Combustion Melting Characterisation of Solid Fuel Obtained from Sewage Sludge
Abstract
:1. Introduction
1.1. Sewage Sludge Generation and Treatment Status
1.2. Energisation by Fuelization
1.3. Combustion Melting Technology (CMT)
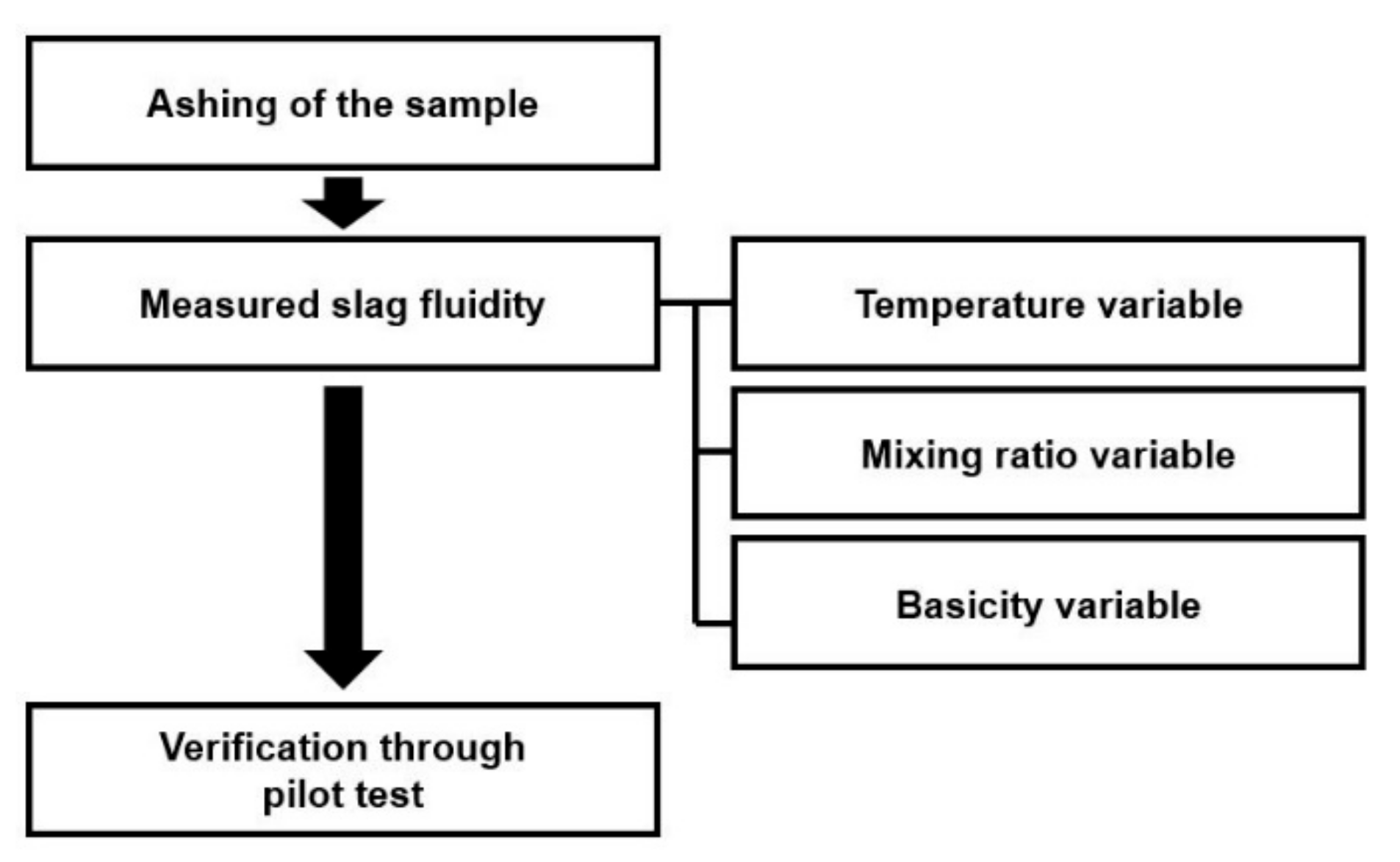
1.4. Evaluation of Slag Flowability by Fluidity Measurement
1.5. Objectives of the Study
2. Materials and Methods
2.1. Sample Preparation and Properties
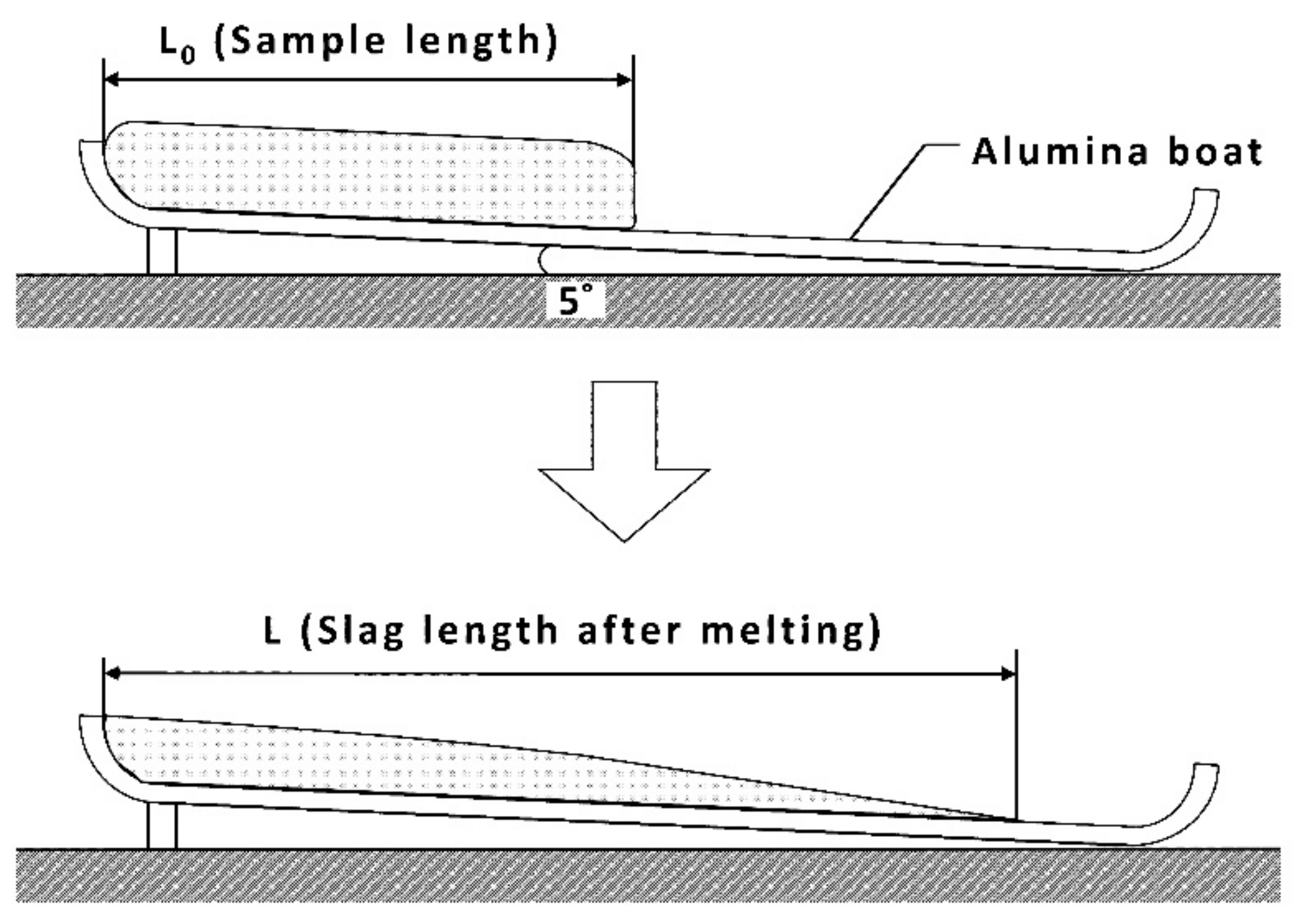
2.2. Pouring Index (PI) Test
2.2.1. Experimental Method
2.2.2. Experimental conditions
2.3. Pilot Plant Experiment
2.3.1. Experimental Method
2.3.2. Experimental Conditions
3. Results and Discussion
3.1. Analysis of the Physicochemical Characteristics
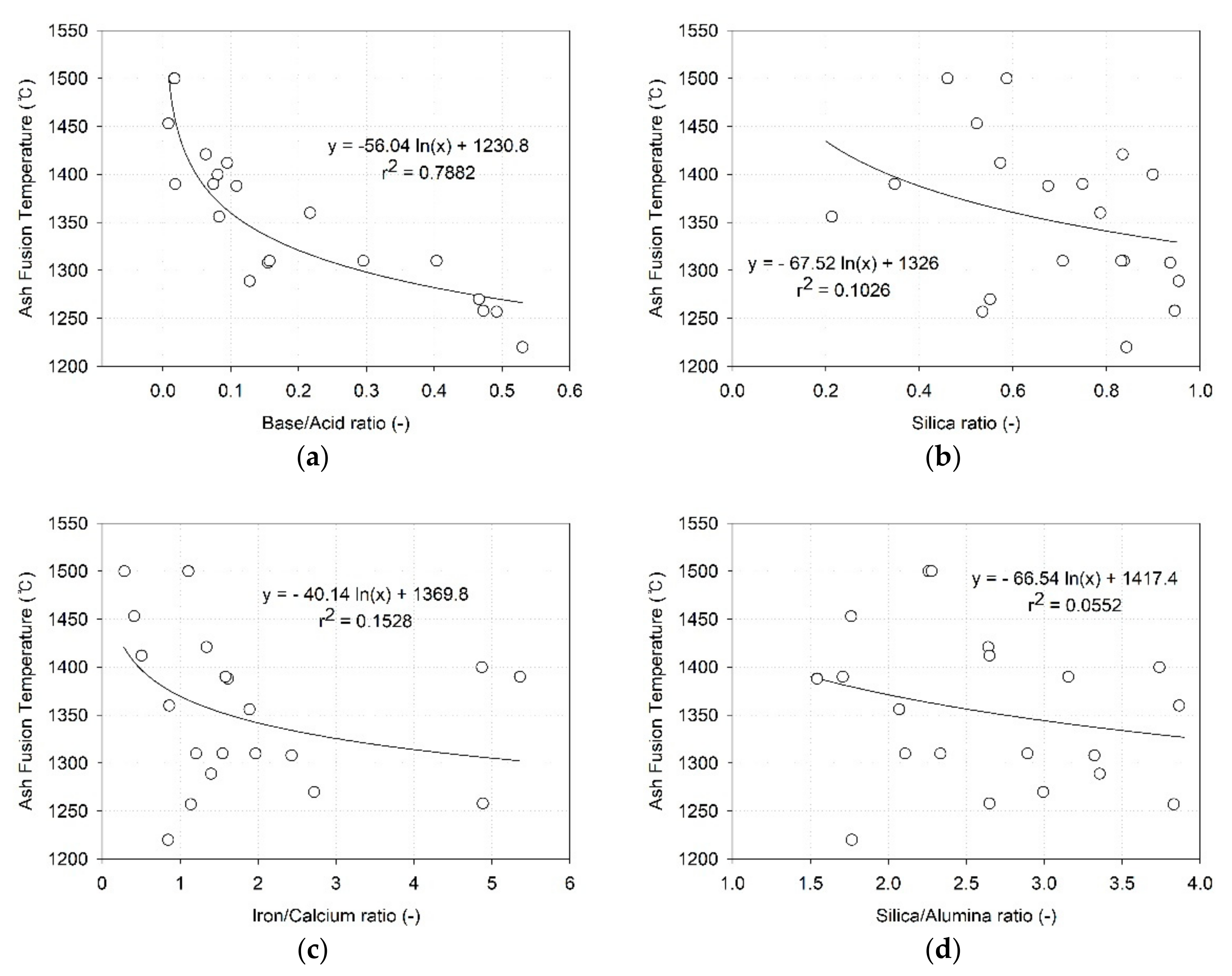
3.2. Correlation between Ash Melting Temperature and Melting Indices
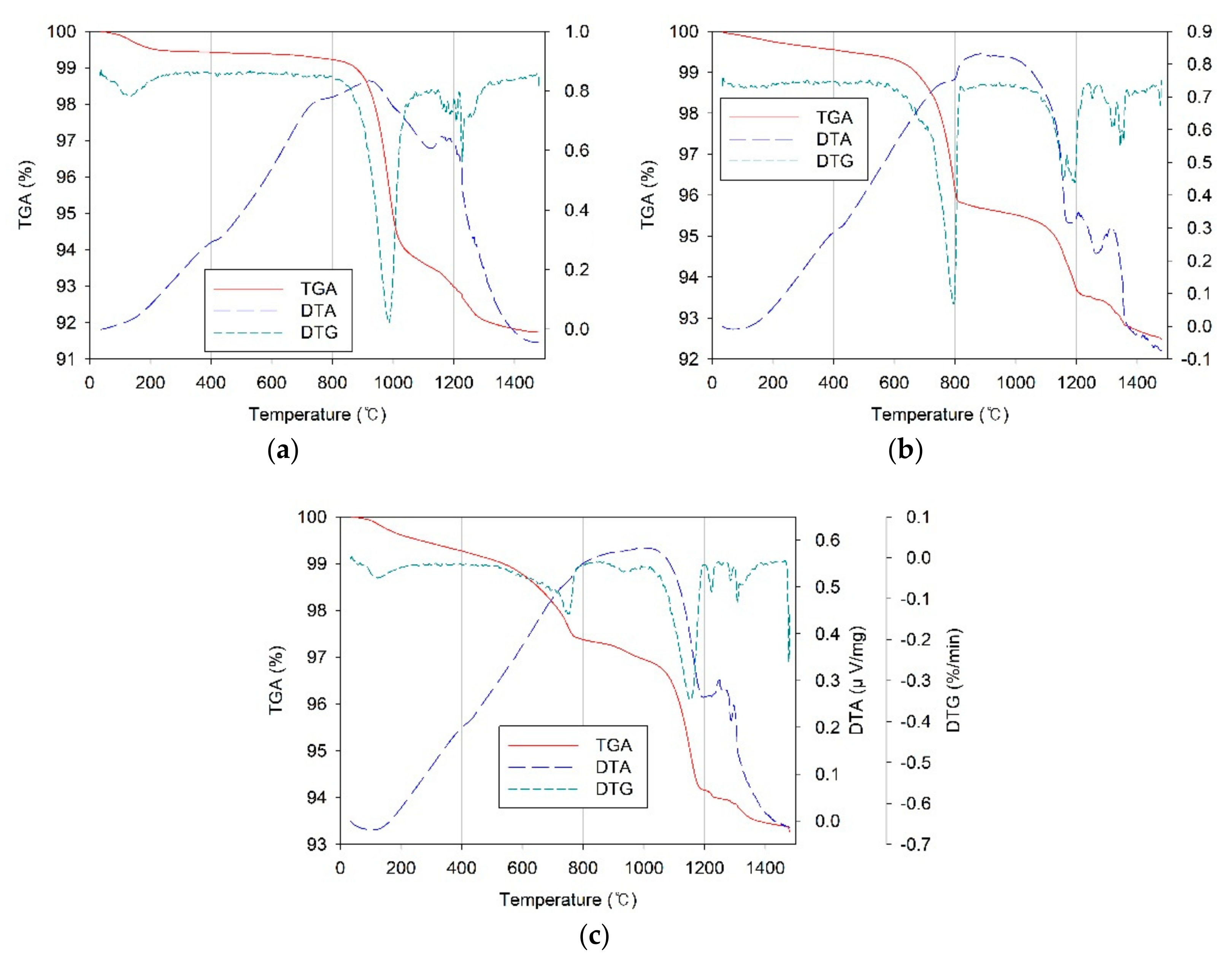
3.3. Thermo-Gravimetric Analysis–Differential Thermal Analysis
3.4. Results of the Pouring Index Test
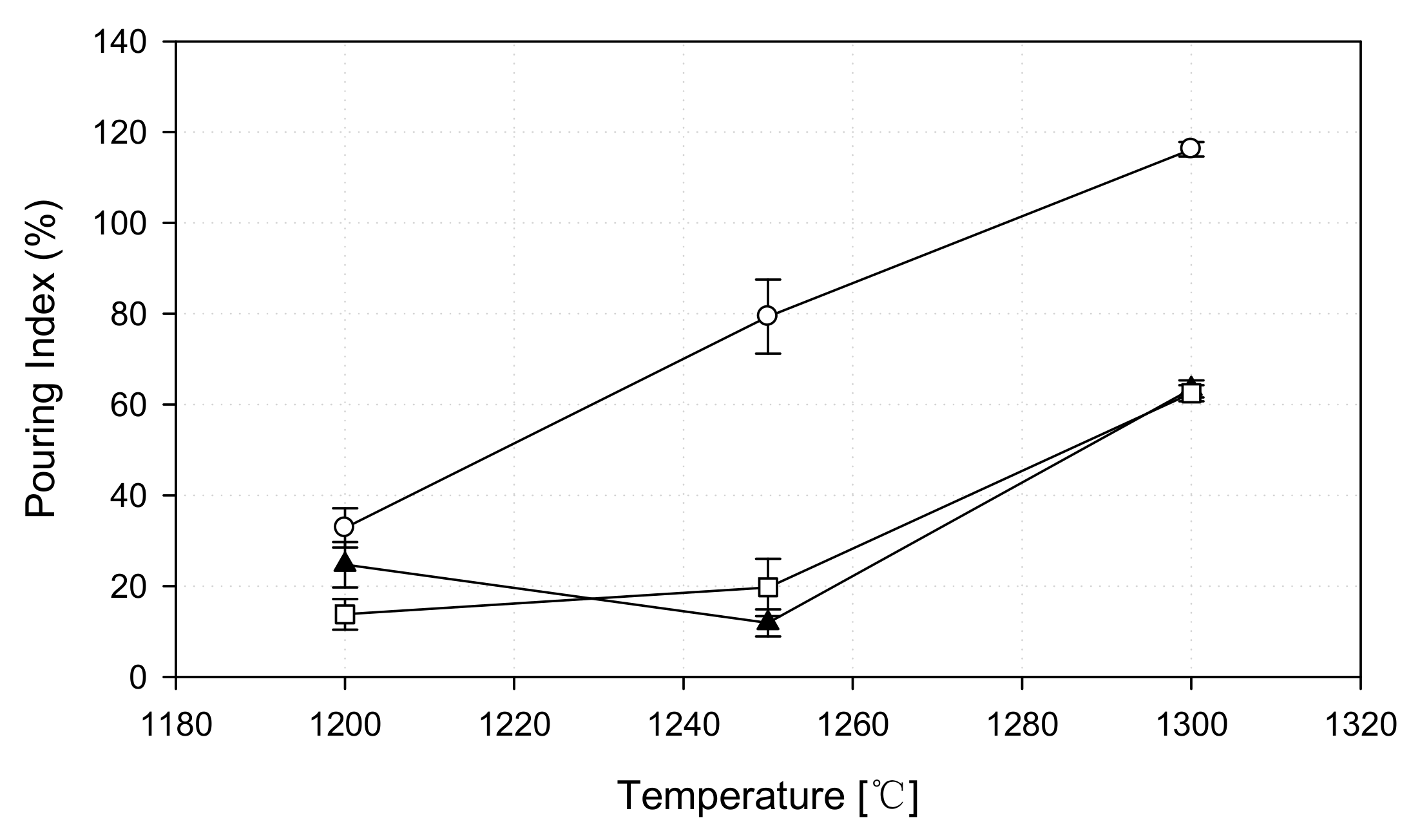
3.4.1. Pouring Index as a Function of Temperature
3.4.2. Pouring Index as a Function of Sawdust Mixing Ratio
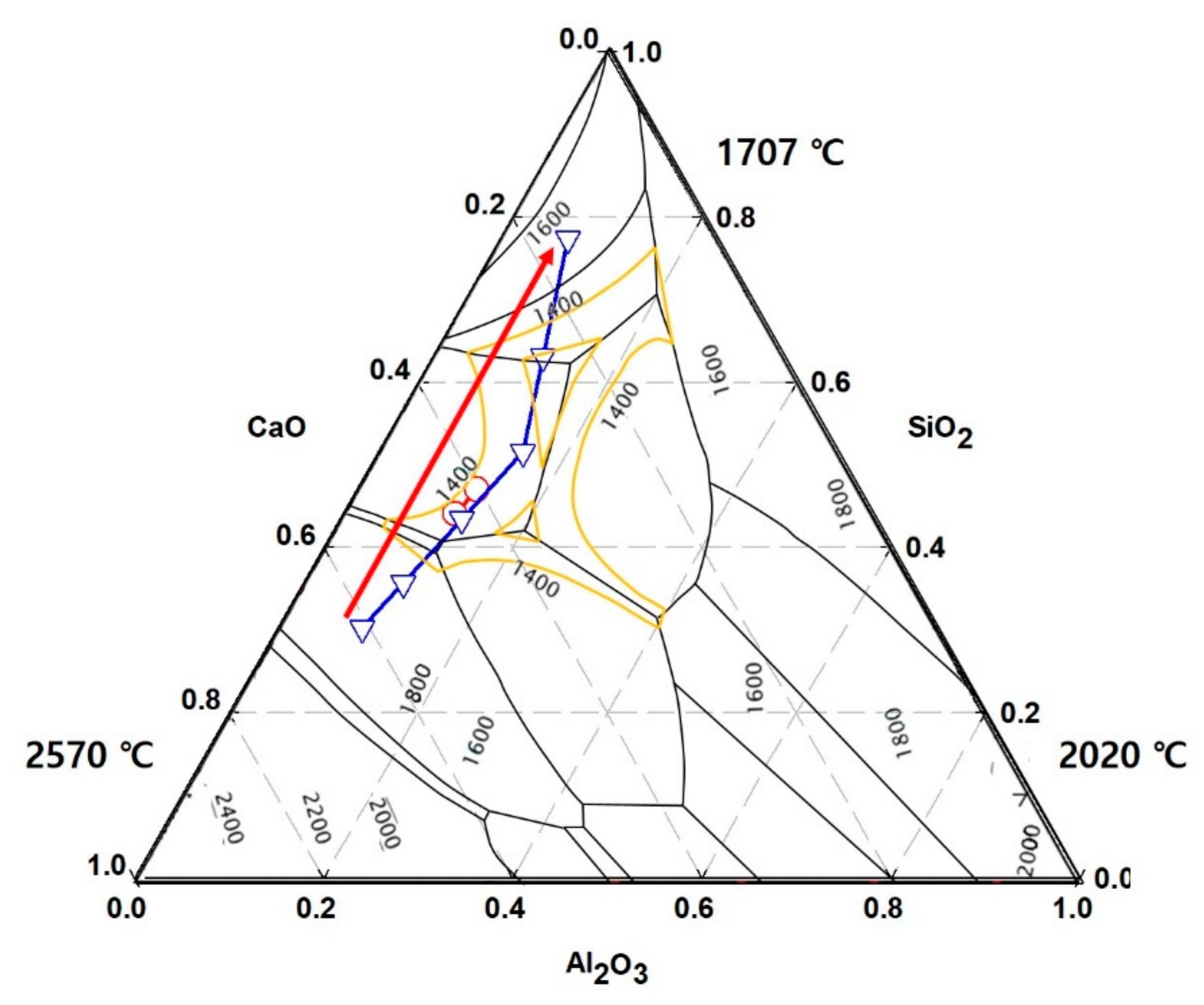
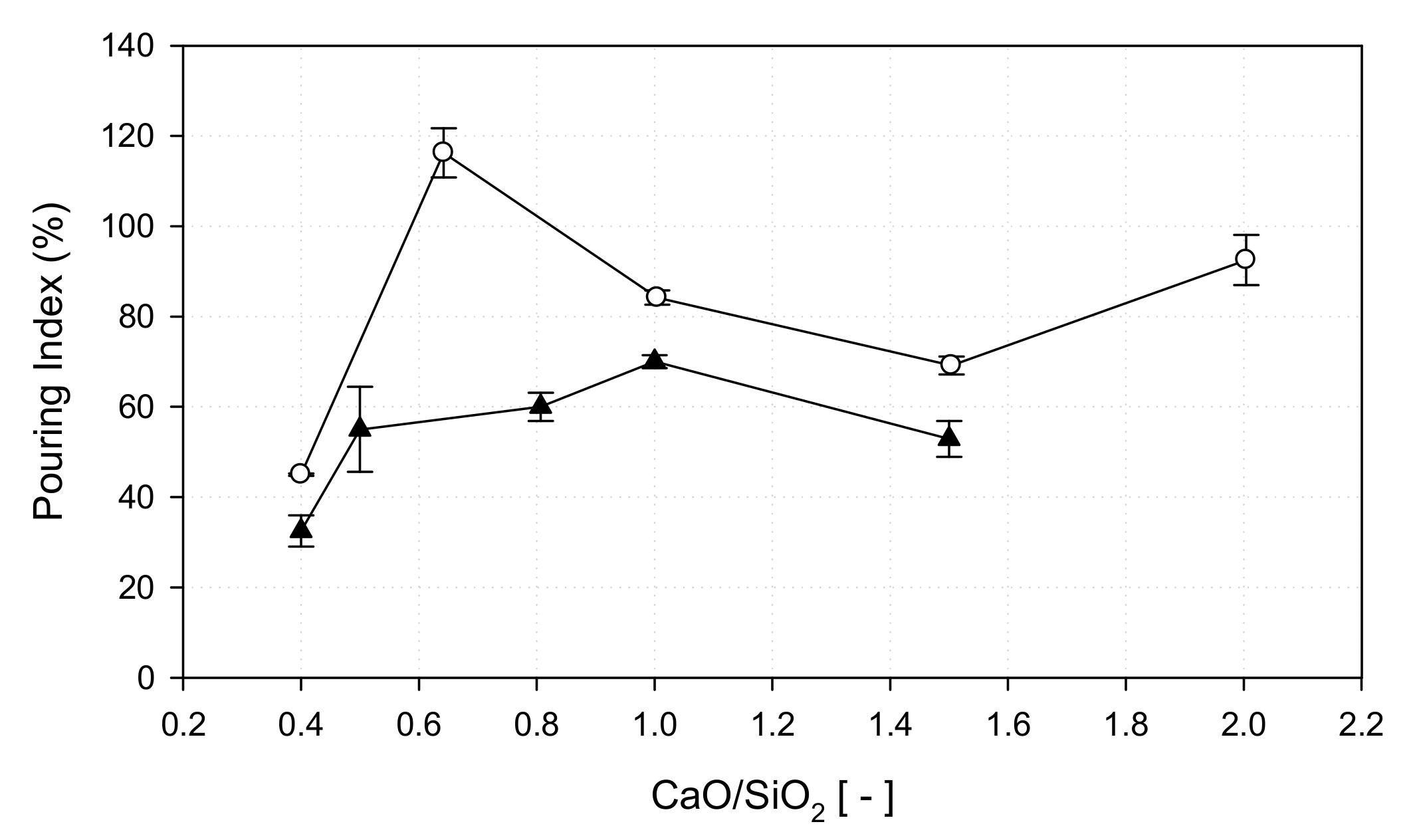
3.4.3. Pouring Index as a Function of Basicity
3.5. Results of the Pilot Plant Test
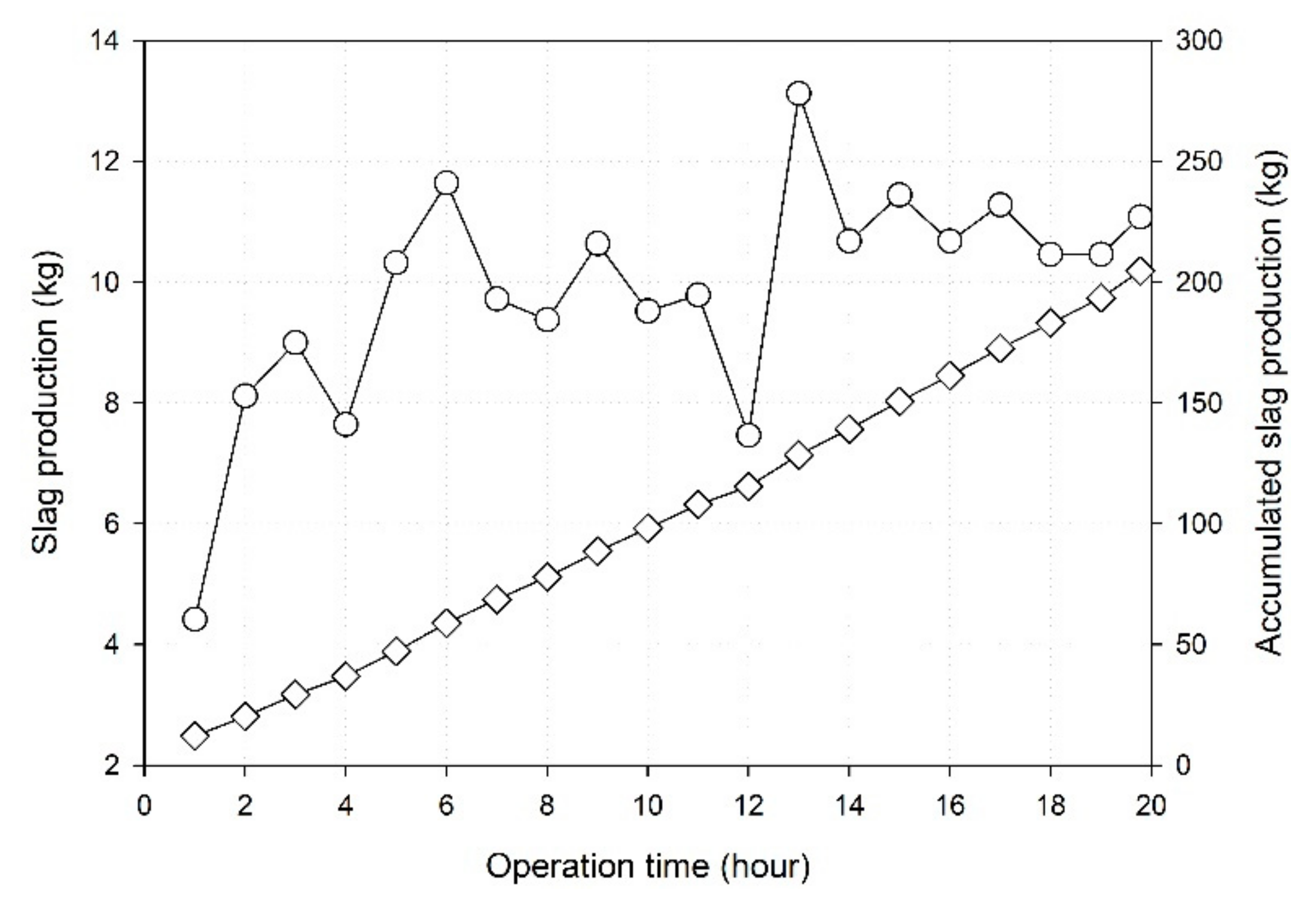
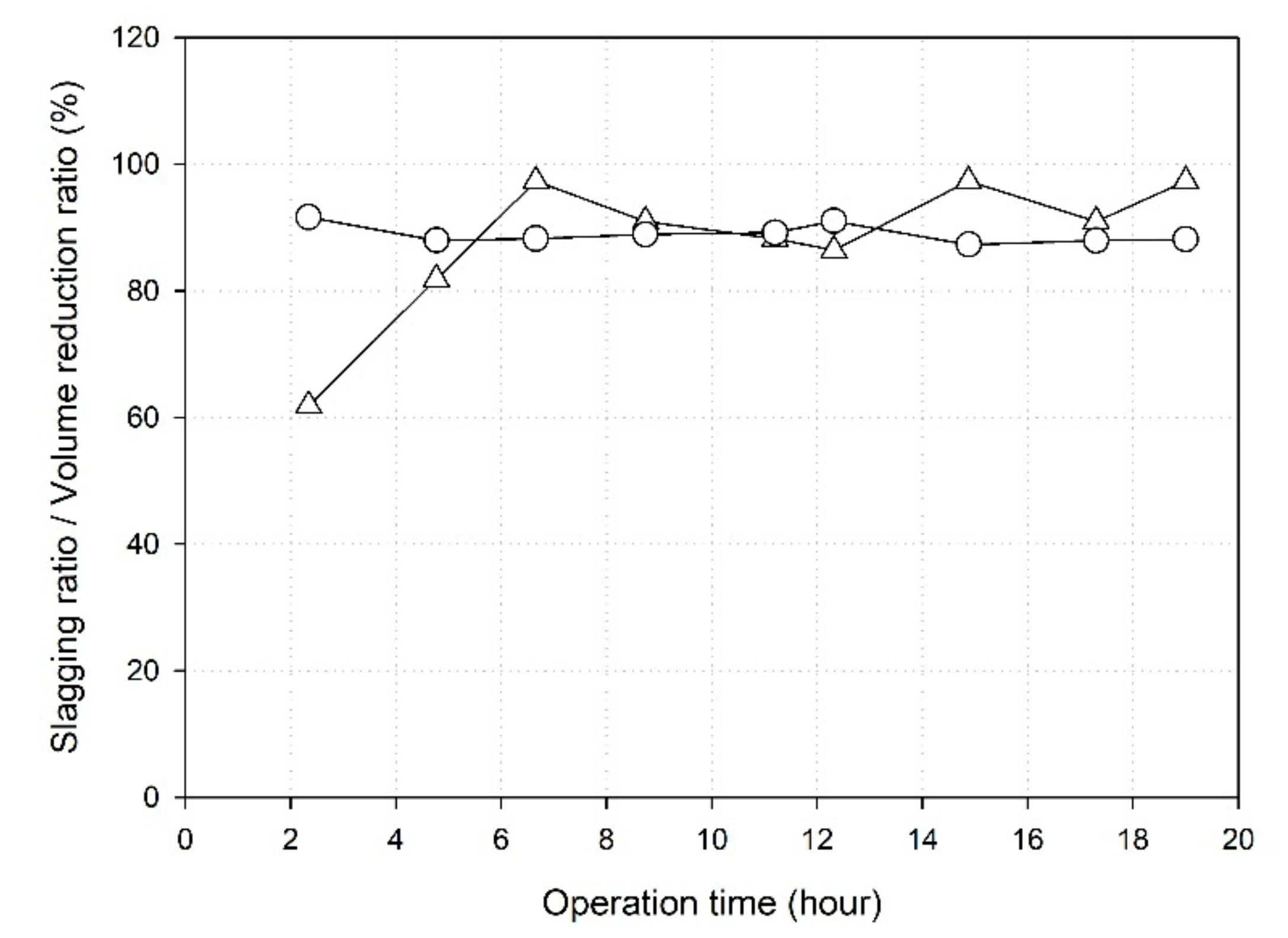
3.5.1. Slag Exhaust Characteristics
3.5.2. Air Pollutants in the Exhaust Gas
3.5.3. Heavy Metal Analysis
4. Conclusions
- The PI of the mixture was lower than that of sewage sludge, and yielded results that were similar to those of sawdust, indicating that the sewage sludge solid fuel in which sawdust was mixed had a negative effect on the PI of ash.
- Basicity degree gradually increased, and the PI decreased as the sawdust mixing ratio increased. In addition, in view of the minimal change in the PI depending on the mixing ratio at 32% or higher, the PI for sewage sludge solid fuel is expected to remain at approximately 60% at a basicity index of 0.8 or higher.
- The increase in fluidity when sawdust is added to adjust the basicity is attributed to the melting temperature being lower than that of a single substance if an inorganic material contained in the ash forms a eutectic compound.
- The slagging rate of the produced slag was 88.03%, and the resulting volume reduction rate was 88.93%, indicating a volume reduction effect on the treatment of sewage sludge in the combustion furnace.
- Analysis of pollutants in exhaust gases generated by the combustion melting pilot plant operation demonstrated that small amounts of CO and SO2 were produced. However, the average NOx concertation was high (853 ppm), indicating that nitrogen was converted to NOx in the oxygen-rich environment. Therefore, to prevent the release of NOx, SNCR and SCR facilities need to be installed in oxygen-enriched combustion melting plants when using sewage sludge solid fuel.
- Analysis of slag containing heavy metals in the eluted solution from sewage sludge solid fuel showed that heavy metals contained in the sewage sludge solid fuel, with the exception of Cu, were retained within the slag. This indicates that heavy metal stability in the environment is ensured in case the sewage sludge solid fuel slag is buried in landfill.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Sewer Information System. Available online: http://www.hasudoinfo.or.kr (accessed on 5 October 2020).
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A Critical review. Prog. Energy Combust. Sci. 2020, 79, 1–32. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.J.; Chang, Y.; Lee, Y.J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A review of sludge-to-energy recovery methods. Energies 2019, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Electric Power Statistics Information System. Available online: http://epsis.kpx.or.kr (accessed on 5 October 2020).
- Korean Statistical Information Service. Available online: http://kosis.kr (accessed on 7 October 2020).
- Wang, Q.; Wei, W.; Gong, Y.; Yu, Q.; Li, Q.; Sun, J.; Yuan, Z. Technologies for reducing sludge production in wastewater treatment plants: State of the art. Sci. Total Environ. 2017, 587–588, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Ishida, T.; Sasabe, K.; Sasaki, K.; Harada, S. Characteristics of melting process for sewage sludge. Water Sci. Technol. 1991, 23, 2019–2028. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Alonso, M.; Folgueras, J.R. Modification of lignite ash fusion temperatures by the addition of different types of sewage sludge. Fuel Process. Technol. 2015, 131, 348–355. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Alonso, M.; Folgueras, J.R. Effects of sludge addition to coal on Na, K and S volatilisation in ashing process and ash fusibility. Fuel Process. Technol. 2015, 138, 714–723. [Google Scholar] [CrossRef]
- Jeong, B.M.; Kim, D.J.; Park, D.K.; Park, Y.S. A study on characteristics analysis of sludge waste by basicity. J. Korea Soc. Waste Manag. 2019, 36, 567–575. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, S.B.; Kim, M.J.; Kim, J.P.; Kim, J.C.; Lee, S.J.; Lee, S.H. Study on the Emission Characteristics of Heavy metals in sewage sludge Incinerator. J. Environ. San. Eng. 2009, 24, 19–27. [Google Scholar]
- Zhao, Z.; Wang, R.; Wu, J.; Yin, Q.; Wang, C. Bottom ash characteristics and pollutant emission during the co-combustion of pulverized coal with high mass-percentage sewage sludge. Energy 2019, 171, 809–818. [Google Scholar] [CrossRef]
- Yu, T.; Yang, W.; Jeon, K.H.; Shin, D.; Hwang, J.H. Combustion and pyrolysis characteristics of solid wastes in a 30 kg/hr capacity pyrolysis melting incinerator. J Korean Soc. Combust. 2006, 32, 172–180. [Google Scholar]
- Kong, L.X.; Jin, B.A.I.; Wen, L.I.; Bai, Z.Q.; Guo, Z.X. Effect of lime addition on slag fluidity of coal ash. J. Fuel Chem. Technol. 2011, 39, 407–411. [Google Scholar] [CrossRef]
- Kim, M.R.; Kim, K.H.; Sung, H.J.; Kang, D.H.; Park, H.S.; Lee, J.K. Relationship between Basicity and Fluidity in Ash Melting Processes. J. Korea Soc. Waste Manag. 2007, 24, 714–723. [Google Scholar]
- Higman, C.; van der Burgt, M. Gasification; Gulf Professional Publishing: Amsterdam, The Netherlands, 2008; p. 456. [Google Scholar]
- Yan, T.; Bai, J.; Kong, L.; Li, H.; Wang, Z.; Bai, Z.; Zhao, H.; Li, W. Improved prediction of critical-viscosity temperature by fusion behavior of coal ash. Fuel 2019, 253, 1521–1530. [Google Scholar] [CrossRef]
- Watanaba, H.; Shiraishi, T. Improvement of melting temperature and viscosity in sewage sludge melting process. Int. Symp. Environ. Tech. Dev. 1992, 15, 393–404. [Google Scholar]
- Park, J.K.; Seo, Y.C. Studies on physicochemical characteristics and optimal melting condition of automobile shredder residue in a melting furnace. Korea Soc. Waste Manag. 2013, 30, 189–198. [Google Scholar] [CrossRef]
- Kim, M.R.; Jang, J.G.; Lee, S.K.; Hwnag, B.Y.; Lee, J.K. Correlation between the ash composition and melting temperature of waste incineration residue. Korean J. Chem. Eng. 2010, 27, 1028–1034. [Google Scholar] [CrossRef]
- Logachev, G.N.; Gostenin, V.A.; Pishnograevm, S.N.; Seleznev, D.I.; Gridasov, V.P. Mobility of blast-furnace slag. Steel Transl. 2013, 43, 4–6. [Google Scholar] [CrossRef]
- Wu, X.; Ji, H.; Dai, B.; Zhang, L. Xinjiang lignite ash slagging and flowability under the weak reducing environment at 1300 °C—A new method to quantify slag flow velocity and its correlation with slag properties. Fuel Process. Technol. 2018, 171, 173–182. [Google Scholar] [CrossRef]
- American Society for Testing Materials International. ASTM D7582-15 Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Ministry of Environment. Act on The Promotion of Saving and Recycling of Resources; Ministry of Environment: Washington, DC, USA, 2014. [Google Scholar]
- United States Environmental Protection Agency. SW-846 Test Method 3052, Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; United States Environmental Protection Agency: Washington, DC, USA, 1996. [Google Scholar]
- Ministry of Environment. Environmental Testing and Inspection Act; Ministry of Environment: Washington, DC, USA, 2017. [Google Scholar]
- United States Environmental Protection Agency. Municipal Solid Waste Combustion Ash Database; United States Environmental Protection Agency: Washington, DC, USA, 1994. [Google Scholar]
- Lee, K.H.; Lim, J.H. Investigation of the mixing melting incineration treatment of sewage sludge and municipal solid waste cinder. J. Appl. Chem. 2009, 13, 285–288. [Google Scholar]
- Park, H.Y.; Im, H.S.; Kim, E.H.; Kim, Y.J.; Kim, K.S.; Lee, J.E. Advanced slagging propensity of coal and its assessment with the conventional indices. J. Energy Eng. 2012, 21, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhai, M.; Wang, Z.; Zhang, Y.; Dong, P. Comprehensive coal quality index for evaluation of coal agglomeration characteristics. Fuel 2018, 231, 379–386. [Google Scholar] [CrossRef]
- Namkung, H.; Kim, H.T.; Wang, F.; Lin, K.; Yu, G. Multilateral approaches for investigation of particle stickiness of coal ash at low temperature fouling conditions. Korean J. Chem. Eng. 2017, 34, 3102–3110. [Google Scholar] [CrossRef]
- Namkung, H.; Kim, C.H.; Kim, D.; Yuan, X.; Kang, T.J.; Kim, H.T. Effect of bed agglomeration by mineral component with different coal type. J. Energy Inst. 2016, 89, 172–181. [Google Scholar] [CrossRef]




| Sawdust Mixing Ratio (%) | Temperature (°C) | Reagents Added | Basicity (-) | |
|---|---|---|---|---|
| Test 1 | 0, 32 *, 100 | 1200, 1250, 1300 | - | - |
| Test 2 | 0, 12.5, 32 *, 80, 90, 100 | 1300 | - | - |
| Test 3 | - | 1300 | SiO2, CaO | 0.4–2.0 |
| Test Condition | Equivalent Ratio(-) | Oxygen Enrichment Ratio (%) | Oxidant Distribution Ratio (%) | |||||
| 1st | 2nd | 3rd | Total | 1st | 2nd | 3rd | ||
| 1.4 | 70 | 70 | 100 | 80 | 80 | 15 | 5 | |
| Properties | Sawdust | Sewage Sludge | Sludge Solid Fuel | |
|---|---|---|---|---|
| Proximate Analysis (Wet Basis, wt.%) | Moisture | 12.98 | 82.55 | 7.48 |
| Combustible | 67.49 | 13.49 | 73.72 | |
| Ash | 19.54 | 3.97 | 18.80 | |
| Proximate Analysis (Dry Basis, wt.%) | Combustible | 77.55 | 77.26 | 79.68 |
| Ash | 22.45 | 22.74 | 20.32 | |
| High Heating Value (HHV; Dry Basis, MJ/kg) | - | 15.67 | 20.49 | 14.94 |
| Ultimate Analysis (Dry Basis, wt.%) | C | 41.48 | 44.69 | 36.95 |
| H | 4.74 | 6.18 | 4.84 | |
| O | 28.71 | 20.07 | 25.61 | |
| N | 2.63 | 5.24 | 5.27 | |
| S | 0.00 | 1.09 | 0.74 | |
| Component | Sawdust Ash (Dry Basis, wt.%) | Sewage Sludge Ash (Dry Basis, wt.%) | Sludge Solid Fuel Ash (Dry Basis, wt.%) |
|---|---|---|---|
| SiO2 | 33.2 | 24.0 | 30.5 |
| Fe2O3 | 12.9 | 20.7 | 15.2 |
| CaO | 28.4 | 15.4 | 24.6 |
| P2O5 | 0.8 | 12.7 | 4.3 |
| Al2O3 | 8.9 | 7.1 | 8.4 |
| SO3 | 4.6 | 6.8 | 5.3 |
| CuO | 0.1 | 3.3 | 1.0 |
| K2O | 4.2 | 2.8 | 3.8 |
| ZnO | 0.3 | 2.1 | 0.8 |
| MgO | 1.6 | 1.1 | 1.5 |
| TiO2 | 2.3 | 1.1 | 1.9 |
| NiO | 0.1 | 0.6 | 0.2 |
| Cr2O3 | 0.2 | 0.5 | 0.3 |
| CeO2 | N/D | 0.4 | 0.2 |
| MnO | 0.5 | 0.3 | 0.5 |
| PbO | 0.1 | 0.3 | 0.2 |
| Na2O | 0.3 | 0.2 | 0.3 |
| SnO2 | N/D | 0.1 | 0.0 |
| BaO | 0.2 | 0.1 | 0.2 |
| SrO | 0.1 | 0.1 | 0.1 |
| WO3 | N/D | 0.1 | 0.0 |
| Cl | 1.0 | 0.1 | 0.7 |
| ZrO2 | 0.0 | 0.1 | 0.0 |
| Co2O3 | N/D | 0.0 | 0.0 |
| MoO3 | N/D | 0.0 | 0.0 |
| Bi2O3 | N/D | 0.0 | N/D |
| Ga2O3 | N/D | 0.0 | N/D |
| Basicity (CaO/SiO2) | 0.86 | 0.64 | 0.81 |
| Expected Melting Temperature (°C) | Ash Fusion Temperature (°C) | |
|---|---|---|
| Sewage Sludge | 1255.6 | 1260 |
| Sawdust | 1239.5 | 1270 |
| Sludge Solid Fuel | 1242.8 | 1290 |
| Temperature (°C) | |||
|---|---|---|---|
| 1200 | 1250 | 1300 | |
| Sewage Sludge | 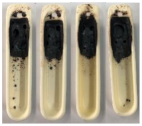 | 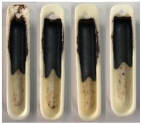 | 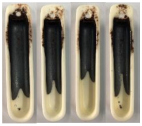 |
| Sawdust |  |  | 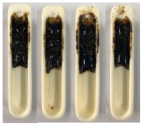 |
| Mixture (68:32) | 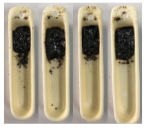 |  | 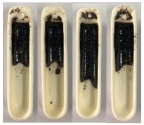 |
| Component | Slag #1 (Dry Basis, wt.%) | Slag #2 (Dry Basis, wt.%) | Slag #3 (Dry Basis, wt.%) | Slag #4 (Dry Basis, wt.%) | Average |
|---|---|---|---|---|---|
| SiO2 | 24.36 | 26.06 | 25.64 | 25.78 | 25.46 |
| Fe2O3 | 24.47 | 17.22 | 19.18 | 19.58 | 20.11 |
| CaO | 19.98 | 22.72 | 23.96 | 23.08 | 22.43 |
| P2O5 | 5.60 | 4.57 | 4.84 | 5.45 | 5.12 |
| Al2O3 | 12.12 | 13.47 | 13.10 | 13.09 | 12.95 |
| SO3 | 0.30 | 0.16 | 0.06 | 0.30 | 0.20 |
| CuO | 0.74 | 0.40 | 0.29 | 0.31 | 0.44 |
| K2O | 1.32 | 1.65 | 1.98 | 2.50 | 1.86 |
| MgO | 2.28 | 2.62 | 2.60 | 2.56 | 2.52 |
| TiO2 | 1.52 | 1.72 | 1.99 | 1.83 | 1.76 |
| NiO | 0.33 | 0.07 | 0.10 | 0.10 | 0.15 |
| Cr2O3 | 3.88 | 6.01 | 2.88 | 2.65 | 3.86 |
| MnO | 0.36 | 0.44 | 0.39 | 0.32 | 0.38 |
| Na2O | 2.24 | 2.04 | 2.04 | 1.96 | 2.07 |
| SrO | 0.21 | 0.25 | 0.25 | 0.18 | 0.22 |
| ZrO2 | 0.29 | 0.60 | 0.72 | 0.30 | 0.48 |
| Basicity (CaO/SiO2) | 0.82 | 0.87 | 0.93 | 0.90 | 0.88 |
| CO2 (%) | CO (ppm) | NOx (ppm) | SO2 (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Max. | Min. | Avg. | Max. | Min. | Avg. | Max. | Min. | Avg. | Max. | Min. | Avg. |
| 62 | 31 | 41 | 60 | 0 | 2 | 1032 | 677 | 853 | 0 | 0 | 0 |
| Sample | Unit | Pb | Cd | Cu | As | Cr | Hg |
|---|---|---|---|---|---|---|---|
| Content Analysis | |||||||
| Sludge Solid Fuel Ash | mg/kg | 711.57 | 28.24 | 6,737.77 | 24.77 | 1137.24 | 0.07 |
| Leaching Analysis | |||||||
| Fly Ash | mg/L | 0.07 | N/D | 0.593 | 0.638 | 0.933 | 0.006 |
| Slag | N/D | N/D | 0.213 | N/D | N/D | N/D | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Park, D.-k.; Lim, Y.-t.; Park, S.-n.; Park, Y.-S.; Kim, K. Combustion Melting Characterisation of Solid Fuel Obtained from Sewage Sludge. Energies 2021, 14, 805. https://doi.org/10.3390/en14040805
Kim D, Park D-k, Lim Y-t, Park S-n, Park Y-S, Kim K. Combustion Melting Characterisation of Solid Fuel Obtained from Sewage Sludge. Energies. 2021; 14(4):805. https://doi.org/10.3390/en14040805
Chicago/Turabian StyleKim, Dongju, Dong-kyoo Park, Yong-taek Lim, Soo-nam Park, Yeong-Su Park, and Kyunghyun Kim. 2021. "Combustion Melting Characterisation of Solid Fuel Obtained from Sewage Sludge" Energies 14, no. 4: 805. https://doi.org/10.3390/en14040805






