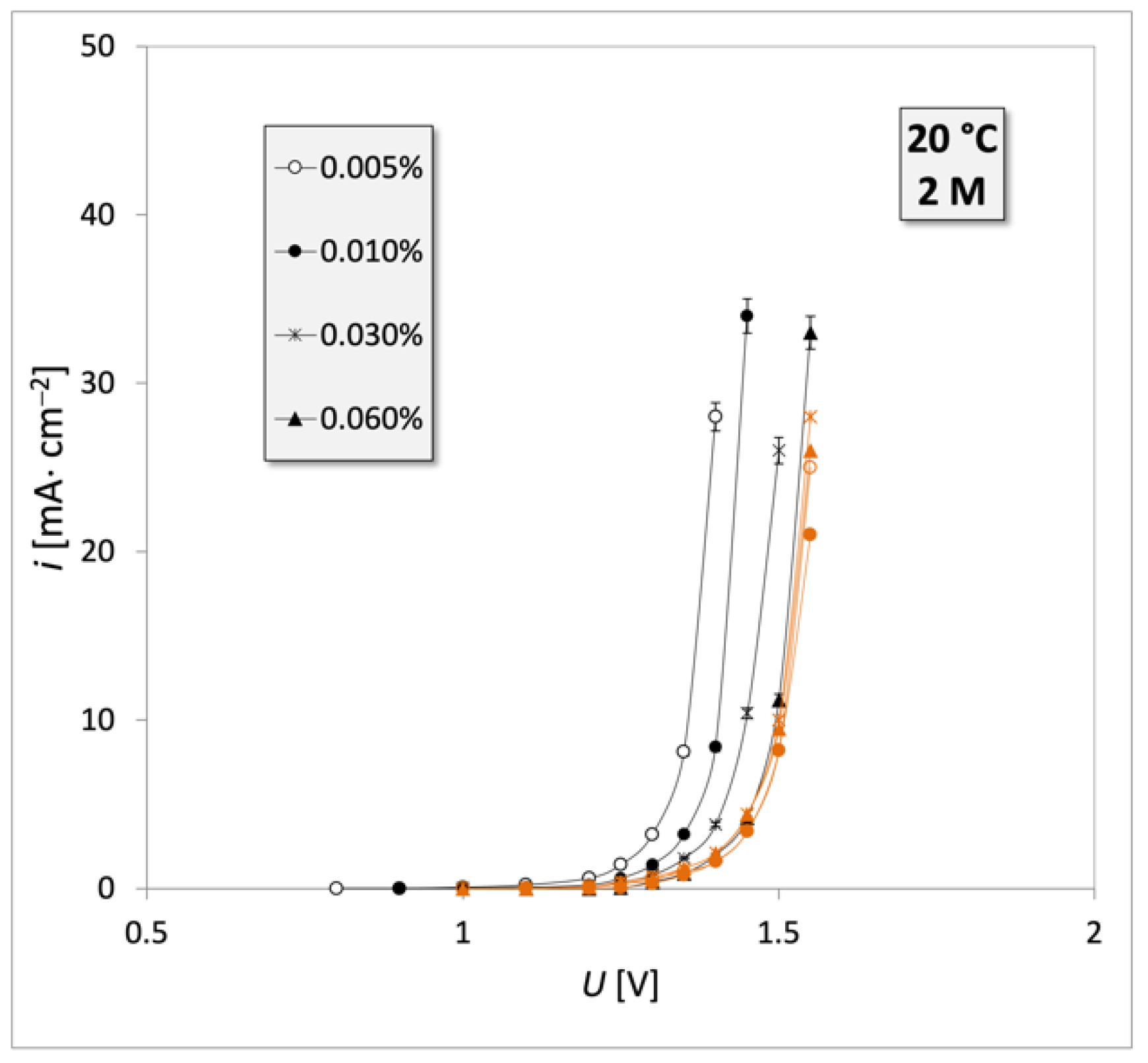
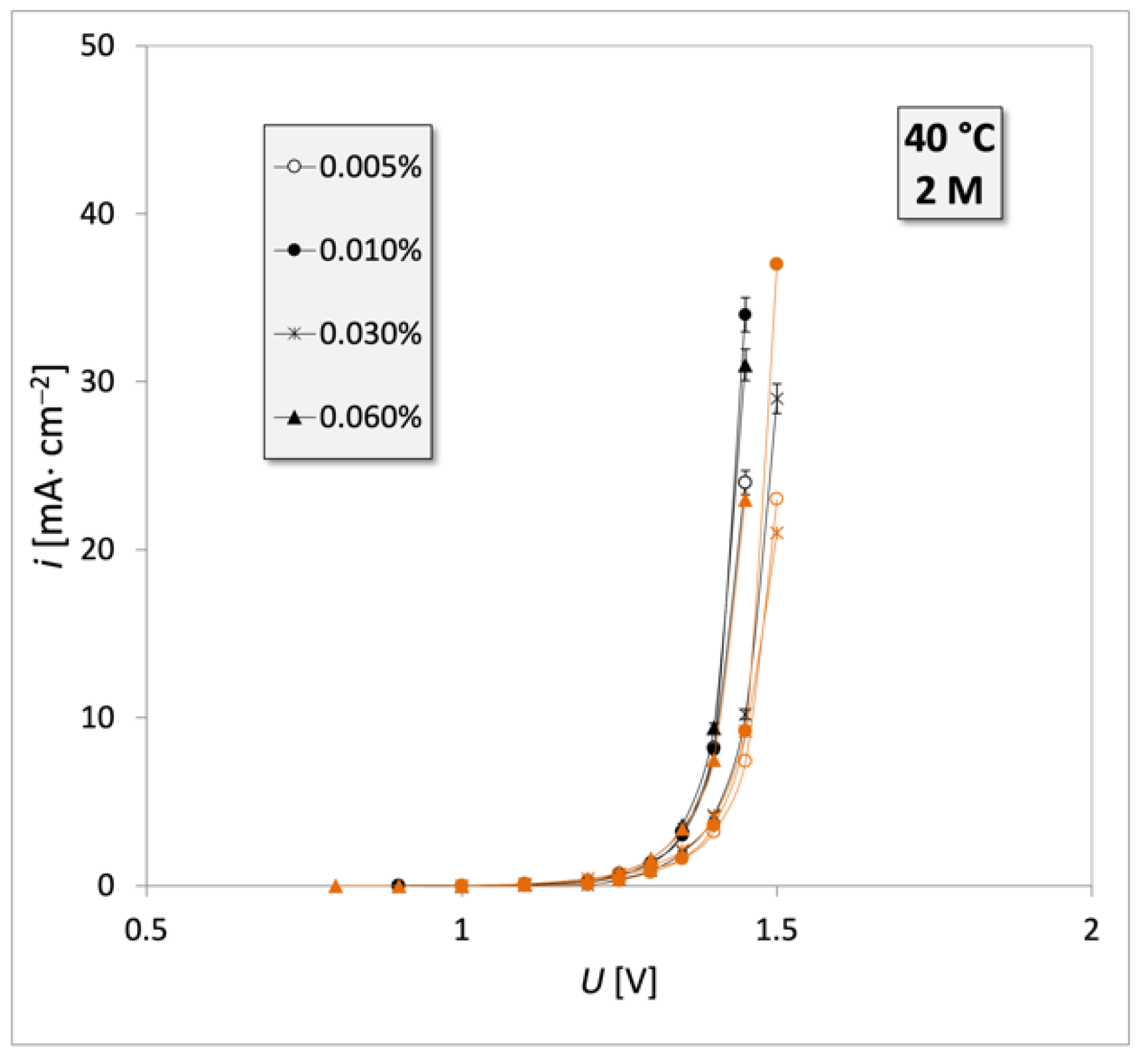
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buchori, L.; Widayat, W.; Muraza, O.; Amali, M.I.; Maulida, R.W.; Prameswari, J. Effect of Temperature and Concentration of Zeolite Catalysts from Geothermal Solid Waste in Biodiesel Production from Used Cooking Oil by Esterification–Transesterification Process. Processes 2020, 8, 1629. [Google Scholar] [CrossRef]
- García-Marino, M.; Rivas-Gonzalo, J.C.; Ibáñez, E.; García-Moreno, C. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal. Chim. Acta 2006, 563, 44–50. [Google Scholar] [CrossRef]
- da Silva, A.L.; Luna, C.B.B.; de Farias, A.F.F.; de Medeiros, S.A.S.L.; Meneghetti, S.M.P.; Rodrigues, A.M.; Costa, A.C.F.M. From disposal to reuse: Production of sustainable fatty acid alkyl esters derived from residual oil using a biphasic magnetic catalyst. Sustainability 2020, 12, 10159. [Google Scholar] [CrossRef]
- Żelazny, S.; Żukowski, W.; Bogdał, D.; Bednarz, S.; Kasprzyk, W.; Świergosz, T. Recovery and characterization studies of post-production alloy waste from the automotive industry. Materials 2020, 13, 5600. [Google Scholar] [CrossRef] [PubMed]
- Jackowski, M.; Niedźwiecki, Ł.; Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s spent grains—Valuable beer industry by-product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef] [PubMed]
- Hamawand, I.; Yusaf, T.; Rafat, S. Recycling of waste engine oils using a new washing agent. Energies 2013, 6, 1023–1049. [Google Scholar] [CrossRef]
- Fuentes, M.J.; Font, R.; Gómez-Rico, M.F.; Martín-Gullón, I. Pyrolysis and combustion of waste lubricant oil from diesel cars: Decomposition and pollutants. J. Anal. Appl. Pyrol. 2007, 79, 215–226. [Google Scholar] [CrossRef]
- Kajdas, C. Major pathways for used oil disposal and recycling, Part 1. Tribotest J. 2000, 7, 61–74. [Google Scholar] [CrossRef]
- Hopmans, J.J. The Problem of the Processing of Spent Oil in the Member States of EEC; Report for the European Economic Community (EEC); National Institute for Wastewater Treatment: Dordrecht, The Netherlands, 1974. [Google Scholar]
- Naima, K.; Liazid, A. Waste oils as alternative fuel for diesel engine: A review. J. Pet. Technol. Altern. Fuels 2013, 4, 30–43. [Google Scholar] [CrossRef]
- Chen, G.; He, G. Separation of water and oil from water-in-oil emulsion by freeze/thaw method. Sep. Purif. Technol. 2003, 31, 83–89. [Google Scholar] [CrossRef]
- Kwon, W.-T.; Park, K.; Han, S.D.; Yoon, S.M.; Kim, J.Y.; Bae, W.; Rhee, Y.W. Investigation of water separation from water-in-oil emulsion using electric field. J. Ind. Eng. Chem. 2010, 16, 684–687. [Google Scholar] [CrossRef]
- Whisman, M.L.; Reynolds, J.W.; Goetzinger, J.W.; Cotton, F.O.; Brinkman, D.W. Re-refining makes quality oils. Hydrocarb. Process. 1978, 57, 141–145. [Google Scholar]
- Nerin, C.; Domeno, C.; Moliner, R.; Lazaro, M.J.; Suelves, I.; Valderrama, J. Behavior of different industrial waste oils in a pyrolysis process: Metals distribution and valuable products. J. Anal. Appl. Pyrol. 2000, 55, 171–183. [Google Scholar] [CrossRef]
- Von Fuchs, G.H.; Diamond, H. Oxidation characteristics of lubricating oils. Ind. Eng. Chem. 1942, 34, 927–937. [Google Scholar] [CrossRef]
- Rahimi, B.; Semnani, A.; Nezamzadeh-Ejhieh, A.; Shakoori Langeroodi, H.; Davood, M.H. Monitoring of the physical and chemical properties of a gasoline engine oil during its usage. J. Anal. Methods Chem. 2012, 819524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y. Oil Analysis Handbook for Predictive Equipment Maintenance, 3rd ed.; Spectro Scientific: Chelmsford, MA, USA, 2016. [Google Scholar]
- Demirbas, A.; Demirbas, I. Importance of rural bioenergy for developing countries. Energy Convers. Manag. 2007, 48, 2386–2398. [Google Scholar] [CrossRef]
- Bhaskar, T.; Uddin, M.A.; Muto, A.; Sakata, Y.; Omura, Y.; Kimura, K.; Kawakami, Y. Recycling of waste lubricant oil into chemical feedstock or fuel oil over supported iron oxide catalysts. Fuel 2004, 83, 9–15. [Google Scholar] [CrossRef]
- Boughton, B.; Horvath, A. Environmental assessment of waste oil management methods. Environ. Sci. Technol. 2004, 38, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Maceiras, R.; Alfonsín, V.; Morales, F.J. Recycling of waste engine oil for diesel production. Waste Manag. 2017, 60, 351–356. [Google Scholar] [CrossRef]
- El-Fadel, M.; Khoury, R. Strategies for vehicle waste-oil management: A case study. Resour. Conserv. Recycl. 2001, 33, 75–91. [Google Scholar] [CrossRef]
- Rincón, J.; Cañizares, P.; García, M.T. Waste oil recycling using mixtures of polar solvents. Ind. Eng. Chem. Res. 2005, 44, 7854–7859. [Google Scholar] [CrossRef]
- Littlepage, M. Waste Oil Recycling Apparatus. US Patent 5188156, 23 February 1993. [Google Scholar]
- Betts, H.S. Diesel Engine Waste Oil Recycling System. US Patent 5476073, 19 December 1995. [Google Scholar]
- Arpa, O.; Yumrutas, R.; Demirbas, A. Production of diesel-like fuel from waste engine oil by pyrolitic distillation. Appl. Energy 2010, 87, 122–127. [Google Scholar] [CrossRef]
- Beck, B.D.; Brain, J.D.; Wolfthal, S.F. Assessment of lung injury produced by particulate emissions of space heaters burning automotive waste oil. In Inhaled Particles VI, Proceedings of an International Symposium and Workshop on Lung Dosimetry Organised by the British Occupational Hygiene Society in Co-Operation with the Commission of the European Communities, Cambridge; Elsevier: Amsterdam, The Netherlands, 1988; pp. 257–265. [Google Scholar]
- Delistraty, D.; Stone, A. Dioxins, metals, and fish toxicity in ash residue from space heaters burning used motor oil. Chemosphere 2007, 68, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Russell, D.; Chasea, H.A. Microwave pyrolysis, a novel process for recycling waste automotive engine oil. Energy 2010, 35, 2985–2991. [Google Scholar] [CrossRef]
- Arpa, O.; Recep, Y.; Zeki, A. Experimental investigation of the effects of diesel-like fuel obtained from waste lubrication oil on engine performance and exhaust emission. Fuel Process. Technol. 2010, 91, 1241–1249. [Google Scholar] [CrossRef]
- Arpa, O.; Yumrutas, R.; Alma, M.H. Effects of turpentine and gasoline-like fuel obtained from waste lubrication oil on engine performance and exhaust emission. Energy 2010, 35, 3603–3613. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Chase, H.A. Catalytic microwave pyrolysis of waste engine oil using metallic pyrolysis char. Appl. Catal. B Environ. 2015, 176–177, 601–617. [Google Scholar] [CrossRef] [Green Version]
- DeDene, C.D.; You, Z. The performance of aged asphalt materials rejuvenated with waste engine oil. Int. J. Pavement Res. Technol. 2014, 7, 145–152. [Google Scholar] [CrossRef]
- Qurashi, I.A.; Swamy, A.K. Viscoelastic properties of recycled asphalt binder containing waste engine oil. J. Clean. Prod. 2018, 182, 992–1000. [Google Scholar] [CrossRef]
- Zhang, K.; Cao, Q.; Jin, L.; Li, P.; Zhang, X. A novel route to utilize waste engine oil by blending it with water and coal. J. Hazard. Mater. 2017, 332, 51–58. [Google Scholar] [CrossRef]
- Fang, C.S.; Lai, P.M.C. Microwave heating and separation of water-in-oil emulsions. J. Microw. Power Electromagn. Energy 1995, 30, 46–57. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Stolten, D. Hydrogen and Fuel Cells. Fundamentals, Technologies and Applications; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Redey, L. Ogniwa Paliwowe; Wydawnictwa Naukowo-Techniczne: Warszawa, Poland, 1973. [Google Scholar]
- Hoogers, G. Fuel Cell Technology Handbook; CRC Press: Boca Raton, FA, USA, 2003. [Google Scholar]
- Larminie, J.; Dicks, A. Fuel Cell System Explained, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Grove, W.R. On voltaic series and the combination of gases by platinum. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1839, 14, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, J.; Zięcina, K. Samoloty Kosmiczne; Wydawnictwa Naukowo-Techniczne: Warszawa, Poland, 1989. [Google Scholar]
- Kakaç, S.; Pramuanjaroenkij, A.; Vasilev, L. (Eds.) Mini-Micro Fuel Cells: Fundamentals and Applications; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Lokurlu, A.; Grube, T.; Höhlein, B.; Stolten, D. Fuel cells for mobile and stationary applications-cost analysis for combined heat and power stations on the basis of fuel cells. Int. J. Hydrogen Energy 2003, 28, 703–711. [Google Scholar] [CrossRef]
- Scott, K.; Taama, W.M.; Argyropoulo, P. Engineering aspects of the direct methanol fuel cell syste. J. Power Sources 1999, 79, 43–59. [Google Scholar] [CrossRef]
- Nahar, G.; Kendall, K. Biodiesel formulations as fuel for internally reforming solid oxide fuel cell. Fuel Process. Technol. 2011, 92, 1345–1354. [Google Scholar] [CrossRef]
- Andújar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Sakharov, I.I.; Rastiannikov, E.G.; Verbitskaia, G.M.; Tarasova, L.N. Washability of syntanol DS-10 from kitchen utensils (article in Russian). Vopr. Pitan. 1975, 4, 75–77. [Google Scholar]
- Survila, A.; Mockus, Z.; Kanapeckaitė, S.; Samulevičienė, M. Effect of syntanol DS-10 and halides on tin(II) reduction kinetics. Electrochim. Acta 2005, 50, 2879–2885. [Google Scholar] [CrossRef]
- Paraska, O.; Karvan, S. Mathematical modelling in scientific research of chemical technology processes. Tech. Trans. Mech. Crac. Univ. Technol. Press 2010, 8, 203–210. [Google Scholar]
- Kravchenko, A.V.; Rudnitskii, A.G.; Nesterenko, A.F.; Kublanovskii, V.S. Degradation of Syntanol DS-10 promoted by energy transfer reactions. Ukr. Chem. J. C/C Ukr. Khimicheskii Zhurnal 1994, 60, 11–13. [Google Scholar]
- Ignatov, O.V.; Shalunova, I.V.; Panchenko, L.V.; Turkovskaia, O.V.; Ptichkina, N.M. Degradation of Syntanol DS-10 by bacteria immobilized in polysaccharide gels (article in Russian). Prikl Biokhim Mikrobiol. 1995, 31, 220–223. [Google Scholar]
- Bockris, J.O.M.; Reddy, A.K.N. Modern Electrochemistry; Kulwer Academic/Plenum Publishers: New York, NY, USA, 2000. [Google Scholar]
- Holtzer, M.; Staronka, A. Chemia Fizyczna. Wprowadzenie; Wydawnictwo AGH: Kraków, Poland, 2000. [Google Scholar]
- Bielański, A. Podstawy Chemii Nieorganicznej; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2013. [Google Scholar]
- Włodarczyk, P.P.; Włodarczyk, B. Wastewater Treatment and Electricity Production in a Microbial Fuel Cell with Cu–B Alloy as the Cathode Catalyst. Catalysts 2019, 9, 572. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, P.P.; Włodarczyk, B. Preparation and Analysis of Ni–Co Catalyst Use for Electricity Production and COD Reduction in Microbial Fuel Cells. Catalysts 2019, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Maya-Cornejo, J.; Guerra-Balcázar, M.; Arjona, N.; Álvarez-Contreras, L.; Rodríguez Valadez, F.J.R.; Gurrola, M.P.; Ledesma-García, J.; Arriaga, L.G. Electrooxidation of crude glycerol as waste from biodiesel in a nanofluidic fuel cell using Cu@Pd/C and Cu@Pt/C. Fuel 2016, 183, 195–205. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, Y.; Hu, B.; Zhang, Q.; Xu, Z.J. Ethylene glycol and ethanol oxidation on spinel Ni-Co oxides in alkaline. J. Electrochem. Soc. 2015, 163, H99–H104. [Google Scholar] [CrossRef]
- Grdén, M.; Łukaszewski, M.; Jerkiewicz, G.; Czerwinski, A. Electrochemical behavior of palladium electrode: Oxidation, electrodissolution and ionic adsorption. Electrochim. Acta 2008, 53, 7583–7598. [Google Scholar] [CrossRef]
- Liu, C.; Hirohara, M.; Maekawa, T.; Chang, R.; Hayashi, T.; Chiang, C.-Y. Selective electro-oxidation of glycerol to dihydroxyacetone by a non-precious electrocatalyst—CuO. Appl. Catal. B Environ. 2020, 265, 118543. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Microbial fuel cell with Ni-Co cathode powered with yeast wastewater. Energies 2018, 11, 3194. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, B.; Włodarczyk, P.P. The membrane-less microbial fuel cell (ML-MFC) with Ni-Co and Cu-B cathode powered by the process wastewater from yeast production. Energies 2020, 13, 3976. [Google Scholar] [CrossRef]



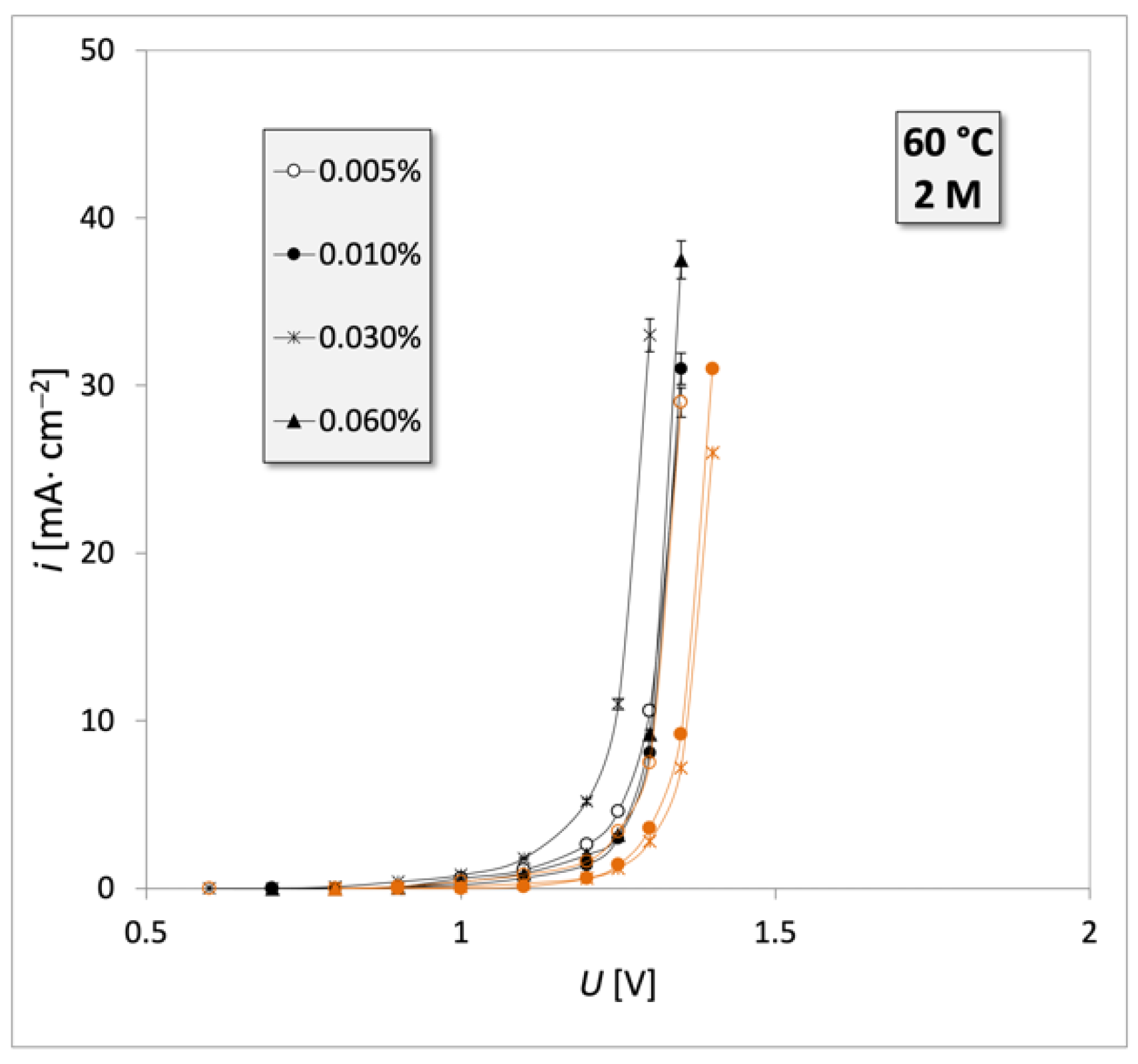
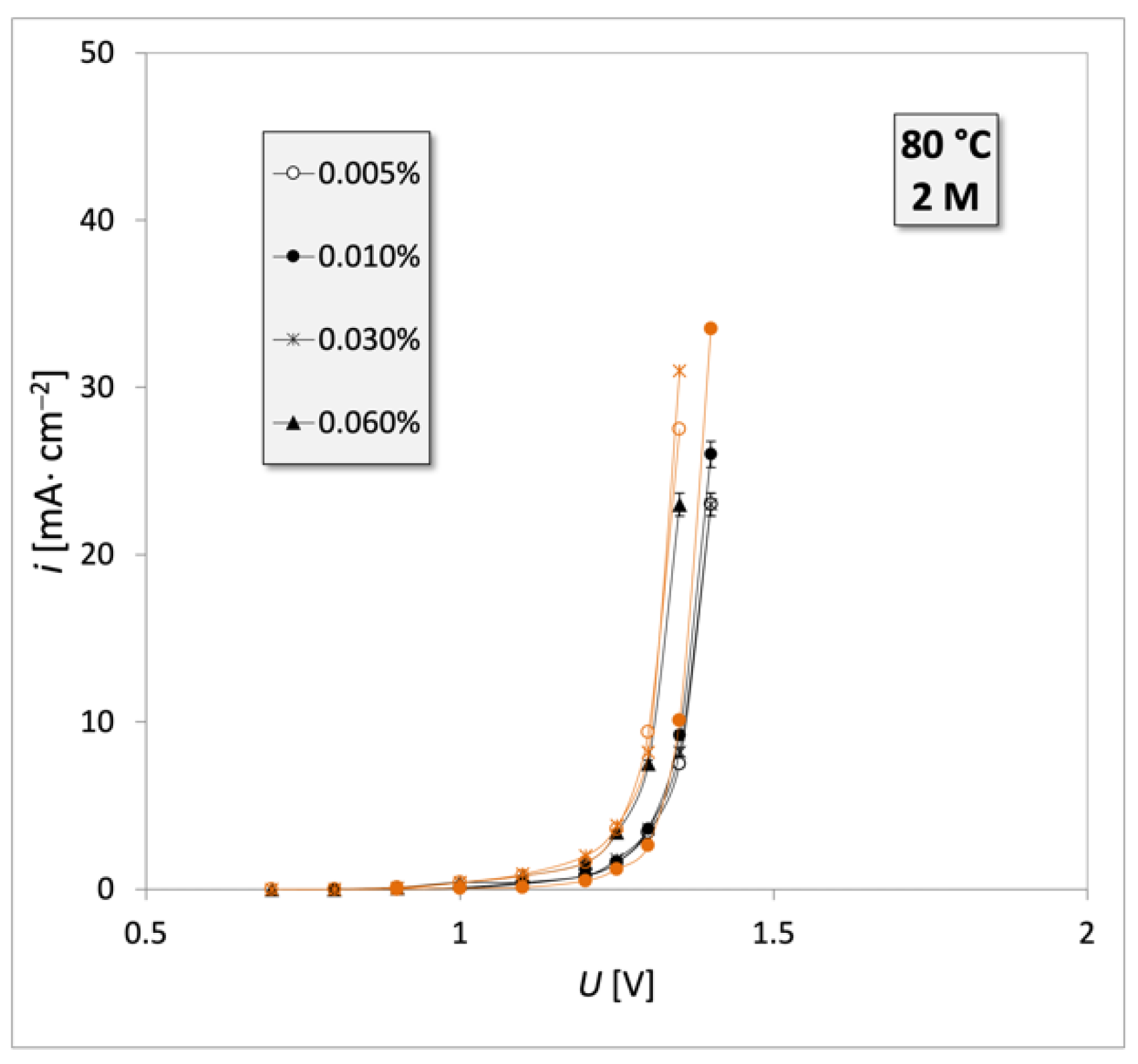
| Engine Oil Grade | Type of Engine 1 | Oil Mileage |
|---|---|---|
| 5W50 | petrol engine (naturally aspirated) 2.0i 155 KM | 15,000 km |
| 10W40 | LPG engine (naturally aspirated) 2.0i 125 KM | 20,000 km |
| 15W40 | petrol engine (naturally aspirated) 1.1 55 KM | 7500 km |
| 10W40 | petrol engine (naturally aspirated) 2.0 105 KM | 10,000 km |
| 5W40 | petrol engine (naturally aspirated) 1.0i 68 KM | 15,000 km |
| 5W30 | petrol engine (turbo) 1.5 150 KM | 15,000 km |
| 5W30 | petrol engine (turbo) 1.3 130 KM | 30,000 km |
| 5W30 | diesel engine (turbo) 1.5 115 KM | 15,000 km |
| 5W30 | diesel engine (turbo) 1.6 92 KM | 15,000 km |
| 5W30 | diesel engine (turbo) 1.5 115 KM | 30,000 km |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, P.P.; Włodarczyk, B. Applicability of Waste Engine Oil for the Direct Production of Electricity. Energies 2021, 14, 1100. https://doi.org/10.3390/en14041100
Włodarczyk PP, Włodarczyk B. Applicability of Waste Engine Oil for the Direct Production of Electricity. Energies. 2021; 14(4):1100. https://doi.org/10.3390/en14041100
Chicago/Turabian StyleWłodarczyk, Paweł P., and Barbara Włodarczyk. 2021. "Applicability of Waste Engine Oil for the Direct Production of Electricity" Energies 14, no. 4: 1100. https://doi.org/10.3390/en14041100






