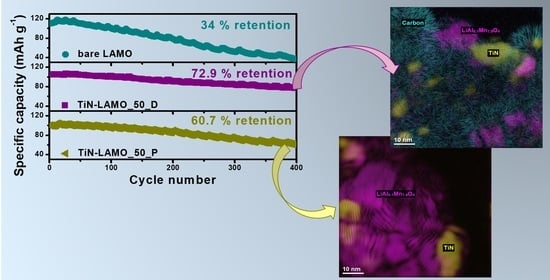
Enhanced Performance of LiAl0.1Mn1.9O4 Cathode for Li-Ion Battery via TiN Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Physicochemical Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Physicochemical Characterization
3.2. Electrochemical Characterization
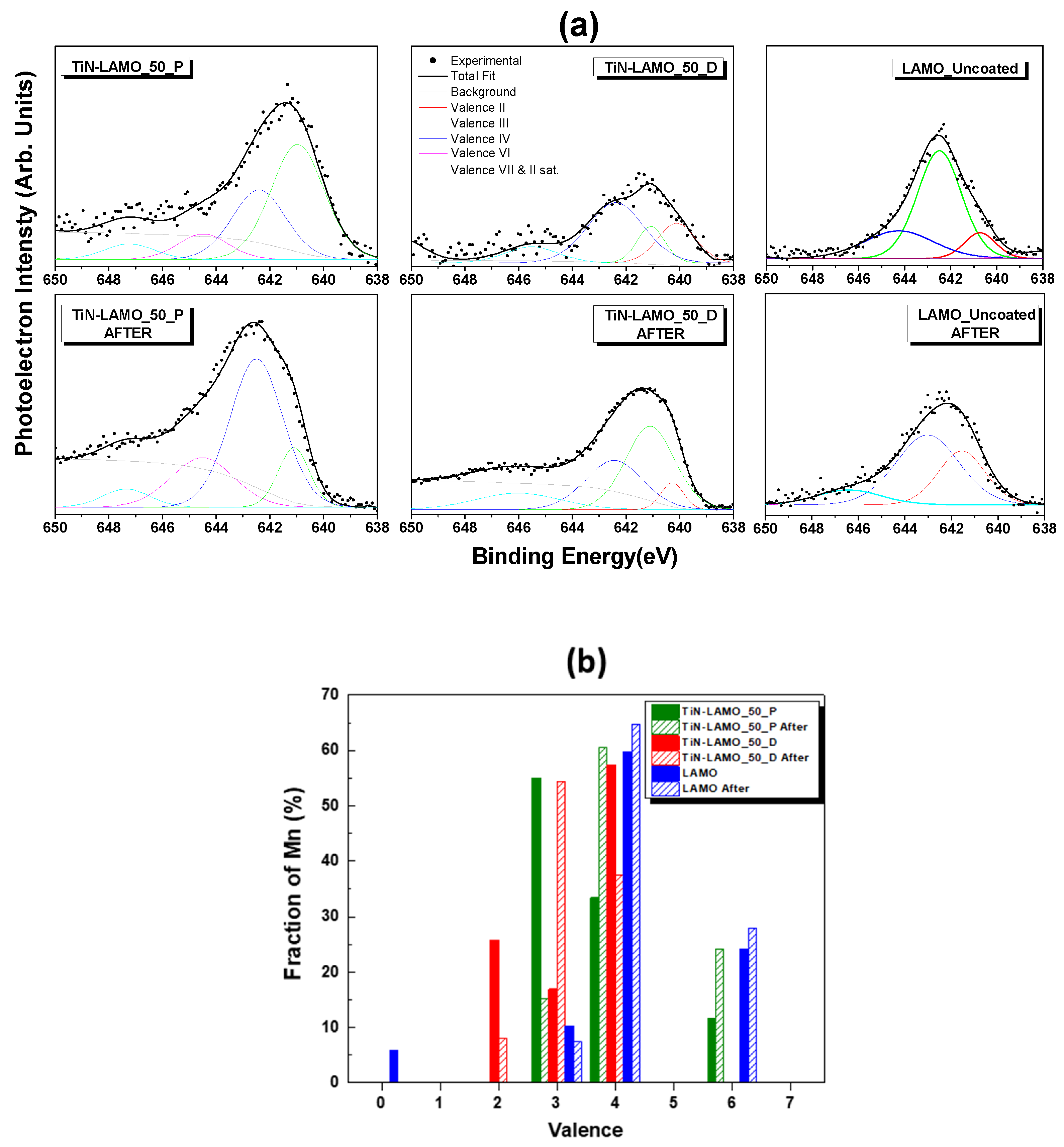
3.3. EDS/XPS Analysis after Cycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Long Gao, X.; Zhang, W.; Chen, J.; Zhou, H.; Zhang, X. Improvement of the high-temperature, high-voltage cycling performance of LiNi0.5Co0.2Mn0.3O2 cathode with TiO2 coating. J. Alloy. Compd. 2012, 543, 181–188. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; El-Hady, D.A.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Jang, D.H.; Shin, Y.J.; Oh, S.M. Dissolution of Spinel Oxides and Capacity Losses in 4 V Li / LixMn2O4 Cells. J. Electrochem. Soc. 1996, 143, 2204–2211. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Developments in Nanostructured Cathode Materials for High-Performance Lithium-Ion Batteries. Adv. Mater. 2008, 20, 2251–2269. [Google Scholar] [CrossRef]
- Yi, T.; Zhu, Y.; Zhu, R.; Zhou, L.; Li, P.; Shu, J. Physicochemical properties of LiAlxMn2 − xO4 and LiAl0.05Mn1.95O4 − yFy cathode material by the citric acid-assisted sol–gel method. Ionics 2009, 15, 177–182. [Google Scholar] [CrossRef]
- Das, S.R.; Majumder, S.B.; Katiyar, R.S. Kinetic analysis of the Li+ ion intercalation behavior of solution derived nano-crystalline lithium manganate thin films. J. Power Sources 2005, 139, 261–268. [Google Scholar] [CrossRef]
- Gummow, R.J.; de Kock, A.; Thackeray, M.M. Improved capacity retention in rechargeable 4 V lithium/lithium-manganese oxide (spinel) cells. Solid State Ion. 1994, 69, 59–67. [Google Scholar] [CrossRef]
- Park, O.K.; Cho, Y.; Yoo, H.C.; Song, H.K.; Cho, J. Who will drive electric vehicles, olivine or spinel? Energy Environ. Sci. 2011, 4, 1621–1633. [Google Scholar] [CrossRef]
- Lucas, P.; Angell, C.A. Synthesis and Diagnostic Electrochemistry of Nanocrystalline Li1+x Mn2− xO4 Powders of Controlled Li Content. J. Electrochem. Soc. 2000, 147, 4459–4463. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Chen, X.; Wu, H.; Zhang, Y. Mg2+ and Ti4+ Co–Doped Spinel LiMn2O4 as Lithium-Ion Battery Cathode. ChemistrySelect 2019, 4, 9583–9589. [Google Scholar] [CrossRef]
- Sakunthala, A.; Reddy, M.V.; Selvasekarapandian, S.; Chowdari, B.V.R.; Selvin, P.C. Synthesis of compounds, Li(MMn11/6)O4 (M = Mn1/6, Co1/6, (Co1/12Cr1/12), (Co1/12Al1/12), (Cr1/12Al1/12)) by polymer precursor method and its electrochemical performance for lithium-ion batteries. Electrochim. Acta 2010, 55, 4441–4450. [Google Scholar] [CrossRef]
- Amaral, F.A.; Bocchi, N.; Brocenschi, R.F.; Biaggio, S.R.; Rocha-Filho, R.C. Structural and electrochemical properties of the doped spinels Li1.05M0.02Mn1.98O3.98N0.02 (M = Ga3+, Al3+, or Co3+; N = S2− or F−) for use as cathode material in lithium batteries. J. Power Sources 2010, 195, 3293–3299. [Google Scholar] [CrossRef]
- Angelopoulou, P.; Paloukis, F.; Słowik, G.; Wójcik, G.; Avgouropoulos, G. Combustion-synthesized LixMn2O4-based spinel nanorods as cathode materials for lithium-ion batteries. Chem. Eng. J. 2017, 311, 191–202. [Google Scholar] [CrossRef]
- Hunter, J.C. Preparation of a new crystal form of manganese dioxide: λ-MnO2. J. Solid State Chem. 1981, 39, 142–147. [Google Scholar] [CrossRef]
- Tsunekawa, H.; Tanimoto, S.; Marubayashi, R.; Fujita, M.; Kifune, K.; Sano, M. Capacity fading of graphite electrodes due to the deposition of manganese ions on them in Li-ion batteries. J. Electrochem. Soc. 2002, 149, A1326–A1331. [Google Scholar] [CrossRef]
- Amine, K.; Liu, J.; Kang, S.; Belharouak, I.; Hyung, Y.; Vissers, D.; Henriksen, G. Improved lithium manganese oxide spinel/graphite Li-ion cells for high-power applications. J. Power Sources 2004, 129, 14–19. [Google Scholar] [CrossRef]
- Benedek, R.; Thackeray, M.M. Reaction energy for LiMn2O4 spinel dissolution in acid. Electrochem. Solid State Lett. 2006, 9, A265–A267. [Google Scholar] [CrossRef]
- Myung, S.T.; Amine, K.; Sun, Y.K. Surface modification of cathode materials from nano-to microscale for rechargeable lithium-ion batteries. J. Mater. Chem. 2010, 20, 7074–7095. [Google Scholar] [CrossRef]
- Kalluri, S.; Yoon, M.; Jo, M.; Liu, H.K.; Dou, S.X.; Cho, J.; Guo, Z. Feasibility of Cathode Surface Coating Technology for High-Energy Lithium-ion and Beyond-Lithium-ion Batteries. Adv. Mater. 2017, 29, 1605807. [Google Scholar] [CrossRef]
- Cho, J.; Kim, T.-J.; Kim, Y.J. Complete blocking of Mn3+ ion dissolution from a LiMn2O4 spinel intercalation compound by Co3O4 coating. Chem. Commun. 2001, 12, 1074–1075. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Pol, V.G.; Gedanken, A.; Aurbach, D. Improving the high-temperature performance of LiMn2O4 spinel electrodes by coating the active mass with MgO via a sonochemical method. Electrochem. Commun. 2003, 5, 940–945. [Google Scholar] [CrossRef]
- Lin, Y.M.; Wu, H.C.; Yen, Y.C.; Guo, Z.Z.; Yang, M.H.; Chen, H.M.; Sheu, H.S.; Wu, N.L. Enhanced high-rate cycling stability of LiMn2O4 cathode by ZrO2 coating for Li-ion battery. J. Electrochem. Soc. 2005, 152, A1526–A1532. [Google Scholar] [CrossRef]
- Lim, S.; Cho, J. PVP-functionalized nanometre scale metal oxide coatings for cathode materials: Successful application to LiMn2O4 spinel nanoparticles. Chem. Commun. 2008, 37, 4472–4474. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Guo, S.; He, X.; Jiang, C.; Wan, C. Investigation of SnO2-modified LiMn2O4 Composite as Cathode Material for Lithium-ion Batteries. Int. J. Electrochem. Sci. 2010, 5, 1113–1126. [Google Scholar]
- Guan, D.; Jeevarajan, J.A.; Wang, Y. Enhanced cycleability of LiMn2O4 cathodes by atomic layer deposition of nanosized-thin Al2O3 coatings. Nanoscale 2011, 3, 1465–1469. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, K.; Yic, C.W. Characteristics and electrochemical performance of the LiMn2O4 with TiO2 surface layer in lithium secondary batteries. J. Ceram. Process. Res. 2015, 16, 232–236. [Google Scholar]
- Park, S.C.; Kim, Y.M.; Han, S.C.; Ahn, S.; Ku, C.H.; Lee, J.Y. The elevated temperature performance of LiMn2O4 coated with LiNi1−XCoXO2 (X = 0.2 and 1). J. Power Sources 2002, 107, 42–47. [Google Scholar] [CrossRef]
- Liu, D.Q.; Liu, X.Q.; He, Z.Z. The elevated temperature performance of LiMn2O4 coated with Li4Ti5O12 for lithium ion battery. Mater. Chem. Phys. 2007, 105, 362–366. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y. Enhanced cycling performance of spinel LiMn2O4 coated with ZnMn2O4 shell. J. Solid State Electrochem. 2008, 12, 851–855. [Google Scholar] [CrossRef]
- Jaber-Ansari, L.; Puntambekar, K.P.; Kim, S.; Aykol, M.; Luo, L.; Wu, J.; Myers, B.D.; Iddir, H.; Russell, J.T.; Saldaña, S.J.; et al. Suppressing Manganese Dissolution from Lithium Manganese Oxide Spinel Cathodes with Single-Layer Graphene. Adv. Energy Mater. 2015, 5, 1500646. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Zhu, D.; Huang, L.W.; Chen, Y.G. Improvement of the overall performances of LiMn2O4 via surface-modification by polypyrrole. Mater. Res. Bull. 2015, 71, 91–97. [Google Scholar] [CrossRef]
- Shi, T.; Dong, Y.; Wang, C.; Tao, F.; Chen, L. Enhanced cycle stability at high rate and excellent high-rate capability of La0.7Sr0.3Mn0.7Co0.3O3-coated LiMn2O4. J. Power Sources 2015, 273, 959–965. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, H.; Zhao, C.; Qian, X. Structure and cycle stability of SrHPO4-coated LiMn2O4 cathode materials for lithium-ion batteries. Electrochim. Acta 2014, 145, 201–208. [Google Scholar] [CrossRef]
- Su, L.; Smith, P.M.; Anand, P.; Jayan, B.R. Surface Engineering of a LiMn2O4 Electrode Using Nanoscale Polymer Thin Films via Chemical Vapor Deposition Polymerization. ACS Appl. Mater. Interfaces 2018, 10, 27063–27073. [Google Scholar] [CrossRef]
- Mattelaer, F.; Vereecken, P.M.; Dendooven, J.; Detavernier, C. The influence of ultrathin amorphous ALD alumina and Titania on the rate capability of anatase TiO2 and LiMn2O4 Lithium ion battery electrodes. Adv. Mater. Interfaces 2017, 4, 1601237. [Google Scholar] [CrossRef]
- Chae, Y.; Lee, J.K.; Choi, W. Surface coating of spinel LiMn2O4 cathode electrode with lithium–nickel–manganese-oxide by RF sputtering method for lithium-ion batteries. J. Electroanal. Chem. 2014, 730, 20–25. [Google Scholar] [CrossRef]
- Tang, D.; Yi, R.; Gordin, M.L.; Melnyk, M.; Dai, F.; Chen, S.; Song, J.; Wang, D. Titanium nitride coating to enhance the performance of silicon nanoparticles as a lithium-ion battery anode. J. Mater. Chem. A 2014, 2, 10375–10378. [Google Scholar] [CrossRef]
- Qiu, Y.; Yan, K.; Yang, S.; Jin, L.; Deng, H.; Li, W. Synthesis of Size-Tunable Anatase TiO2 Nanospindles and Their Assembly into Anatase@Titanium Oxynitride/Titanium Nitride−Graphene Nanocomposites for Rechargeable Lithium Ion Batteries with High Cycling Performance. ACS Nano 2010, 4, 6515–6526. [Google Scholar] [CrossRef]
- Han, H.; Song, T.; Bae, J.Y.; Nazar, L.F.; Kim, H.; Paik, U. Nitridated TiO2 hollow nanofibers as an anode material for high power lithium ion batteries. Energy Environ. Sci. 2011, 4, 4532–4536. [Google Scholar] [CrossRef]
- Gao, Y.; Park, J.; Liang, X. Synergic Titanium Nitride Coating and Titanium Doping by Atomic Layer Deposition for Stable- and High-Performance Li-Ion Battery. J. Electrochem. Soc. 2018, 165, A3871–A3877. [Google Scholar] [CrossRef]
- Li, G.R.; Wang, F.; Jiang, Q.W.; Gao, X.P.; Shen, P.W. Carbon Nanotubes with Titanium Nitride as a Low-Cost Counter-Electrode Material for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2010, 49, 3653–3656. [Google Scholar] [CrossRef]
- Milosev, I.; Strehblow, H.H.; Navinsek, B.; Metikoshukovic, M. Electrochemical and thermal oxidation of TiN coatings studied by XPS. Surf. Interface Anal. 1995, 23, 529–539. [Google Scholar] [CrossRef]
- Avasarala, B.; Haldar, P. Electrochemical oxidation behavior of titanium nitride based electrocatalysts under PEM fuel cell conditions. Electrochim. Acta 2010, 55, 9024–9034. [Google Scholar] [CrossRef]
- Patsalas, P.; Logothetidis, S. Optical, electronic, and transport properties of nanocrystalline titanium nitride thin films. J. Appl. Phys. 2001, 90, 4725–4734. [Google Scholar] [CrossRef]
- Angelopoulou, P.; Avgouropoulos, G. Effect of electrode loading on the electrochemical performance of LiAl0.1Mn1.9O4 cathode for lithium-ion batteries. Mater. Res. Bull. 2019, 119, 110562. [Google Scholar] [CrossRef]
- Patsalas, P.; Charitidis, C.; Logothetidis, S. The effect of substrate temperature and biasing on the mechanical properties and structure of sputtered titanium nitride thin films. Surf. Coat. Technol. 2000, 125, 335–340. [Google Scholar] [CrossRef]
- Patsalas, P.; Logothetidis, S. Interface properties and structural evolution of TiN/Si and TiN/GaN heterostructures. J. Appl. Phys. 2003, 93, 989–998. [Google Scholar] [CrossRef]
- Patsalas, P.; Logothetidis, S. In-situ monitoring of the electronic properties and growth evolution of TiN films. Surf. Coat. Technol. 2004, 180–181, 421–424. [Google Scholar] [CrossRef]
- Patsalas, P.; Gravalidis, C.; Logothetidis, S. Surface kinetics and sub plantation phenomena affecting the texture, morphology, stress, and growth evolution of titanium nitride films. J. Appl. Phys. 2004, 96, 6234–6246. [Google Scholar] [CrossRef]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S. Optical properties and plasmonic performance of titanium nitride. Materials 2015, 8, 3128–3154. [Google Scholar] [CrossRef]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S.; Abadias, G.; Bellas, D.V.; Lekka, C.; Lidorikis, E. Conductive nitrides: Growth principles, optical and electronic properties, and their perspectives in photonics and plasmonics. Mater. Sci. Eng. R Rep. 2018, 123, 1–55. [Google Scholar] [CrossRef]
- Logothetidis, S.; Stergioudis, G.; Patsalas, P. Oxidation and structural changes in fcc TiNx thin films studied with X-ray reflectometry. Surf. Coat. Technol. 1998, 100–101, 295–299. [Google Scholar] [CrossRef]
- Jaeger, D.; Patscheider, J. A complete and self-consistent evaluation of XPS spectra of TiN. J. Elect. Spectrosc. Related Phen. 2012, 185, 523–534. [Google Scholar] [CrossRef]
- Haasch, R.T.; Lee, T.-Y.; Gall, D.; Greene, J.E.; Petrov, I. Epitaxial TiN (001) grown and analyzed in situ by XPS and UPS. II. Analysis of Ar+ sputter etched layers. Surf. Sci. Spec. 2000, 7, 204–212. [Google Scholar] [CrossRef]
- Bender, H.; Chen, W.; Portillo, J.; Van den Hove, L.; Wandervorst, W. AES and XPS analysis of the interaction of Ti with Si and SiO2 during RTA. Appl. Surf. Sci. 1989, 38, 37–47. [Google Scholar] [CrossRef]
- Pliatsikas, N.; Siozios, A.; Kassavetis, S.; Vourlias, G.; Patsalas, P. Optical properties of nanostructured Al-rich Al1 − xTixN films. Surf. Coat. Technol. 2014, 257, 63–69. [Google Scholar] [CrossRef]
- Bertoti, I.; Mohai, M.; Sullivan, J.L.; Saied, S.O. Surface characterisation of plasma-nitrided titanium: An XPS study. Appl. Surf. Sci. 1995, 84, 357–371. [Google Scholar] [CrossRef]
- Wolf, M.; Schultze, J.W.; Strehblow, H.-H. Low-energy implantation and sputtering of TiO2 by nitrogen and argon and the electrochemical reoxidation. Surf. Interf. Anal. 1991, 17, 726–736. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Brivio, C.; Musolino, V.; Alet, P.-J.; Merlo, M.; Hutter, A.; Ballif, C. Application-independent protocol for predicting the efficiency of lithium-ion battery cells in operations. J. Energy Storage 2018, 15, 415–422. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novák, P. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Yi, T.F.; Xie, Y.; Wu, Q.J.; Liu, H.P.; Jiang, L.J.; Ye, M.F.; Zhu, R.S. High rate cycling performance of lanthanum-modified Li4Ti5O12 anode materials for lithium-ion batteries. J. Power Sources 2012, 214, 220–226. [Google Scholar] [CrossRef]
- Militello, M.C.; Gaarenstroom, S.W. Lithium Manganese Oxide (LiMn2O4) by XPS. Surf. Sci. Spec. 2001, 8, 207–213. [Google Scholar] [CrossRef]
- Militello, M.C.; Gaarenstroom, S.W. Manganese Dioxide (MnO2) by XPS. Surf. Sci. Spec. 2001, 8, 200–206. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulou, P.; Kassavetis, S.; Papavasiliou, J.; Karfaridis, D.; Słowik, G.; Patsalas, P.; Avgouropoulos, G. Enhanced Performance of LiAl0.1Mn1.9O4 Cathode for Li-Ion Battery via TiN Coating. Energies 2021, 14, 825. https://doi.org/10.3390/en14040825
Angelopoulou P, Kassavetis S, Papavasiliou J, Karfaridis D, Słowik G, Patsalas P, Avgouropoulos G. Enhanced Performance of LiAl0.1Mn1.9O4 Cathode for Li-Ion Battery via TiN Coating. Energies. 2021; 14(4):825. https://doi.org/10.3390/en14040825
Chicago/Turabian StyleAngelopoulou, Pinelopi, Spyros Kassavetis, Joan Papavasiliou, Dimitris Karfaridis, Grzegorz Słowik, Panos Patsalas, and George Avgouropoulos. 2021. "Enhanced Performance of LiAl0.1Mn1.9O4 Cathode for Li-Ion Battery via TiN Coating" Energies 14, no. 4: 825. https://doi.org/10.3390/en14040825
APA StyleAngelopoulou, P., Kassavetis, S., Papavasiliou, J., Karfaridis, D., Słowik, G., Patsalas, P., & Avgouropoulos, G. (2021). Enhanced Performance of LiAl0.1Mn1.9O4 Cathode for Li-Ion Battery via TiN Coating. Energies, 14(4), 825. https://doi.org/10.3390/en14040825