Effects of Supercritical CO2 on Matrix Permeability of Unconventional Formations
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
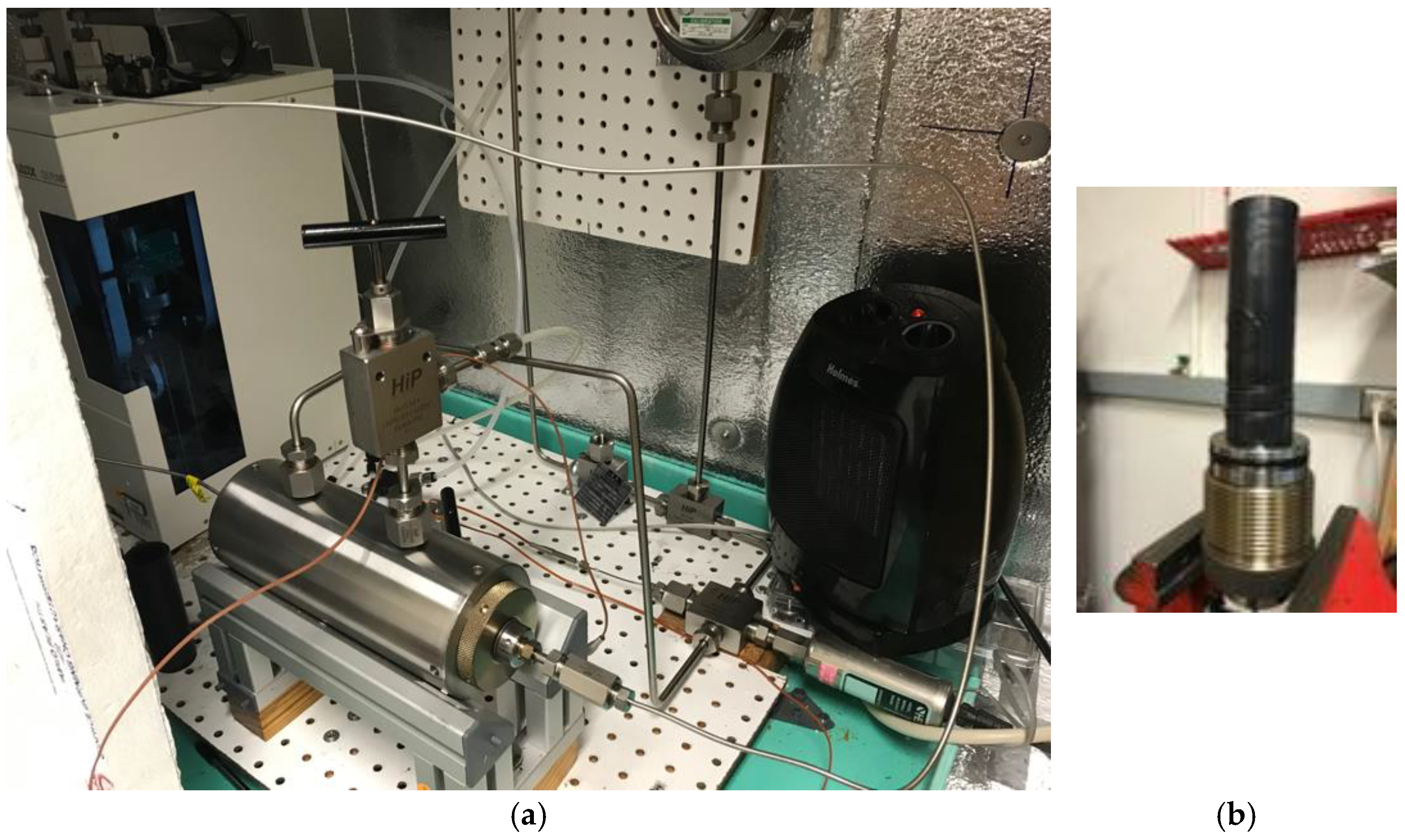
2.2. Experimental Procedure
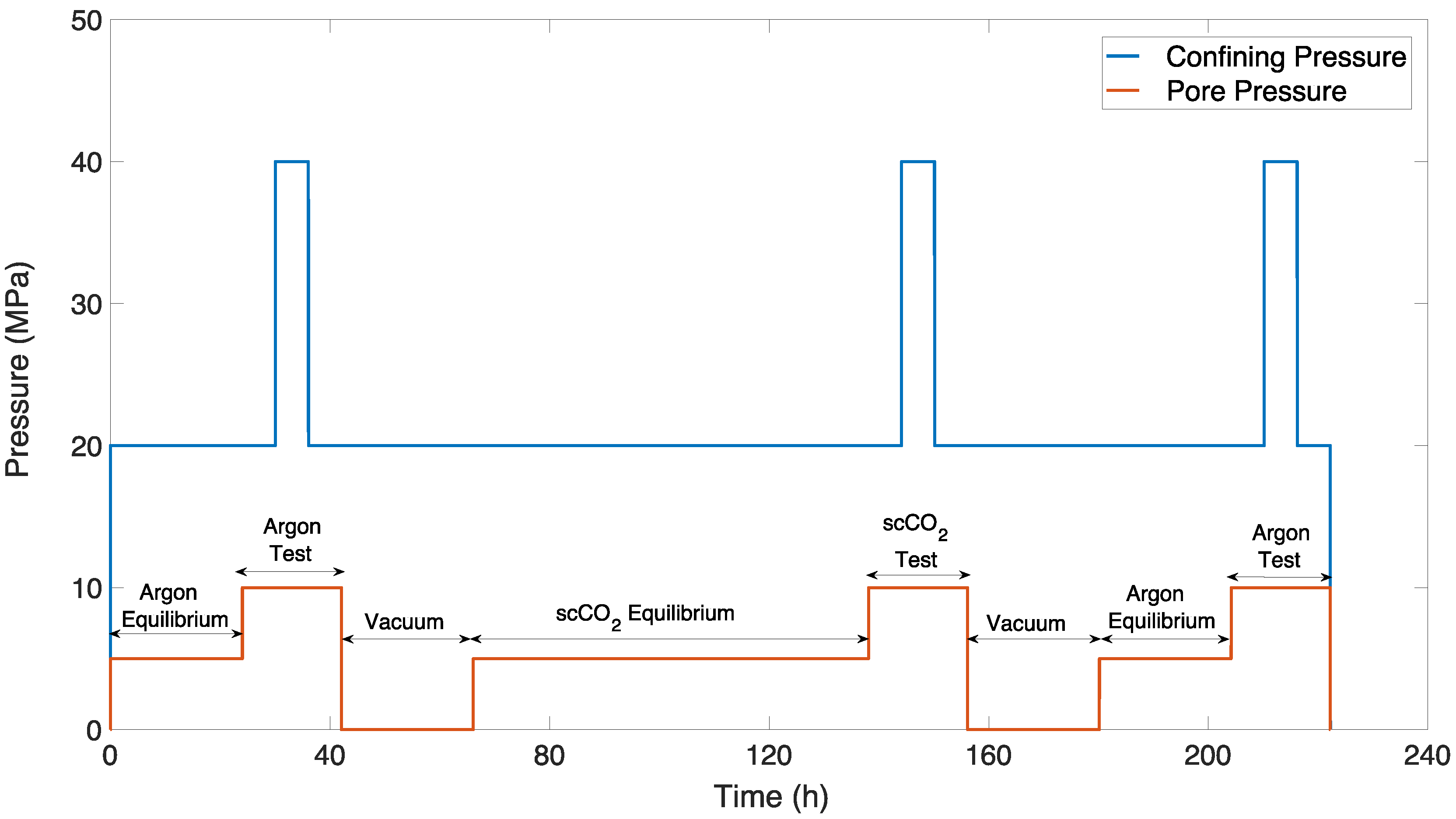
2.3. Experimental Program
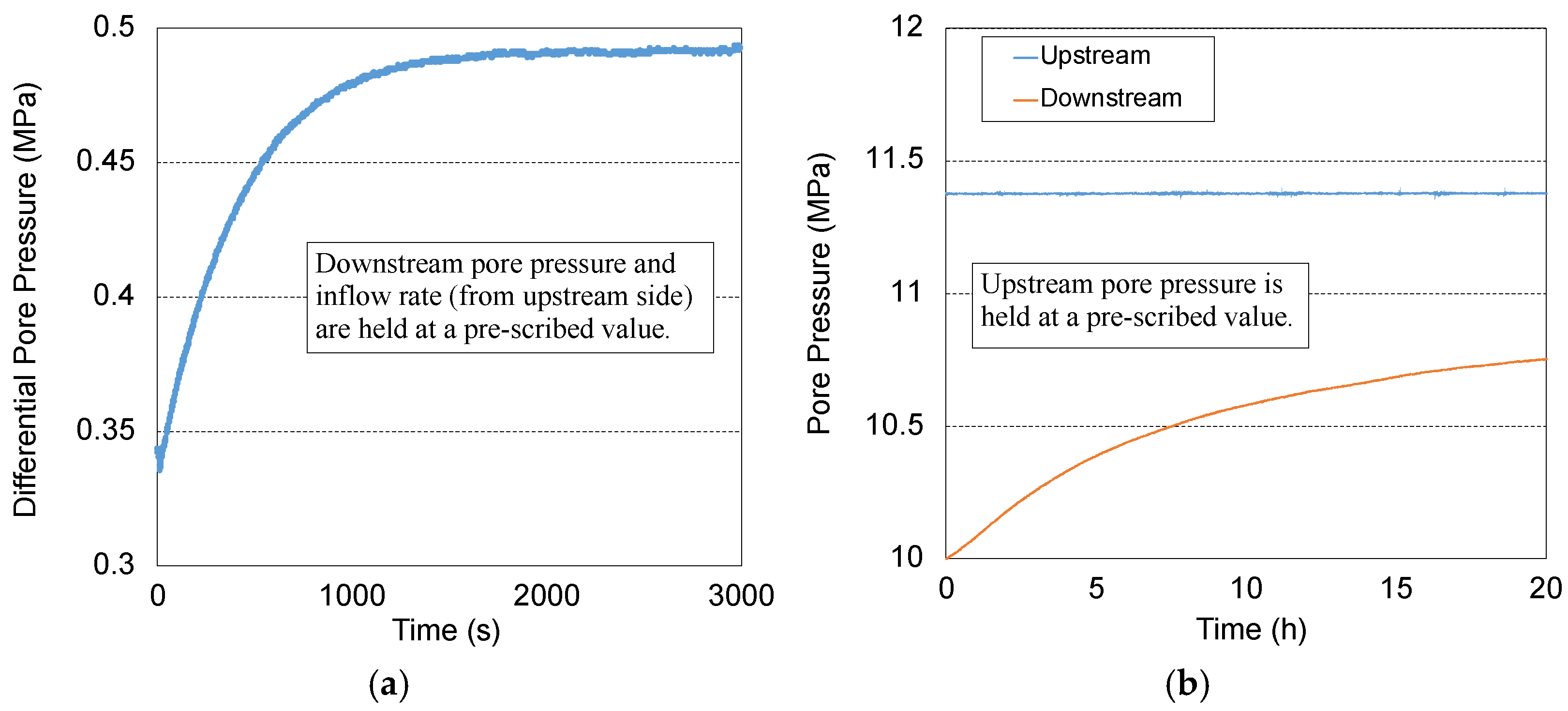
2.4. Permeability Measurement
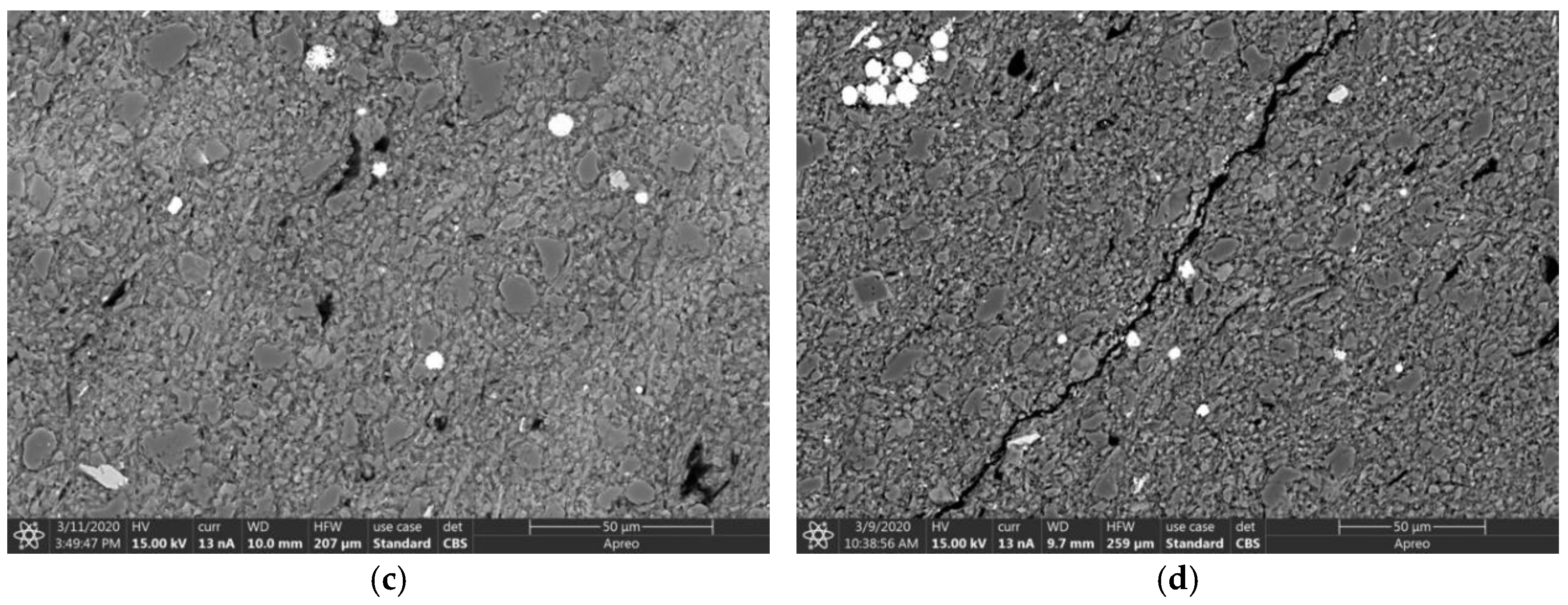
2.5. Imaging
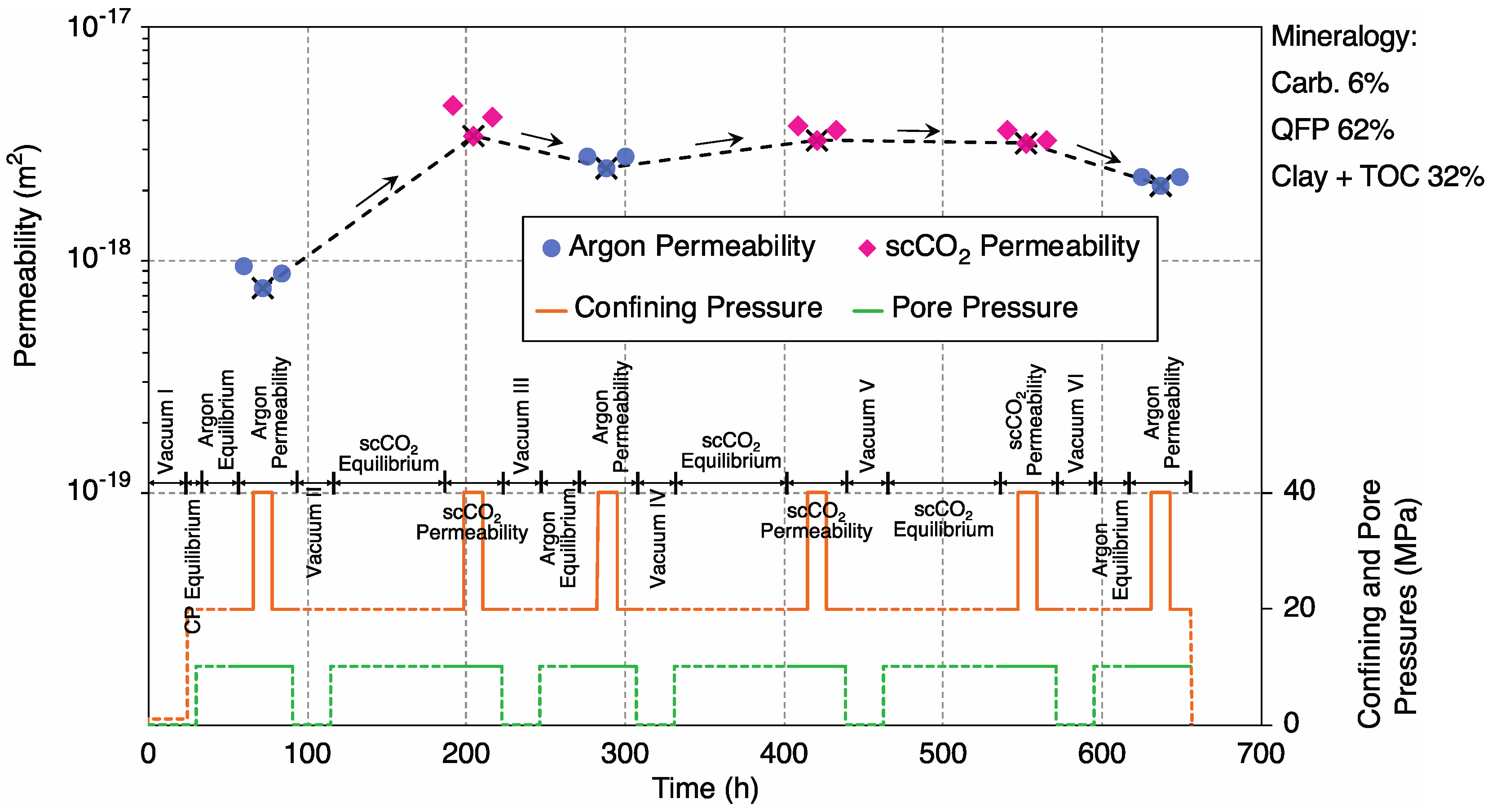
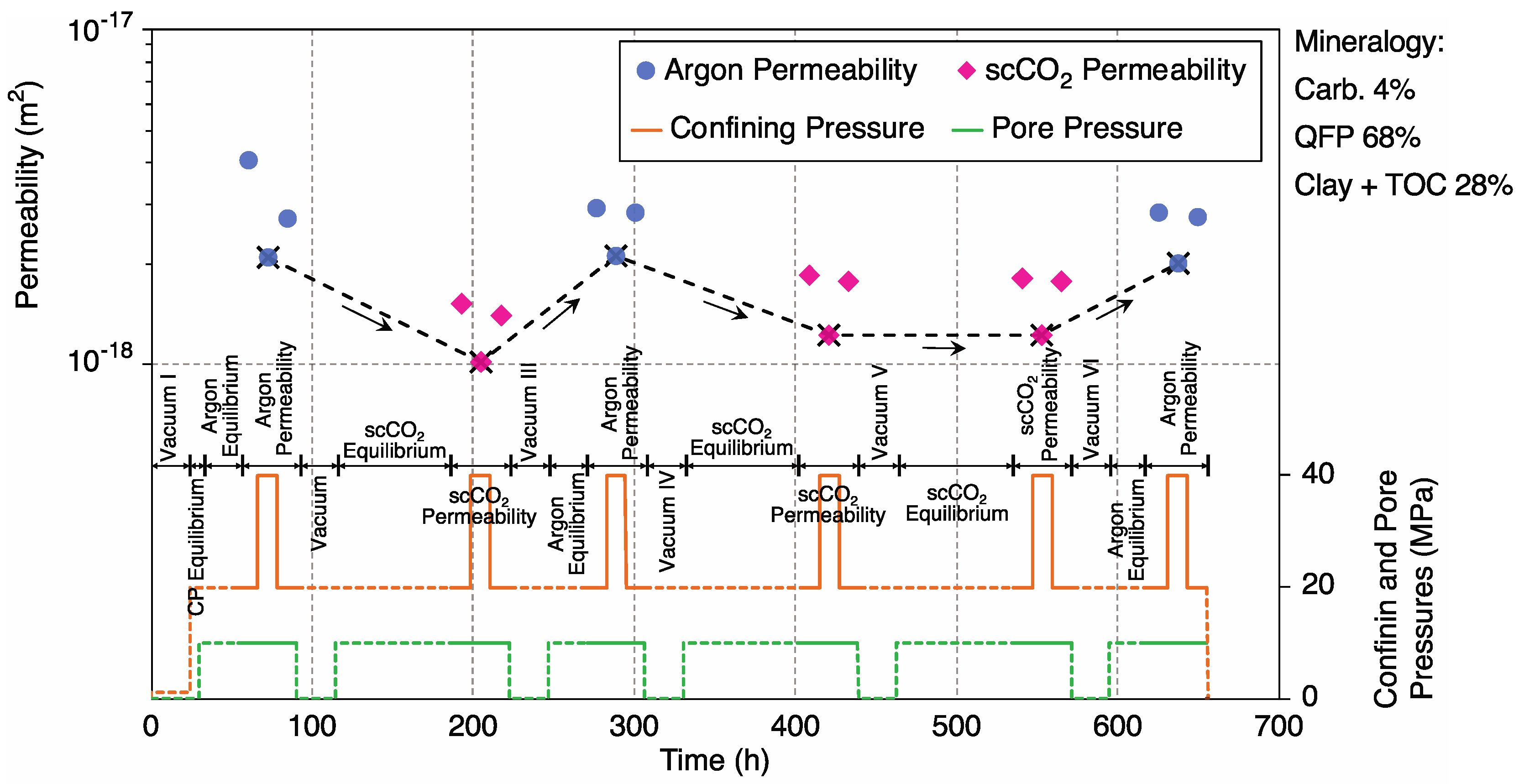
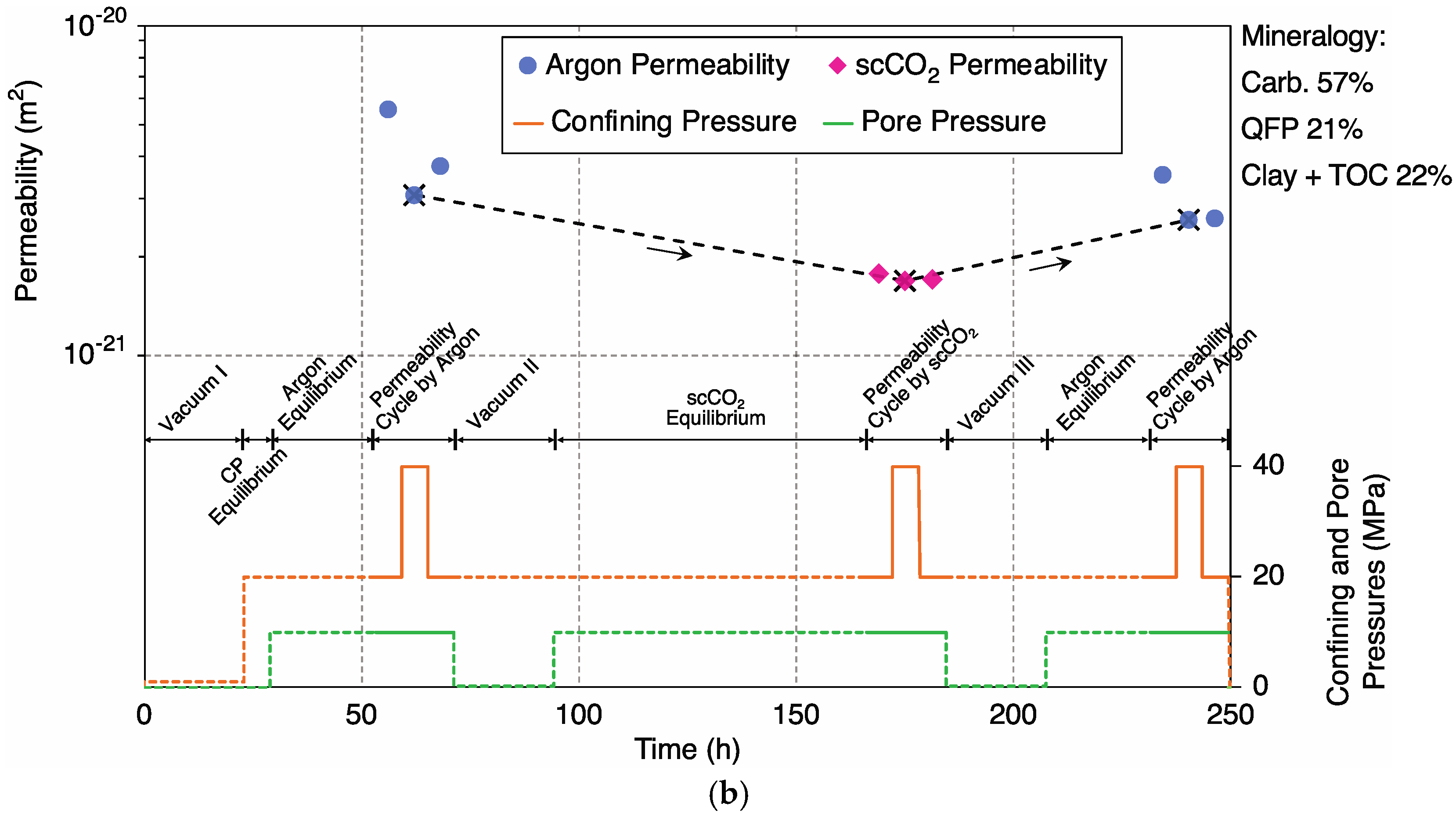
3. Results and Data Analysis
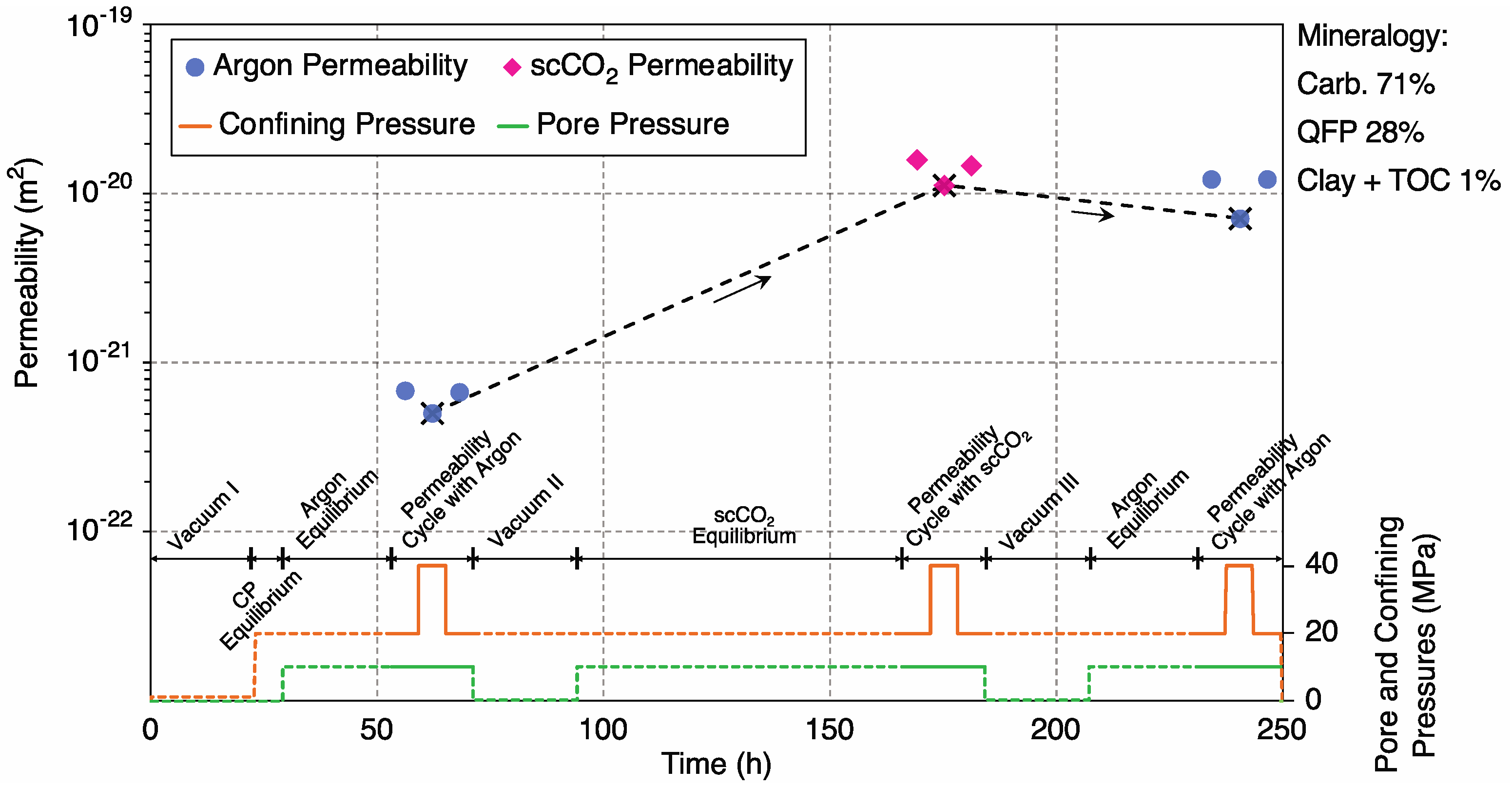
3.1. Mineral Dissolution
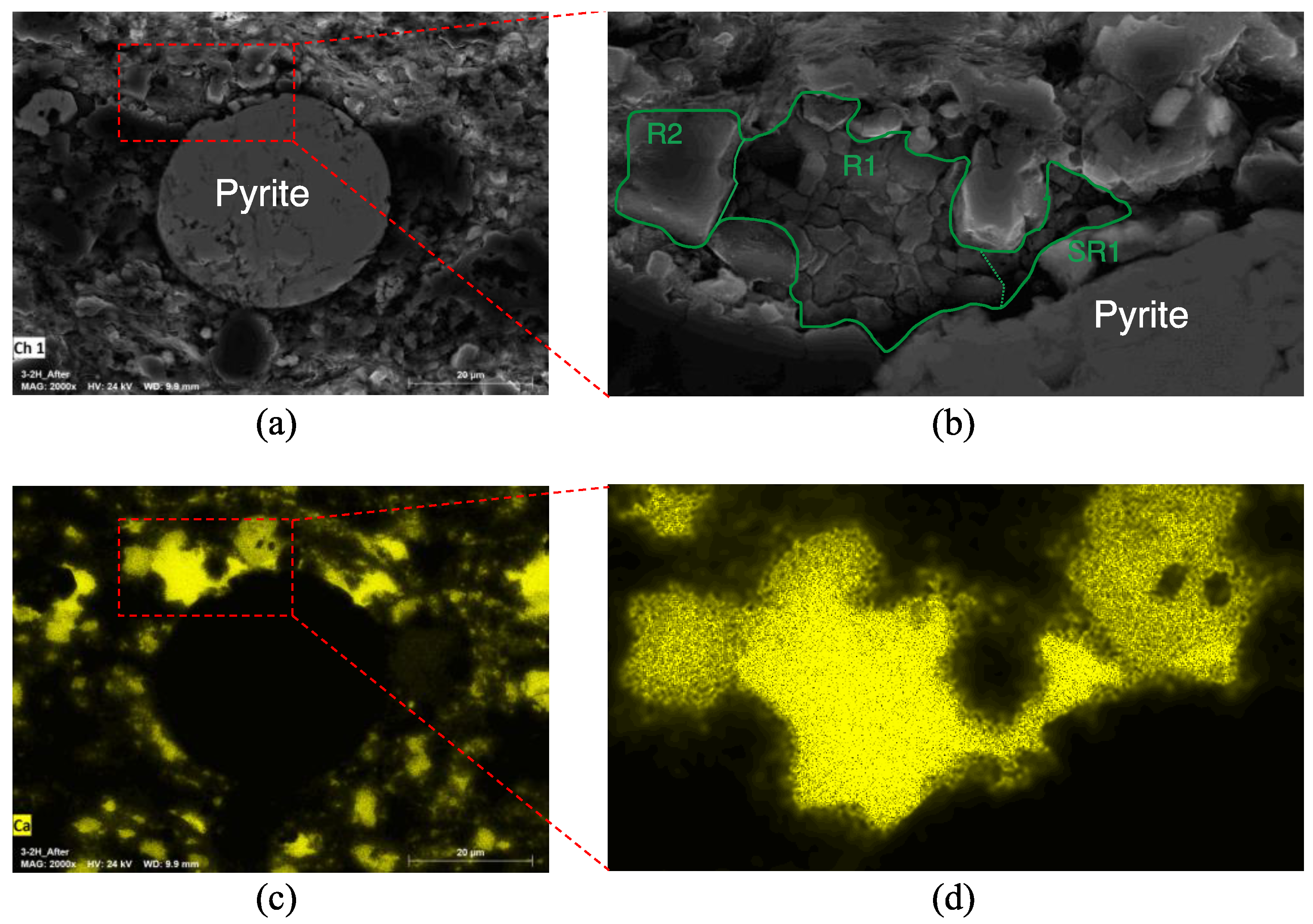
3.1.1. Sample 2-1H; Ultralow-Permeability, Carbonate-Rich
3.1.2. Sample 4-2H; High-Permeability, Carbonate-Poor
3.2. Adsorption into Clays and Organic Matter
3.2.1. Sample 4-4H; High “Clay + TOC”; Regular Cycles
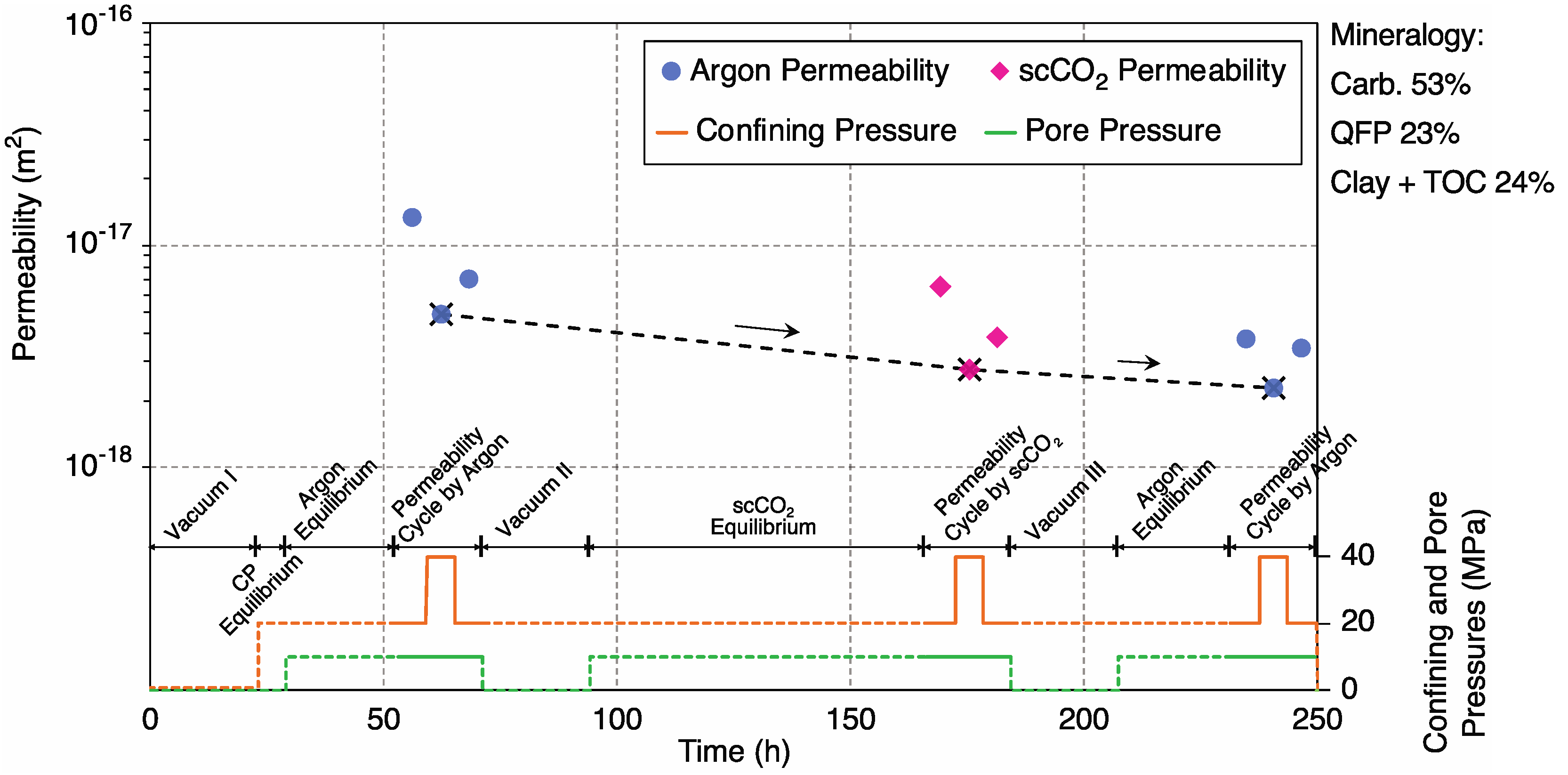
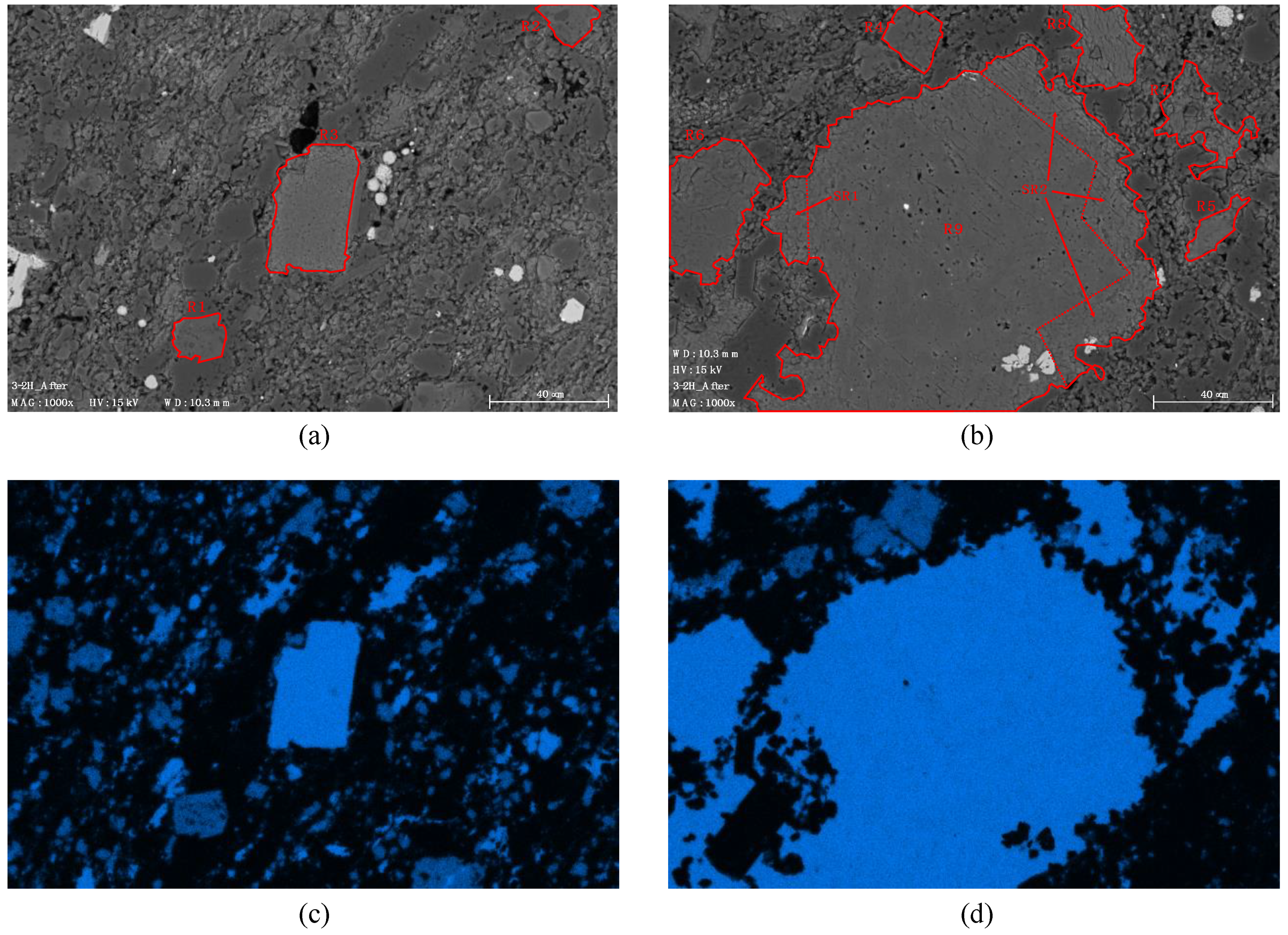
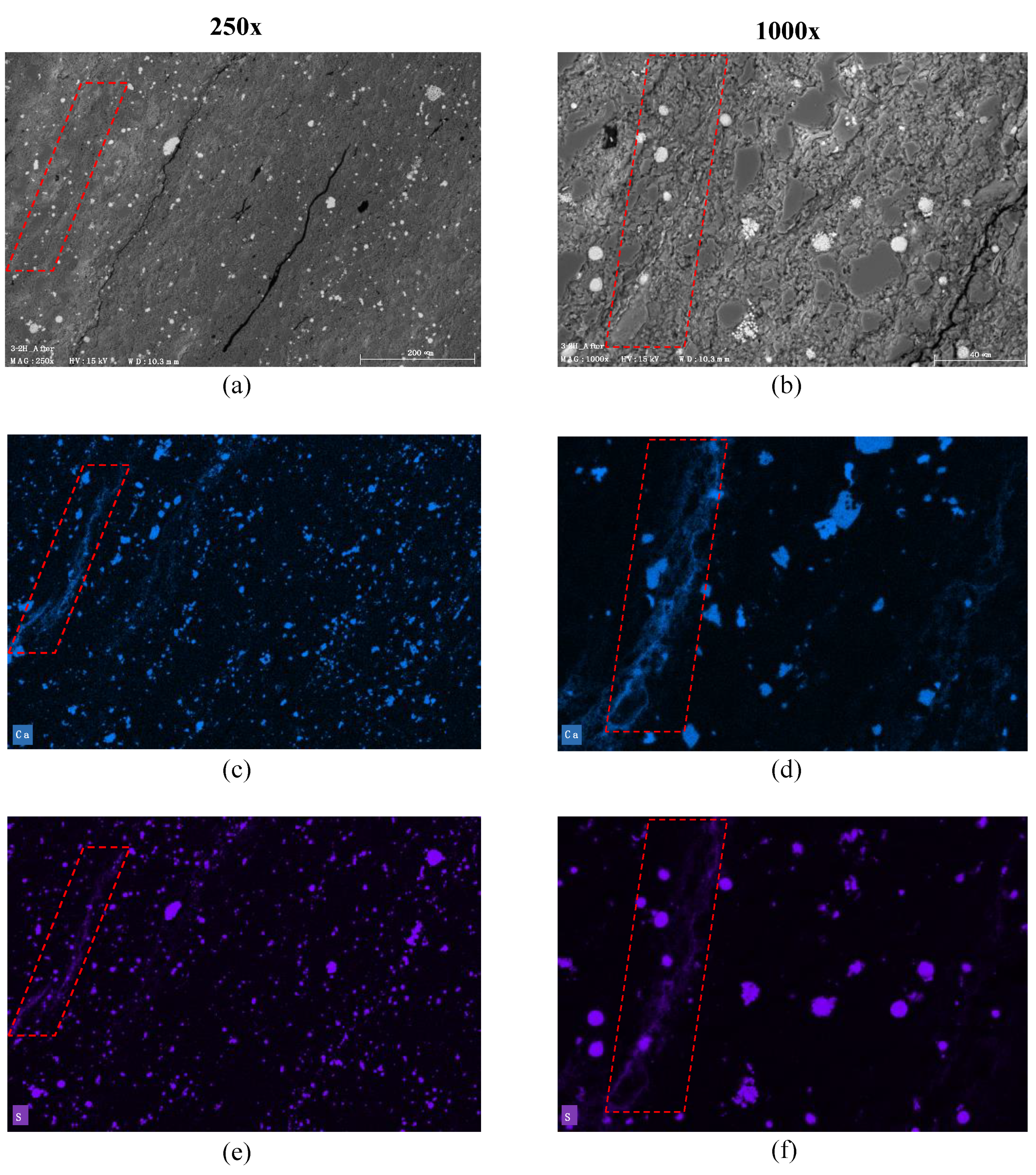
3.2.2. Sample 3-2H; Extended Cycles
3.3. Competition between Dissolution and Adsorption
3.3.1. Samples 1-63H and 3-110H; Shorter-Term Experiment; Combined Effects
3.3.2. Sample 1-42-3H; Longer-Term Experiment
3.4. Sample 4-6H; Matrix Weakening
4. Discussions
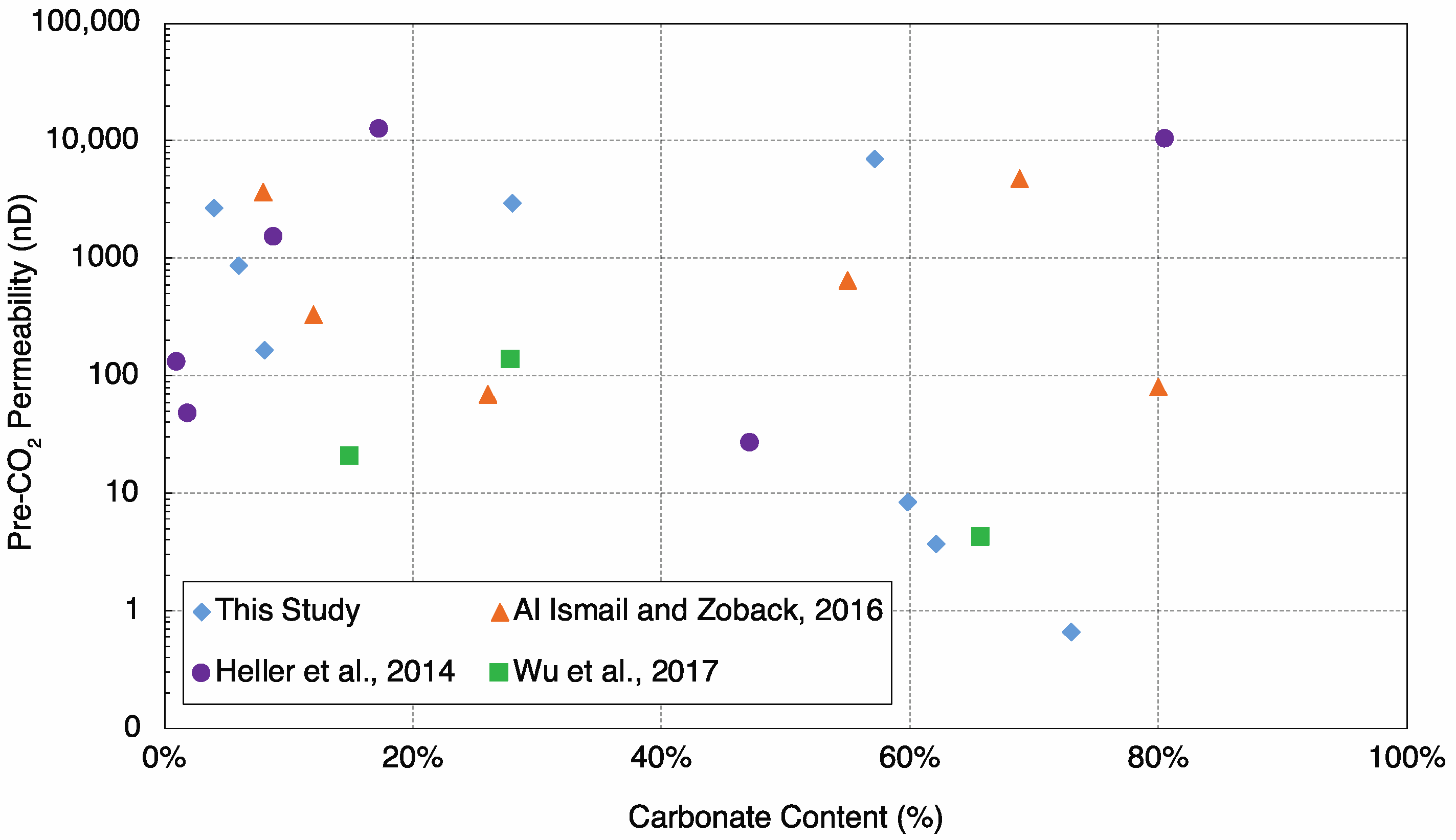
4.1. Control of Initial (Unreacted) Argon Permeability by Carbonate Content
4.2. Argon vs. scCO2 Permeability
4.3. Competing Effects of Dissolution and Adsorption
4.4. Micro-Structural Changes
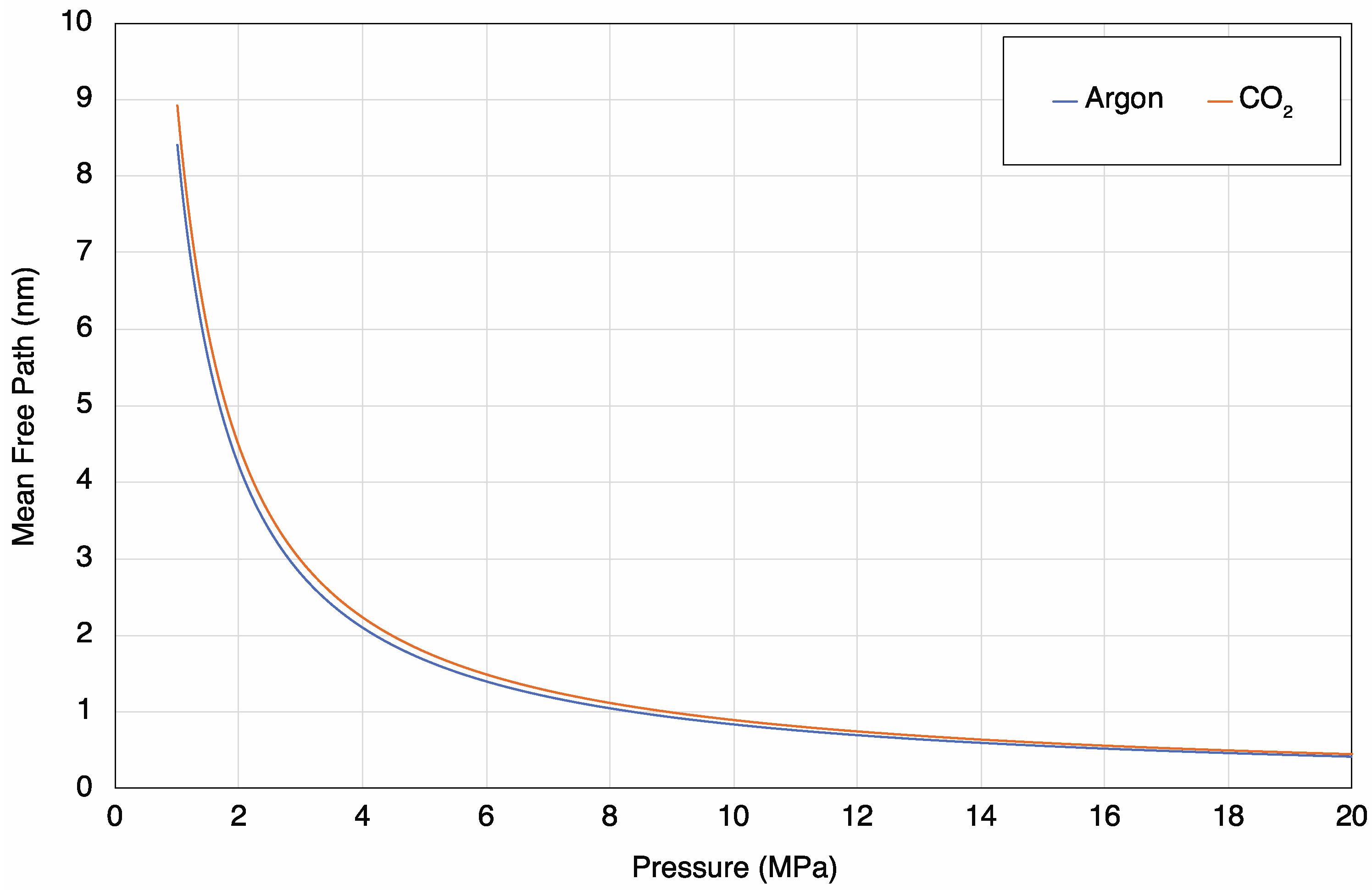
4.5. Pressure-Dependency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA Report. Exploring Clean Energy Pathways: The Role of CO2 Storage. 2019. Available online: https://www.iea.org/reports/the-role-of-co2-storage (accessed on 12 April 2020).
- NETL Report. Carbon Sequestration Atlas of the United States and Canada, Fifth ed. National Energy Technology Laboratory; U.S. Department of Energy, 2015. Available online: https://www.netl.doe.gov/sites/default/files/2018-10/ATLAS-V-2015.pdf (accessed on 28 September 2020).
- Kang, S.M.; Fathi, E.; Ambrose, R.J.; Akkutlu, I.Y.; Sigal, R.F. Carbon dioxide storage capacity of organic-rich shales. SPE J. 2011, 16, 842–855. [Google Scholar] [CrossRef]
- Jin, L.; Hawthorne, S.; Sorensen, J.; Pekot, L.; Kurz, B.; Smith, S.; Heebink, L.; Herdegen, V.; Bosshart, N.; Torres, J.; et al. Advancing CO2 enhanced oil recovery and storage in unconventional oil play—experimental studies on Bakken shales. Appl. Energy 2017, 208, 171–183. [Google Scholar] [CrossRef]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Zoback, M.D.; Kovscek, A.R.; Wilcox, J. Interdisciplinary Investigation of CO2 Sequestration in Depleted Shale Gas Formations; The Leland Stanford Junior University: Stanford, CA, USA, 2013. [Google Scholar]
- Heller, R.; Vermylen, J.; Zoback, M. Experimental investigation of matrix permeability of gas shales. AAPG Bull. 2014, 98, 975–995. [Google Scholar] [CrossRef]
- Zoback, M.D.; Kohli, A.H. Unconventional Reservoir Geomechanics; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Zhang, C.; Ranjith, P.G. Experimental study of matrix permeability of gas shale: An application to CO2-based shale fracturing. Energies 2018, 11, 702. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, J.; Jiang, Y.; Xian, X.; Liu, Q. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, D.; Luo, P.; Zhang, Y.; Zhang, L.; Sun, L. Influences of subcritical and supercritical CO2 treatment on the pore structure characteristics of marine and terrestrial shales. J. CO2 Util. 2018, 28, 152–167. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, D.; Luo, P.; Zhang, Y.; Sun, L.; Wang, K. Experimental investigation of the geochemical interactions between supercritical CO2 and shale: Implications for CO2 storage in gas-bearing shale formations. Energy Fuels 2018, 32, 1963–1978. [Google Scholar] [CrossRef]
- Hadian, P.; Rezaee, R. The Effect of Supercritical CO2 on Shaly Caprocks. Energies 2020, 13, 149. [Google Scholar] [CrossRef]
- Rohmer, J.; Pluymakers, A.; Renard, F. Mechano-chemical interactions in sedimentary rocks in the context of CO2 storage: Weak acid, weak effects? Earth-Sci. Rev. 2016, 157, 86–110. [Google Scholar] [CrossRef]
- Lu, J.; Nicot, J.P.; Mickler, P.J.; Ribeiro, L.H.; Darvari, R. Alteration of Bakken reservoir rock during CO2-based fracturing—An autoclave reaction experiment. J. Unconv. Oil Gas Resour. 2016, 14, 72–85. [Google Scholar] [CrossRef]
- Sanguinito, S.; Goodman, A.; Tkach, M.; Kutchko, B.; Culp, J.; Natesakhawat, S.; Fazio, J.; Fukai, I.; Crandall, D. Quantifying dry supercritical CO2-induced changes of the Utica Shale. Fuel 2018, 226, 54–64. [Google Scholar] [CrossRef]
- Goodman, A.; Sanguinito, S.; Tkach, M.; Natesakhawat, S.; Kutchko, B.; Fazio, J.; Cvetic, P. Investigating the role of water on CO2-Utica Shale interactions for carbon storage and shale gas extraction activities–Evidence for pore scale alterations. Fuel 2019, 242, 744–755. [Google Scholar] [CrossRef]
- Kutchko, B.; Sanguinito, S.; Natesakhawat, S.; Cvetic, P.; Culp, J.T.; Goodman, A. Quantifying pore scale and matrix interactions of SCCO2 with the Marcellus shale. Fuel 2020, 266, 116928. [Google Scholar] [CrossRef]
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar] [CrossRef]
- Lindner, E.N. Review of the Effects of CO2 on Very-Fine-Grained Sedimentary Rock/Shale–Part II: Clay Mineral & Shale Response to Hydration (No. 68913329-03d5-45bc-b32a-a7e8e763b10a); National Energy Technology Laboratory-Energy Data eXchange; NETL: 2016. Available online: https://www.osti.gov/scitech/search/filter-results:FD/semantic:10.18141/1432993 (accessed on 3 March 2020).
- Al Ismail, M.; Reece, J.S.; Hol, S.; Zoback, M. The effect of CO2 adsorption on permeability anisotropy in the Eagle Ford Shale. In Unconventional Resources Technology Conference (URTEC); 2014; Available online: https://onepetro.org/URTECONF/proceedings-abstract/14URTC/All-14URTC/URTEC-1921520-MS/151592 (accessed on 15 November 2019).
- Wu, W.; Zoback, M.D.; Kohli, A.H. The impacts of effective stress and CO2 sorption on the matrix permeability of shale reservoir rocks. Fuel 2017, 203, 179–186. [Google Scholar] [CrossRef]
- Klewiah, I.; Berawala, D.S.; Walker, H.C.A.; Andersen, P.Ø.; Nadeau, P.H. Review of experimental sorption studies of CO2 and CH4 in shales. J. Nat. Gas Sci. Eng. 2020, 73, 103045. [Google Scholar] [CrossRef]
- van Noort, R.; Yarushina, V. Water, CO2 and argon permeabilities of intact and fractured shale cores under stress. Rock Mech. Rock Eng. 2019, 52, 299–319. [Google Scholar] [CrossRef]
- Play, W.S. Permian Basin. 2018. Available online: https://www.eia.gov/maps/pdf/PermianBasin_Wolfcamp_EIAReport_Oct2018.pdf (accessed on 24 May 2020).
- Scheidegger, A.E. The Physics of Flow through Porous Media, 3rd ed.; University of Toronto Press: Toronto, ON, Canada, 1974. [Google Scholar]
- Brace, W.; Walsh, J.B.; Frangos, W.T. Permeability of granite under high pressure. J. Geophys. Res. 1968, 73, 2225–2236. [Google Scholar] [CrossRef]
- Metwally, Y.M.; Sondergeld, C.H. Measuring low permeabilities of gas-sands and shales using a pressure transmission technique. Int. J. Rock Mech. Min. Sci. 2011, 48, 1135–1144. [Google Scholar] [CrossRef]
- Einsele, G. Sedimentary Basins: Evolution, Facies, and Sediment Budget; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Kamali-Asl, A.; Ghazanfari, E.; Newell, P.; Stevens, M. Elastic, viscoelastic, and strength properties of Marcellus Shale specimens. J. Pet. Sci. Eng. 2018, 171, 662–679. [Google Scholar] [CrossRef]






| Formation | Wolfcamp | Eagle Ford | Utica | |||||
|---|---|---|---|---|---|---|---|---|
| Sample ID | 2-1H | 3-2H | 4-2H | 4-4H | 1-63H | 3-110H | 4-6H | 1-42-3H |
| Diameter (mm) | 25.21 | 25.25 | 25.28 | 25.25 | 25.31 | 25.35 | 25.25 | 25.32 |
| Length (mm) | 9.55 | 8.02 | 6.62 | 7.32 | 6.06 | 6.41 | 6.03 | 7.34 |
| Depth (m) | 2859 | 2893 | 2943 | 2949 | 4281 | 4334 | 4348 | 1914 |
| Well Number | Sugg-A-171-6TW | Seidel-A-2MW | Lennington-1H/OH-HH-63992 | |||||
| Carbonate (%) | 71.5 | 3.9 | 5.8 | 3.6 | 55.8 | 56.9 | 53.1 | 15.3 |
| Calcite (%) | 71.5 | <bdl | <bdl | 1.1 | 55.8 | 56.1 | 53.1 | 15.3 |
| Dolomite (%) | <bdl | 3.9 | 5.8 | 2.5 | <bdl | 0.8 | <bdl | <bdl |
| QFP (%) | 27.7 | 67.9 | 61.8 | 52.5 | 21.3 | 21.5 | 22.8 | 32.9 |
| Feldspar (%) | <bdl | 7.2 | 7.2 | <bdl | <bdl | <bdl | <bdl | <bdl |
| Quartz (%) | 24.5 | 53.8 | 52.7 | 51.3 | 14.0 | 14.3 | 14.8 | 28.5 |
| Plagioclase (%) | <bdl | 11.0 | 7.2 | <bdl | 2.5 | 2.5 | 2.9 | <bdl |
| Pyrite (%) | 3.2 | 3.8 | 1.9 | 1.2 | 4.8 | 4.7 | 5.1 | 4.4 |
| Muscovite (%) | <bdl | 24.6 | 29.3 | <bdl | <bdl | <bdl | <bdl | <bdl |
| Illite (%) | <bdl | <bdl | <bdl | 39.5 | 9.7 | 8.5 | 9.9 | 47.2 |
| Mica (%) | <bdl | <bdl | <bdl | <bdl | 5.1 | 5.3 | 5.7 | <bdl |
| Chlorite (%) | <bdl | <bdl | <bdl | <bdl | 1.3 | 0.9 | 1.4 | <bdl |
| Total Clay (%) | <bdl | 24.6 | 29.3 | 39.5 | 16.1 | 14.7 | 17 | 47.2 |
| TOC (%) | 0.8 | 3.6 | 3.1 | 4.4 | 6.8 | 6.9 | 7.1 | 4.6 |
| S1 (mg/g) | 0.59 | 3.83 | 5.7 | 6.57 | 8.72 | 9.94 | 10.67 | N/A |
| S2 (mg/g) | 1.05 | 8.68 | 6.87 | 13.04 | 7.07 | 6.28 | 6.47 | N/A |
| S3 (mg/g) | 0.17 | 0.32 | 0.32 | 0.2 | 0.27 | 0.25 | 0.24 | N/A |
| Tmax (°C) | 442 | 444 | 445 | 446 | 350 | 470 | 479 | N/A |
| HI | 140 | 240 | 222 | 297 | 103 | 91 | 92 | N/A |
| OI | 23 | 9 | 10 | 5 | 4 | 4 | 3 | N/A |
| PI | 0.36 | 0.31 | 0.46 | 0.34 | 0.55 | 0.61 | 0.62 | N/A |
| Sample ID | Meas. Method | Initial Perm (m2) | kC2/kC1 | kC3/kC1 | Net Change | Processes/Phenomena |
|---|---|---|---|---|---|---|
| 2-1H | PD | 0.50 × 10−21 | 22.4 | 14.2 | Significant Increase | Irreversible dissolution of carbonate minerals (D) |
| 3-2H | SS | 2.08 × 10−18 | 0.49 | 1.01 | Constant | Reversible adsorption of scCO2 into clays and organic matter; Slight carbonate dissolution in micro-cracks (A) |
| 4-2H | SS | 0.76 × 10−18 | 4.50 | 3.25 | Significant Increase | Irreversible dissolution of calcite-filled micro-cracks (D);No observable adsorption despite high “clay+TOC” |
| 4-4H | PD | 1.47 × 10−19 | 0.52 | 0.95 | Slight Decrease | Reversible adsorption of scCO2 into clays and organic matter (A) |
| 1-63H | PD | 6.38 × 10−21 | 0.83 | 1.14 | Slight Increase | Dissolution dominates adsorption while both occur to a moderate degree (MAD) |
| 3-110H | PD | 3.08 × 10−21 | 0.55 | 0.84 | Slight Decrease | Adsorption dominates dissolution while both occur to a moderate degree (MAD) |
| 4-6H | SS | 4.87 × 10−18 | 0.57 | 0.47 | Decrease | Mechanical compaction of matrix surrounding micro-cracks overshadowing adsorption and dissolution (W) |
| 1-42-3H | SS | 1.97 × 10−18 | 0.30 | 0.45 | Decrease | Adsorption dominates over dissolution in both short and longer-term with enhanced dissolution in the latter (MAD) |
| Sample ID | Clay + TOC | (k10U-k30)/k10U × 100 | ||
|---|---|---|---|---|
| C1 Cycle; before scCO2 | C3 Cycle; after scCO2 | C6 Cycle; after Extended scCO2 | ||
| 2-1H | 1% | 25.3% | 41.5% | N/A |
| 3-2H | 28% | 23.5% | 26% | 27.3% |
| 4-2H | 32% | 13.1% | 11.6% | 9.2% |
| 4-4H | 44% | 10.8% | 6.7% | N/A |
| 1-63H | 23% | 25.1% | 21.9% | N/A |
| 3-110H | 22% | 17.8% | 0.73% | N/A |
| 4-6H | 24% | 30.6% | 33.4% | N/A |
| 1-42-3H | 52% | 33.3% | 45.7% | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamali-Asl, A.; Zoback, M.D.; Kohli, A.H. Effects of Supercritical CO2 on Matrix Permeability of Unconventional Formations. Energies 2021, 14, 1101. https://doi.org/10.3390/en14041101
Kamali-Asl A, Zoback MD, Kohli AH. Effects of Supercritical CO2 on Matrix Permeability of Unconventional Formations. Energies. 2021; 14(4):1101. https://doi.org/10.3390/en14041101
Chicago/Turabian StyleKamali-Asl, Arash, Mark D Zoback, and Arjun H. Kohli. 2021. "Effects of Supercritical CO2 on Matrix Permeability of Unconventional Formations" Energies 14, no. 4: 1101. https://doi.org/10.3390/en14041101
APA StyleKamali-Asl, A., Zoback, M. D., & Kohli, A. H. (2021). Effects of Supercritical CO2 on Matrix Permeability of Unconventional Formations. Energies, 14(4), 1101. https://doi.org/10.3390/en14041101





