Quantifying the Effect of De-Emulsifiers on Acid Treatment in Carbonate Formations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Indiana Limestone Core
2.1.2. Crude Oil
2.1.3. Hydrochloric Spent-Acid
2.1.4. De-Emulsifiers
2.1.5. Surfactant
2.2. Experimental Setup and Procedures
2.2.1. Bottle Tests for Screening De-Emulsifiers
2.2.2. Bottle Tests for Emulsion Viscosity and Stability
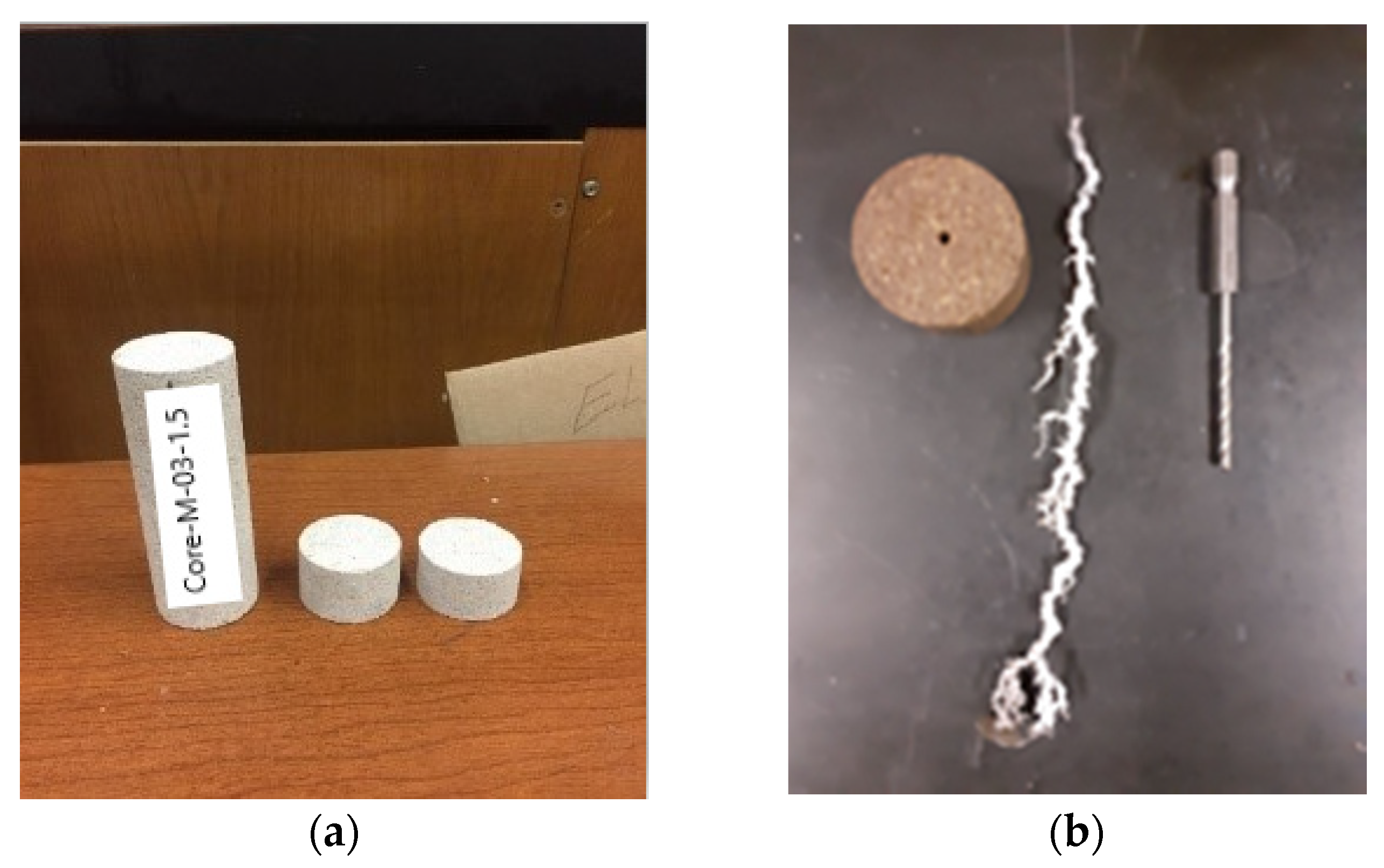
2.2.3. Core Preparation Procedure
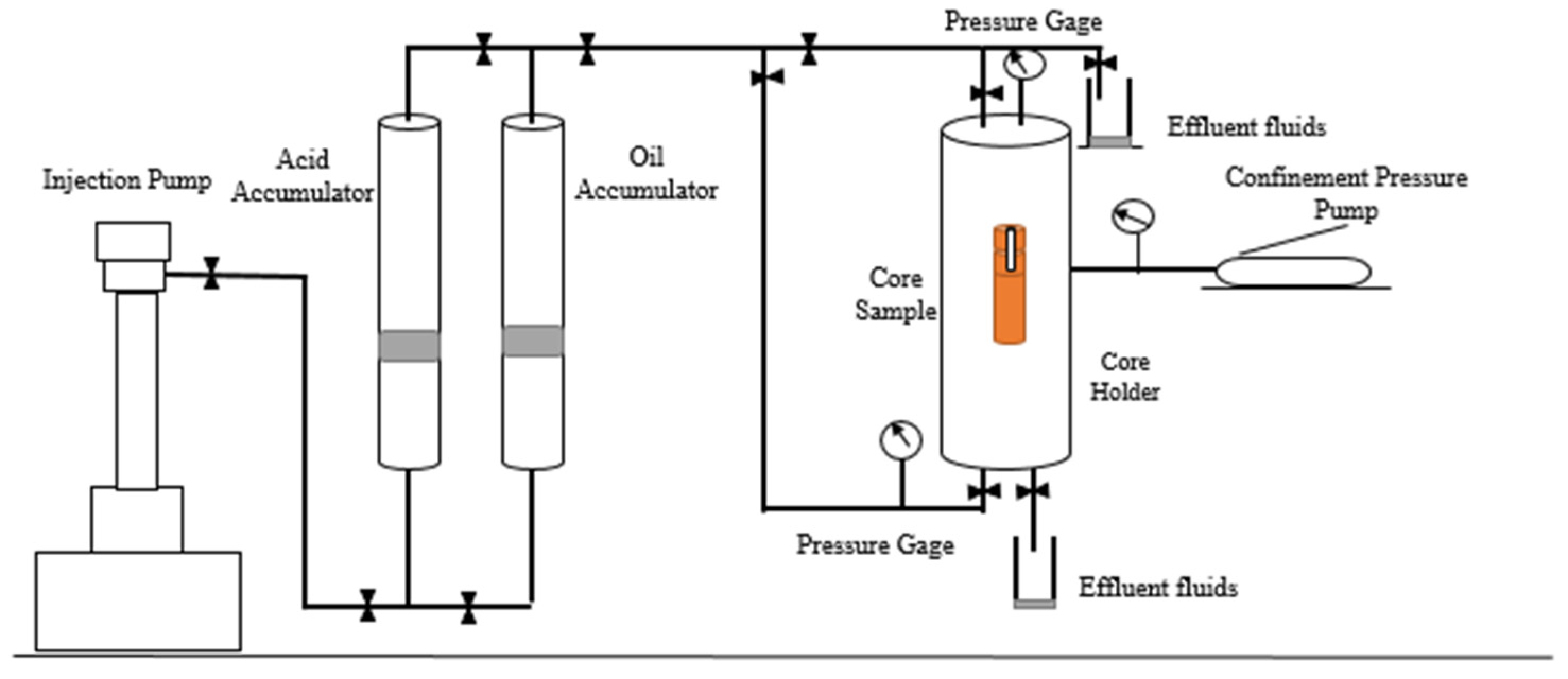
2.2.4. Core Flooding Tests
2.3. Assumptions and Limitations
3. Results and Discussion
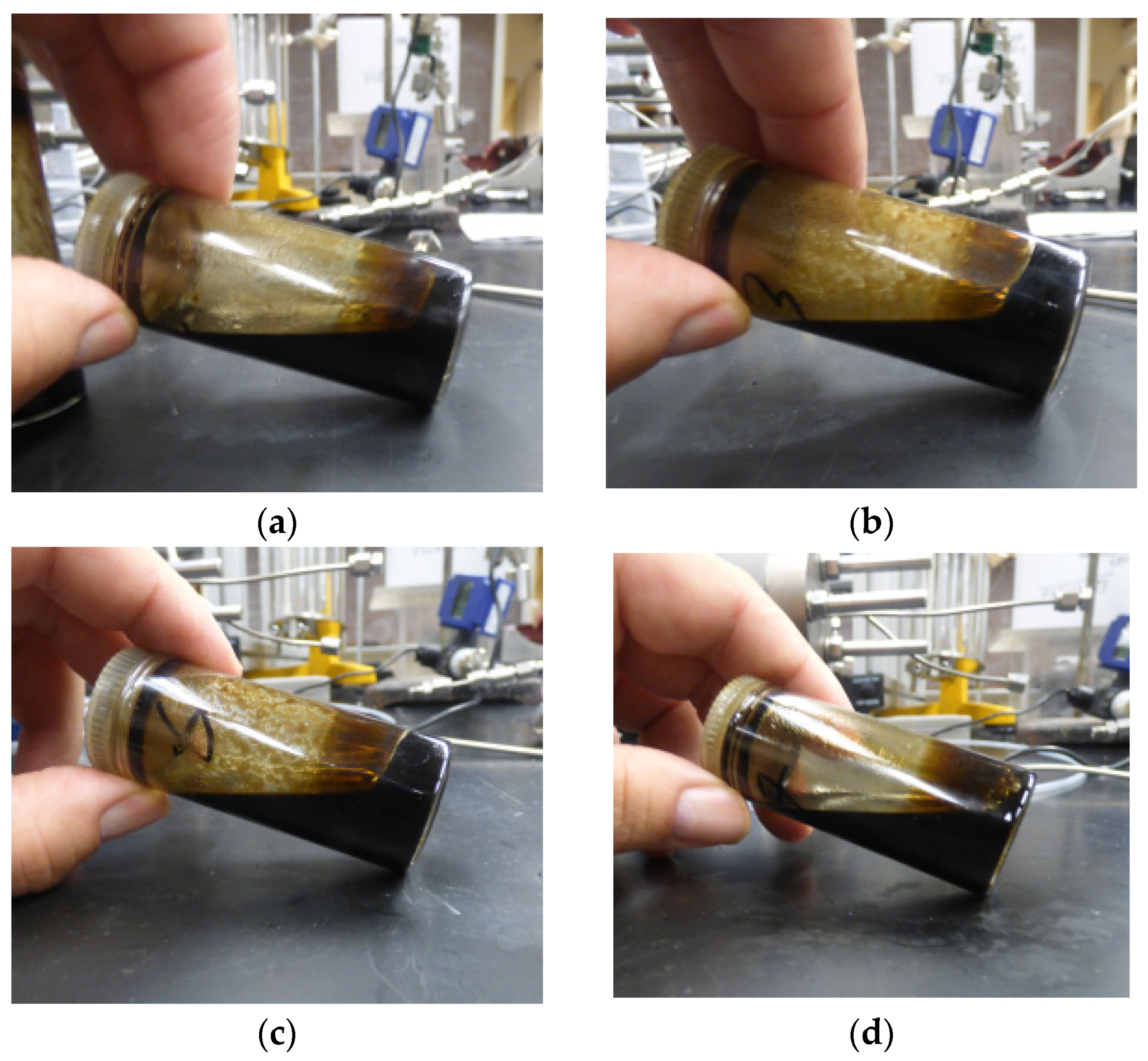
3.1. Bottle Tests for Screening De-Emulsifiers
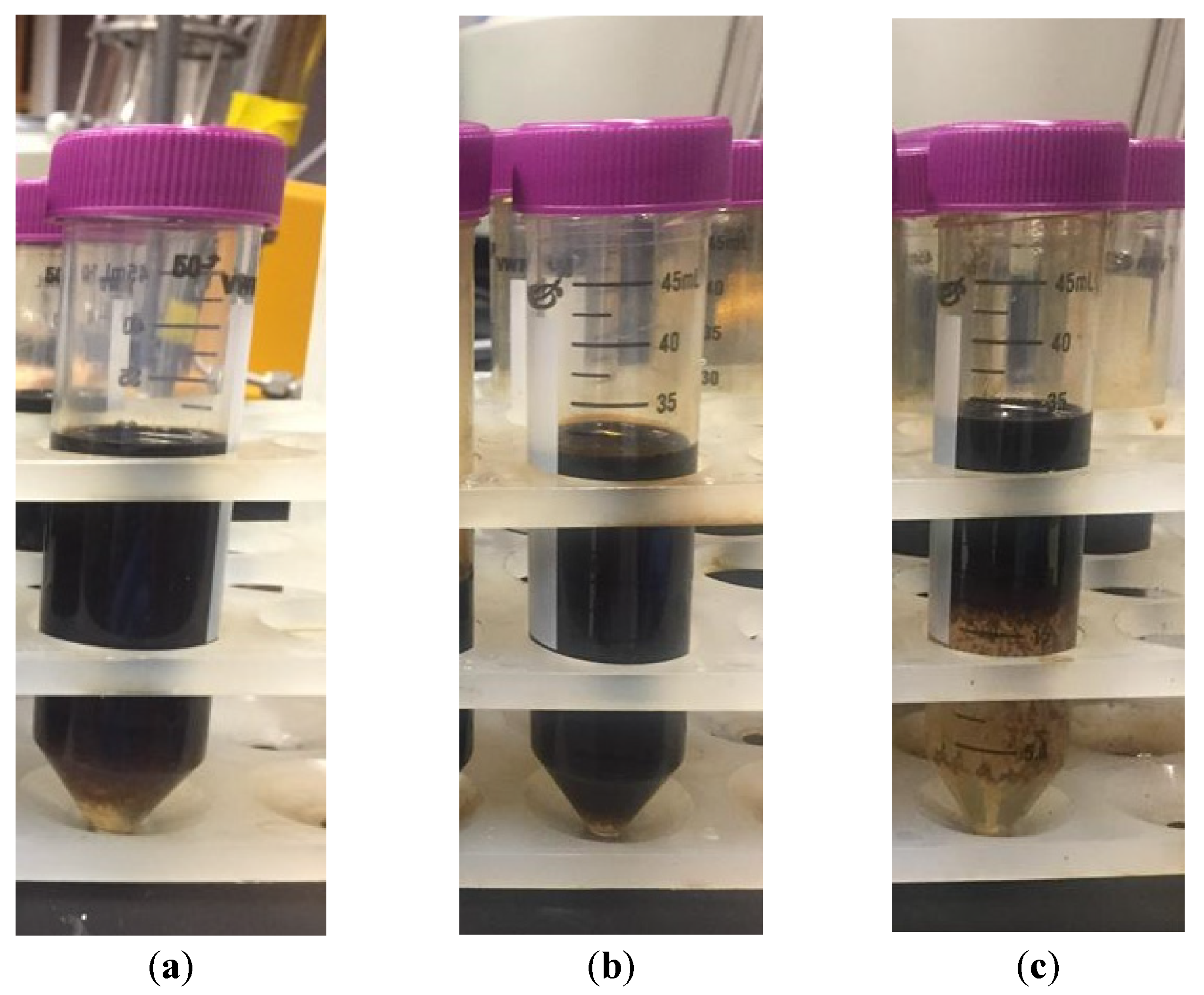
3.2. Bottle Tests for Emulsion Stability and Viscosity
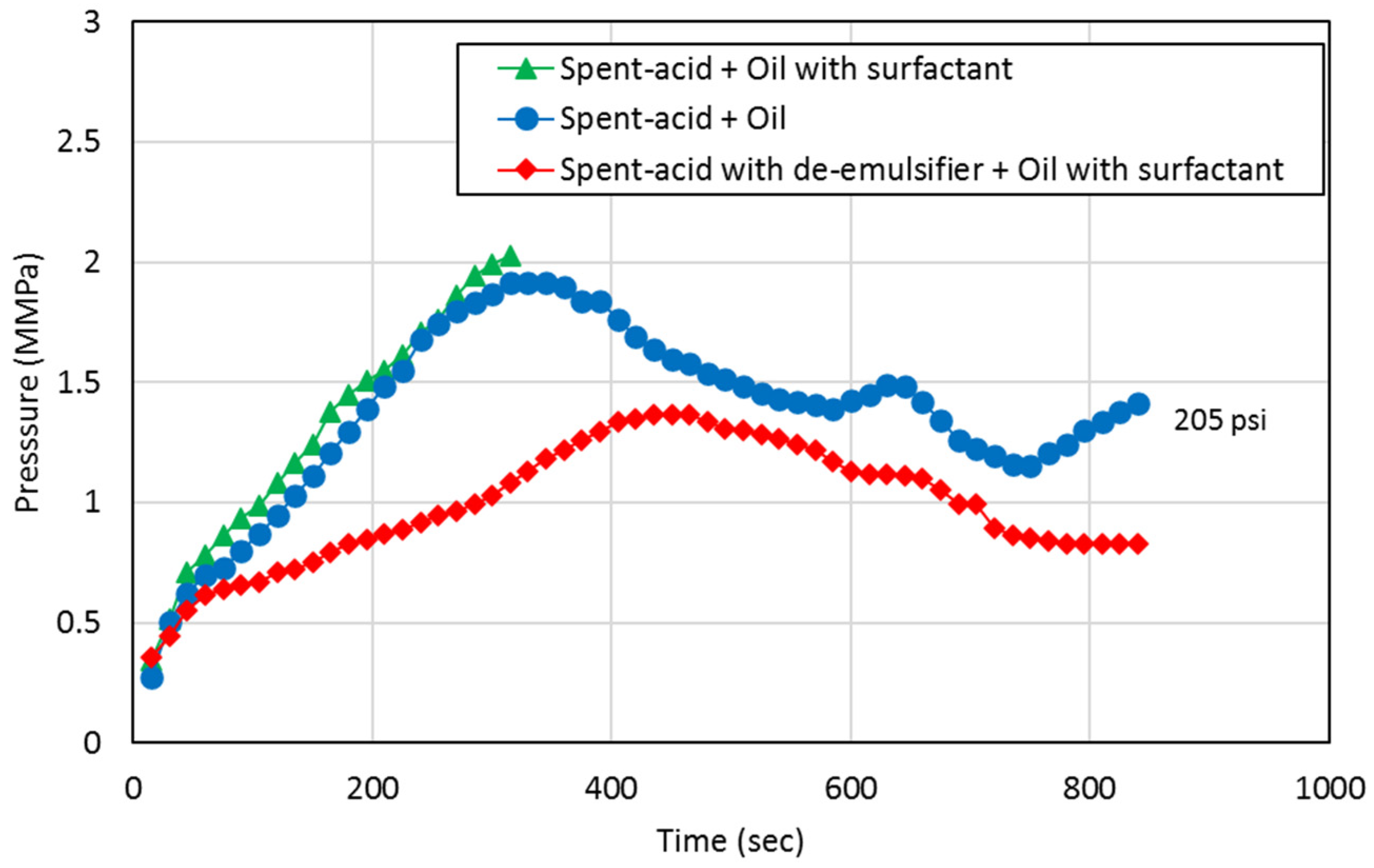
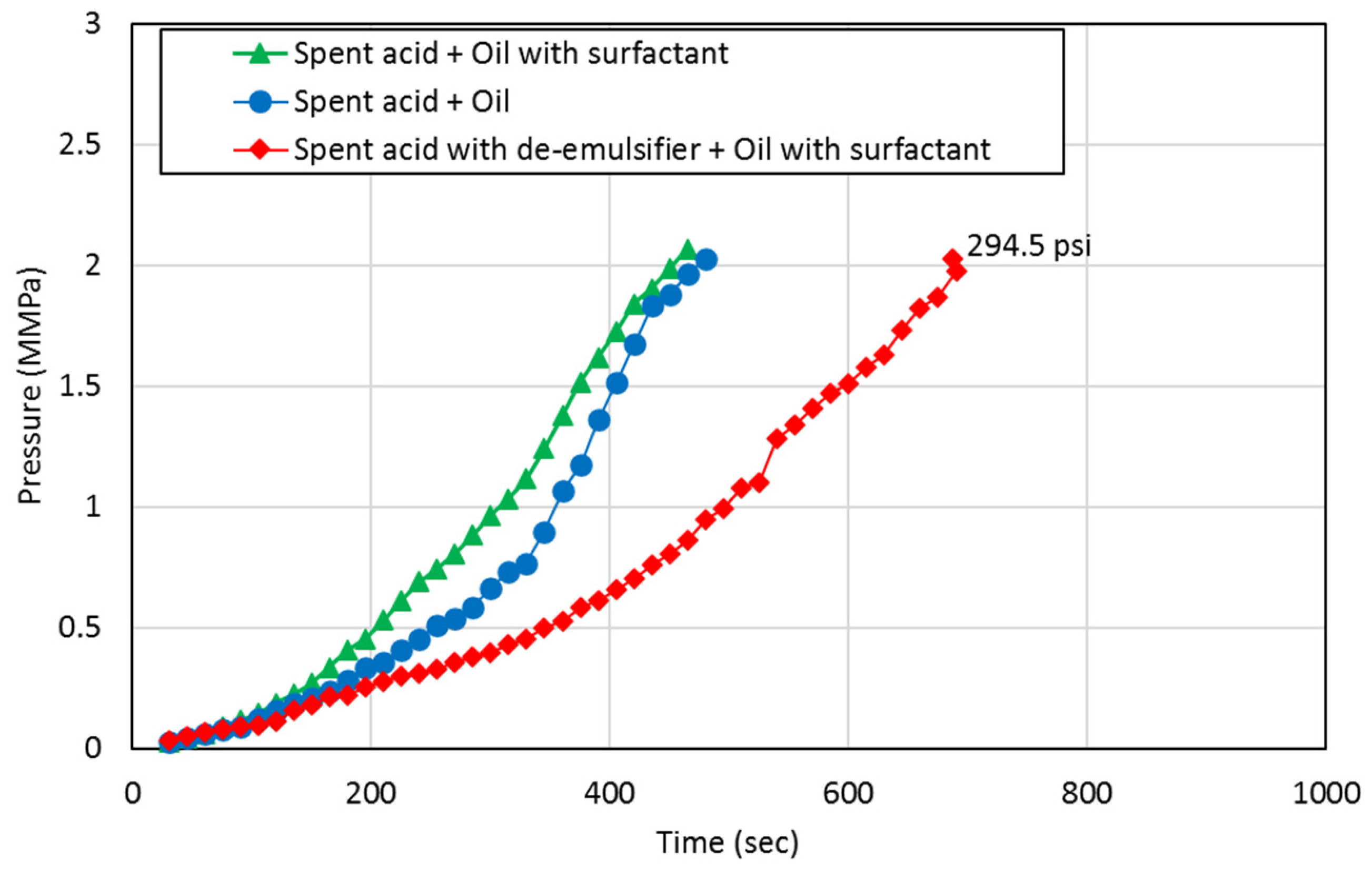
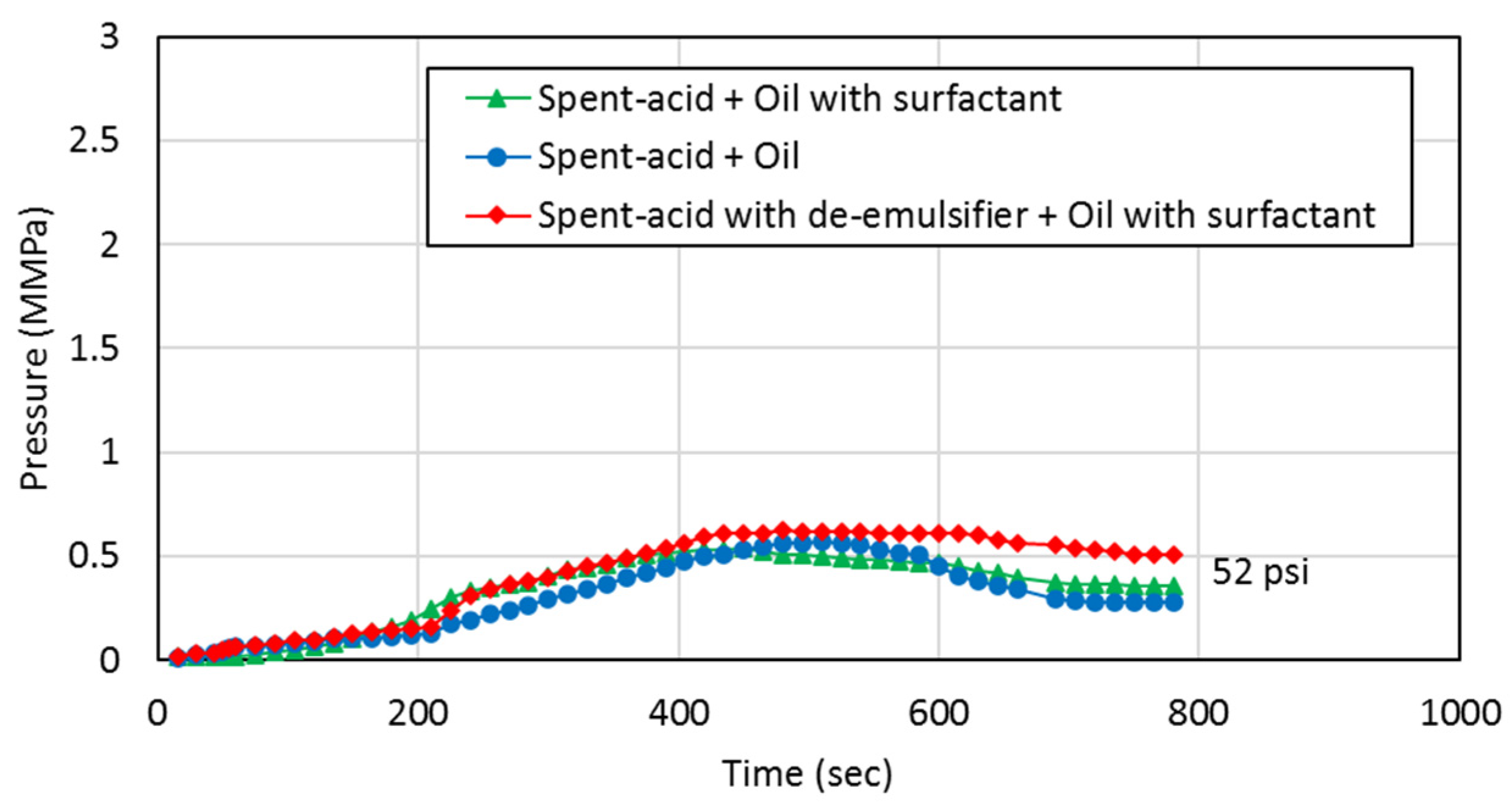
3.3. Core Flooding Tests
4. Conclusions
- Emulsion-prone systems result in higher resistance to flow during both the acid injection phase and the oil production phase indicating reduced flow capacity in the pore space, which can be explained by the presence of emulsions resulting from spent-acid and oil mixing.
- De-emulsifiers that successfully control the formation of emulsions result in doubling the flow capacity for both spent-acid and oil. This can improve the performance of the matrix acidizing treatment in carbonate formations.
- The experimental protocol followed in this study proved to be effective in documenting the de-emulsifiers’ effect on flow properties in acidizing treatments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moore, E.; Crowe, C.; Hendrickson, A. Formation, Effect and Prevention of Asphaltene Sludges During Stimulation Treatments. J. Pet. Technol. 1965, 17, 1023–1028. [Google Scholar] [CrossRef]
- Kokal, S.; Al-Juraid, J. Reducing Emulsion Problems by Controlling Asphaltene Solubility and Precipitation. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 27–30 September 1998. [Google Scholar]
- Kokal, S.L. Crude Oil Emulsions: A State-Of-The-Art Review. SPE Prod. Facil. 2005, 20, 5–13. [Google Scholar] [CrossRef]
- Kokal, S.; Al-Yousif, A.; Meeranpillai, N.; Al-Awaisi, M. Very Thick Crude Emulsions: A Field Case Study of a Unique Crude Production Problem. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–3 October 2001. [Google Scholar]
- Kokal, S.L.; Al-Ghamdi, A.; Meeranpillai, N.S. An Investigative Study of Potential Emulsion Problems Before Field Development. SPE Proj. Facil. Constr. 2007, 2, 1–10. [Google Scholar] [CrossRef]
- Abdulredha, M.M.; Aslina, H.S.; Luqman, C.A. Overview on petroleum emulsions, formation, influence and demulsification treatment techniques. Arab. J. Chem. 2020, 13, 3403–3428. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhru’L-Razi, A.; Abdullah, L.C.; Elnashaie, S.S.; Pendashteh, A. Demulsification techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Kokal, S.; Al-Dawood, N.; Fontanilla, J.; Al-Ghamdi, A.; Nasr-El-Din, H.; Al-Rufaie, Y. Productivity Decline in Oil Wells Related to Asphaltene Precipitation and Emulsion Blocks. SPE Prod. Facil. 2003, 18, 247–256. [Google Scholar] [CrossRef]
- Kokal, S.; Alvarez, C. Reducing Pressure Drop in Offshore Pipelines by Controlling the Viscosities of Pressurized Emulsions. In Proceedings of the Middle East Oil Show, Sanabis, Bahrain, 5–8 April 2003. [Google Scholar]
- Atehortúa, C.M.G.; Pérez, N.; Andrade, M.A.B.; Pereira, L.O.V.; Adamowski, J.C. Water-in-oil emulsions separation using an ultrasonic standing wave coalescence chamber. Ultrason. Sonochem. 2019, 57, 57–61. [Google Scholar] [CrossRef]
- Aziz, H.M.A.; Darwish, S.F.; Abdeen, F.M. Downhole Emulsion Problem, The Causes and Remedy, Ras Budran Field. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Melbourne, Australia, 8–10 October 2002. [Google Scholar]
- Knopp, M. Non-Damaging Matrix and Fracturing Acids: Some Key Considerations; Distinguished Lecturer Program, Society of Petroleum Engineers: Richardson, TX, USA, 2009; Available online: http://www.spe.org/dl/docs/2009/Knopp.pdf (accessed on 12 February 2021).
- Sharma, M.K.; Shah, D.O. Introduction to Macro- and Microemulsions. In ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 1985; Volume 272, pp. 1–18. [Google Scholar]
- Kokal, S.L. Crude Oil Emulsions. In Petroleum Engineering Handbook; Fanchi, J.R., Ed.; General Engineering; Society of Petroleum Engineers: Rich-ardson, TX, USA, 2006; Volume 1. [Google Scholar]
- Czarnecki, J.; Moran, K. On the Stabilization Mechanism of Water-in-Oil Emulsions in Petroleum Systems. Energy Fuels 2005, 19, 2074–2079. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Jia, W.; Li, Z.; Zhao, Y.; Ren, S. Demulsification of Crude Oil-in-Water Emulsions Driven by Graphene Oxide Nanosheets. Energy Fuels 2015, 29, 4644–4653. [Google Scholar] [CrossRef]
- Jones, T.; Neustadter, E.; Whittingham, K. Water-In-Crude Oil Emulsion Stability And Emulsion Destabilization By Chemical Demulsifiers. J. Can. Pet. Technol. 1978, 17. [Google Scholar] [CrossRef]
- Sjöblom, J.; Mingyuan, L.; Christy, A.A.; Gu, T. Water-in-crude-oil emulsions from the Norwegian continental shelf 7. Interfacial pressure and emulsion stability. Colloids Surfaces 1992, 66, 55–62. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Hartland, S. Dynamics of Emulsification and Demulsification of Water in Crude Oil Emulsions. Ind. Eng. Chem. Res. 1994, 33, 1271–1279. [Google Scholar] [CrossRef]
- Mason, S.; May, K.; Hartland, S. Drop size and concentration profile determination in petroleum emulsion separation. Colloids Surfaces A Physicochem. Eng. Asp. 1995, 96, 85–92. [Google Scholar] [CrossRef]
- Wanli, K.; Yi, L.; Baoyan, Q.; Guangzhi, L.; Zhenyu, Y.; Jichun, H. Interactions between alkali/surfactant/polymer and their effects on emulsion stability. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 175, 243–247. [Google Scholar] [CrossRef]
- Scarborough, B.; Elsafih, M.; Fahes, M.; Pournik, M. Characterizing Time-Dependent Stability and Viscosity of Acid–Crude Emulsions for Matrix-Acidizing Applications. Energy Fuels 2019, 33, 10508–10518. [Google Scholar] [CrossRef]
- Houchin, L.; Dunlap, D.; Arnold, B.; Domke, K. The Occurrence and Control of Acid-Induced Asphaltene Sludge. In Proceedings of the SPE Formation Damage Symposium, Lafayette, LA, USA, 22–23 February 1990. [Google Scholar]
- Krueger, R.F. An Overview of Formation Damage and Well Productivity in Oilfield Operations. J. Pet. Technol. 1986, 38, 131–152. [Google Scholar] [CrossRef]
- Oneil, B.J.; Maley, D.M.; Lalchan, C.A. Prevention of Acid-Induced Asphaltene Precipitation: A Comparison of Anionic Vs. Cationic Surfactants. J. Can. Pet. Technol. 2015, 54, 49–62. [Google Scholar] [CrossRef]
- Jacobs, I. Chemical Systems for the Control of Asphaltene Sludge During Oilwell Acidizing Treatments. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 8–10 February 1989. [Google Scholar]
- Opawale, A.O.; Osisanya, S.O. Tool for Troubleshooting Emulsion Problems in Producing Oilfields. SPE Prod. Oper. Symp. 2013. [Google Scholar] [CrossRef]
- Paulis, J.B.; Sharma, M.M. A New Family of Demulsifiers for Treating Oilfield Emulsions. SPE Int. Symp. Oilfield Chem. 1997. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Al-Dirweesh, S.; Samuel, M.M. Development and Field Application of a New, Highly Stable Emulsified Acid. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar]
- Sidaoui, Z.; Sultan, A.S. Formulating a Stable Emulsified Acid at High Temperatures: Stability and Rheology Study. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 14–16 November 2016. [Google Scholar]
- Abdollahi, R.; Esfandyari, H.; Pari, M.N.; Davarpanah, A. Conventional diverting techniques and novel fibr-assisted self-diverting system in carbonate reservoir acidizing with successful case studies. Pet. Res. 2021. [Google Scholar] [CrossRef]
- Oluwatosin, I. Experimental Study of the Rheology and Stability Behavior of Surfactant Stabilized Water-in-oil Emulsion. Master’s Thesis, University of Oklahoma, Norman, OK, USA, 2016. Available online: https://hdl.handle.net/11244/47076 (accessed on 12 February 2021).
- Gomez, S. Experimental Characterization of Brine in Crude Oil Emulsions and Its Performance in Artificial Lift Systems. Master’s Thesis, University of Oklahoma, Norman, OK, USA, 2018. Available online: https://shareok.org/handle/11244/316785 (accessed on 12 February 2021).
- Rabbani, E.; Davarpanah, A.; Memariani, M. An experimental study of acidizing operation performances on the wellbore productivity index enhancement. J. Pet. Explor. Prod. Technol. 2018, 8, 1243–1253. [Google Scholar] [CrossRef]
- Salager, J.-L.; Forgiarini, A.M. Emulsion Stabilization, Breaking, and Inversion Depends upon Formulation: Advantage or Inconvenience in Flow Assurance. Energy Fuels 2012, 26, 4027–4033. [Google Scholar] [CrossRef]
- Umar, A.A.; Saaid, I.B.M.; Sulaimon, A.A.; Pilus, R.B.M. A review of petroleum emulsions and recent progress on water-in-crude oil emulsions stabilized by natural surfactants and solids. J. Pet. Sci. Eng. 2018, 165, 673–690. [Google Scholar] [CrossRef]
- Dong, K.; Zhu, D.; Hill, A.D. Theoretical and Experimental Study on Optimal Injection Rates in Carbonate Acidizing. SPE J. 2017, 22, 892–901. [Google Scholar] [CrossRef]
- Kumar, R.; He, J.; Bataweel, M.; Nasr-El-Din, H.A. New Insights on the Effect of Oil Saturation on the Optimal Acid-Injection Rate in Carbonate Acidizing. SPE J. 2018, 23, 969–984. [Google Scholar] [CrossRef]
- Shukla, S.; Zhu, D.; Hill, A. The Effect of Phase Saturation Conditions on Wormhole Propagation in Carbonate Acidizing. SPE J. 2006, 11, 273–281. [Google Scholar] [CrossRef]
- Saneifar, M.; Fahes, M.M.; Lewis-Hosein, R.; Hassna, B.O.; Hill, D. The Effect of Spent Acid on Carbonate Rock Wettability. In Proceedings of the Trinidad and Tobago Energy Resources Conference, Port of Spain, Trinidad, 27–30 June 2010. [Google Scholar]
- Kokal, S.L.; Al-Dokhi, M. Case Studies of Emulsion Behavior at Reservoir Conditions. SPE Prod. Oper. 2008, 23, 312–317. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsafih, M.; Fahes, M.; Teodoriu, C. Quantifying the Effect of De-Emulsifiers on Acid Treatment in Carbonate Formations. Energies 2021, 14, 1148. https://doi.org/10.3390/en14041148
Elsafih M, Fahes M, Teodoriu C. Quantifying the Effect of De-Emulsifiers on Acid Treatment in Carbonate Formations. Energies. 2021; 14(4):1148. https://doi.org/10.3390/en14041148
Chicago/Turabian StyleElsafih, Mohamed, Mashhad Fahes, and Catalin Teodoriu. 2021. "Quantifying the Effect of De-Emulsifiers on Acid Treatment in Carbonate Formations" Energies 14, no. 4: 1148. https://doi.org/10.3390/en14041148
APA StyleElsafih, M., Fahes, M., & Teodoriu, C. (2021). Quantifying the Effect of De-Emulsifiers on Acid Treatment in Carbonate Formations. Energies, 14(4), 1148. https://doi.org/10.3390/en14041148






