Cobalt Nanoparticle-Embedded Nitrogen-Doped Carbon Catalyst Derived from a Solid-State Metal-Organic Framework Complex for OER and HER Electrocatalysis
Abstract
1. Introduction
2. Materials and Methods
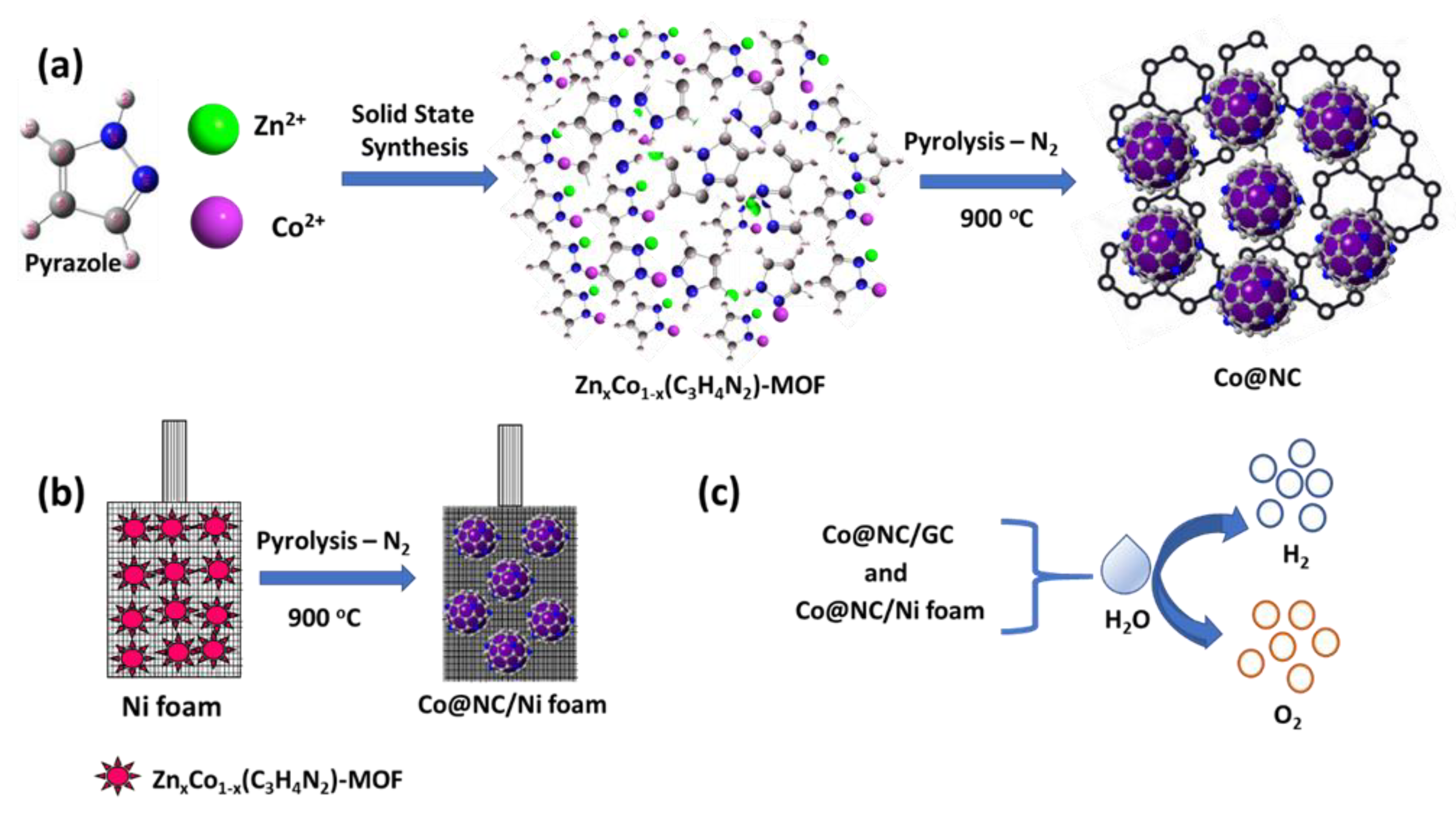
2.1. Synthesis of the Co@NC Catalysts
2.2. Physical and Electrochemical Characterizations
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Li, Y.; Xia, M.; Li, Z.; Chen, Z.; Ma, Z.; Qina, X.; Shao, G. Ni nanoparticles supported on graphene layers: An excellent 3D electrode for hydrogen evolution reaction in alkaline solution. J. Power Sources 2017, 347, 220–228. [Google Scholar] [CrossRef]
- Tian, B.; Zhen, W.; Gao, H.; Zhang, X.; Li, Z.; Lu, G. Carboxyl-assisted synthesis of Co nanorods with high energy facet on graphene oxide sheets for efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2017, 203, 789–797. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yin, X.; Wang, Y.; Lu, L.; Song, A.; Xia, M.; Li, Z.; Qin, X.; Shao, G. Comparison of three nickel-based carbon composite catalysts for hydrogen evolution reaction in alkaline solution. Int. J. Hyd. Energy 2017, 42, 22655–22662. [Google Scholar] [CrossRef]
- Reddy, S.; Song, L.; Kang, L.; Feng, Q.; Du, R.; Zhang, J.; He, L.; Seeram, R. Preparation of Mo2C–carbon nanomaterials for hydrogen evolution reaction. Carbon Lett. 2019, 29, 225–232. [Google Scholar] [CrossRef]
- Zhao, L.; Mingming, L.; Mingwei, C.; Xiaopeng, Q.; Juan, L.; Chao, L.; Peera, S.G.; Tongxiang, L. Exploring the oxygen electrode bi-functional activity of Ni–N–C-doped graphene systems with N, C co-ordination and OH ligand effects. J. Mater. Chem. A 2020, 8, 20453–20462. [Google Scholar]
- Haihong, Z.; Carlos, A.C.R.; Yuan, Z.; Shuwei, Z.; Yongjun, F.; Nicolas, A.V. Recent Advances of Cobalt-Based Electrocatalysts for Oxygen Electrode Reactions and Hydrogen Evolution Reaction. Catalysts 2018, 8, 559. [Google Scholar]
- Jamesh, M.I.; Sun, X. Recent progress on earth abundant electrocatalysts for oxygen evolution reaction (OER) in alkaline medium to achieve efficient water splitting–a review. J. Power Sources 2018, 400, 31–68. [Google Scholar] [CrossRef]
- Ziliang, C.; Huilin, Q.; Kun, Z.; Dalin, S.; Renbing, W. Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Prog. Mat. Sci. 2020, 108, 100618. [Google Scholar]
- Ya, Y.; Bao, Y.X.; Bin, Z.; Xin, W.A. review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar]
- Hele, G.; Qichun, F.; Jixin, Z.; Jingsan, X.; Qianqian, L.; Siliang, L.; Kaiwen, X.; Chao, Z.; Tianxi, L. Cobalt nanoparticle-embedded nitrogen-doped carbon/carbon nanotube frameworks derived from a metal–organic framework for tri-functional ORR, OER and HER electrocatalysis. J. Mater. Chem. A 2019, 7, 3664–3672. [Google Scholar]
- Hao-Fan, W.; Liyu, C.; Huan, P.; Stefan, K.; Qiang, X. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem Soc. Rev. 2020, 49, 1414–1448. [Google Scholar]
- Shaik, G.P.; Kwon, H.J.; Lee, T.G. Highly efficient Co@NCS nanosheet electrocatalyst for oxygen reduction reaction: An environment-friendly, low-cost and sustainable electrocatalyst. Mater. Res. Bull. 2020, 128, 110873. [Google Scholar] [CrossRef]
- Remya, V.R.; Kurian, M. Synthesis and catalytic applications of metal–organic frameworks: A review on recent literature. Int. Nano Lett. 2019, 9, 17–29. [Google Scholar] [CrossRef]
- Available online: www.chemistryworld.com/news/enhancing-solvents-sustainability/3010810.article (accessed on 7 August 2019).
- Peera, S.G.; Jayaraman, B.; Nam, H.K.; Joong, H.L. Sustainable Synthesis of Co@NC Core Shell Nanostructures from Metal Organic Frameworks via Mechanochemical Coordination Self-Assembly: An Efficient Electrocatalyst for Oxygen Reduction Reaction. Small 2018, 14, 1800441–1800456. [Google Scholar] [CrossRef]
- Bo, Y.; Nan, J.; Meili, S.; Walter, S.D.; Junko, Y.; Yujie, S. Bimetal–Organic Framework Self-Adjusted Synthesis of Support-Free Nonprecious Electrocatalysts for Efficient Oxygen Reduction. ACS Catal. 2015, 5, 7068–7076. [Google Scholar]
- Peera, S.G.; Kwon, H.J.; Lee, T.G.; Hussain, A.M. Heteroatom- and metalloid-doped carbon catalysts for oxygen reduction reaction: A mini-review. Ionics 2020, 26, 1563–1589. [Google Scholar] [CrossRef]
- Yunhe, S.; Hongliang, J.; Yihua, Z.; Xiaoling, Y.; Jianhua, S.; Wenjian, Z.; Jianding, C.; Chunzhong, L. Enriched graphitic N-doped carbon-supported Fe3O4 nanoparticles as efficient electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 7281–7287. [Google Scholar]
- Wenxiu, Y.; Xiangjian, L.; Xiaoyu, Y.; Jianbo, J.; Shaojun, G. Bamboo-like Carbon Nanotube/Fe3C Nanoparticle Hybrids and Their Highly Efficient Catalysis for Oxygen Reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar]
- Yuke, S.; Wenfu, X.; Shijin, L.; Jian, G.; Mingfei, S. Hierarchical Hollow Co/N-C@NiCo2O4 Microsphere as an Efficient Bi-functional Electrocatalyst for Rechargeable Zn–Air Battery. Front. Mater. 2019, 6, 261. [Google Scholar]
- Peng, Y.; Lei, W.; Fanfei, S.; Ying, X.; Xu, L.; Jingyuan, M.; Xiuwen, W.; Chungui, T.; Jinghong, L.; Honggang, F. Co Nanoislands Rooted on Co–N–C Nanosheets as Efficient Oxygen Electrocatalyst for Zn–Air Batteries. Adv. Mater. 2019, 31, 1901666–1901675. [Google Scholar]
- Xiaojing, Z.; Jiale, D.; Ligui, L.; Zexing, W.; Shaowei, C. N, S co-doped hierarchical porous carbon spheres embedded with cobalt nanoparticles as efficient bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. Nanoscale 2019, 11, 21302–21310. [Google Scholar]
- Lu, J.; Yin, S.; Shen, P.K. Carbon-Encapsulated Electrocatalysts for the Hydrogen Evolution Reaction. Electrochem. Energ. Rev. 2019, 2, 105–127. [Google Scholar] [CrossRef]
- Bangan, L.; Dianxue, C.; Pan, W.; Guiling, W.; Yinyi, G. Oxygen evolution reaction on Ni-substituted Co3O4 nanowire array electrodes. Int. J. Hyd. Energy 2011, 36, 72–78. [Google Scholar]
- Gang, W.; Ning, L.; De-Rui, Z.; Kurachi, M.; Bo-Qing, X. Anodically electrodeposited CoNi mixed oxide electrode: Preparation and electrocatalytic activity for oxygen evolution in alkaline media. J. Solid State Chem. 2004, 177, 3682–3692. [Google Scholar]
- Rajeshkhanna, G.; Umeshbabu, E.; Rao, G.R. In situ grown nano-architectures of Co3O4 on Ni-foam for charge storage aplication. J. Chem. Sci. 2017, 129, 157–166. [Google Scholar] [CrossRef]
- Yang, H.; Jens, O.J.; Wei, Z.; Lars, N.C.; Wei, X.; Niels, J.B.; Qingfeng, L. Hollow Spheres of Iron Carbide Nanoparticles Encased in Graphite Layers as Oxygen Reduction Catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar]
- Jiao, D.; Liang, Y.; Dehui, D.; Xiaoqi, C.; Fan, Y.; Xinhe, B. Highly active reduction of oxygen on a FeCo alloy catalyst encapsulated in pod-like carbon nanotubes with fewer walls. J. Mater. Chem. A 2013, 1, 14868–14873. [Google Scholar]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed]
- Xiaoxin, Z.; Xiaoxi, H.; Anandarup, G.; Rafael, S.; Bhaskar, R.S.; Eliška, M.; Tewodros, A. Cobalt-embedded nitrogen-rich carbon nanotubes efficiently catalyze hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed. 2014, 53, 4372–4376. [Google Scholar]
- Bo, Y.; Nan, J.; Meili, S.; Sheraz, G.; Junko, Y.; Yujie, S. High-Performance Overall Water Splitting Electrocatalysts Derived from Cobalt-Based Metal–Organic Frameworks. Chem. Mater. 2015, 27, 7636–7642. [Google Scholar]
- Nannan, Y.; Qianqian, J.; Jie, L.; Jianguo, T. A review on non-noble metal based electrocatalysis for the oxygen evolution reaction. Arab. J. Chem. 2020, 13, 4294–4309. [Google Scholar]
- Yaxiao, G.; Zhaoyang, Y.; Changshuai, S.; Erkang, W. Amorphous Co2B Grown on CoSe2 Nanosheets as a Hybrid Catalyst for Efficient Overall Water Splitting in Alkaline Medium. ACS Appl. Mater. Interfaces 2017, 9, 39312–39317. [Google Scholar]
- Hongxiu, Z.; Lecheng, L.; Xingwang, Z. One-Step Synthesis of Cubic Pyrite-Type CoSe2 at Low Temperature for Efficient Hydrogen Evolution Reaction. RSC Adv. 2014, 4, 54344–54348. [Google Scholar]
- Yue, H.H.; Yu, B.; Qi, F.; Zhou, J.H.; Wang, X.Q.; Zheng, B.J.; Zhang, W.L.; Li, Y.R.; Chen, Y.F. Interwoven CoSe2/CNTs Hybrid as a Highly Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2017, 253, 200–207. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, G.D.; Kim, J.H.; Park, S.K.; Kang, Y.C. Rational Design and Synthesis of Extremely Efficient Macroporous CoSe2-CNT Composite Microspheres for Hydrogen Evolution Reaction. Small 2017, 13, 1700068. [Google Scholar] [CrossRef]
- Dai, C.; Tian, X.K.; Nie, Y.L.; Tian, C.; Yang, C.; Zhou, Z.X.; Li, Y.; Gao, X.Y. Successful Synthesis of 3D CoSe2 Hollow Microspheres with High Surface Roughness and its Excellent Performance in Catalytic Hydrogen Evolution Reaction. Chem. Eng. J. 2017, 321, 105–112. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Li, G.D.; Wu, Y.Y.; Gao, R.Q.; Zou, X.X. Vertically Grown CoS Nanosheets on Carbon Cloth as Efficient Hydrogen Evolution Electrocatalysts. Int. J. Hydrogen Energy 2017, 42, 9914–9921. [Google Scholar] [CrossRef]
- Zhang, H.B.; Ma, Z.J.; Duan, J.; Huimin, L.; Guigao, L.; Tao, W.; Kun, C.; Mu, L.; Li, S.; Xianguang, M.; et al. Active sites implanted carbon cages in core shell architecture: Highly active and durable electrocatalyst for hydrogen evolution reaction. ACS Nano 2016, 10, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Xiao, X.X.; Lv, T.T.; Xiaomeng, L.; Botao, L.; Wei, W.; Liu, J. Cobalt encapsulated N-doped defect-rich carbon nanotube as pH universal hydrogen evolution electrocatalyst. Appl. Surf. Sci. 2018, 446, 10–17. [Google Scholar] [CrossRef]
- Fei, H.; Yang, Y.; Peng, Z.W.; Gedeng, R.; Qifeng, Z.; Lei, L.; Errol, L.G.S.; James, M.T. Cobalt nanoparticles embedded in nitrogen-doped carbon for the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2015, 7, 8083–8087. [Google Scholar] [CrossRef]
- Feng, X.G.; Bo, X.J.; Guo, L.P. CoM(M = Fe, Cu, Ni)-embedded nitrogen-enriched porous carbon framework for efficient oxygen and hydrogen evolution reactions. J. Power Sources 2018, 389, 249–259. [Google Scholar] [CrossRef]
- Manikandan, A.; Lee, L.; Wang, Y.C.; Chen, C.W.; Chen, Y.Z.; Henry, M.; Tseng, J.Y.; Zhiming, M.W.; Chueh, Y.L. Graphene-coated copper nanowire networks as a highly stable transparent electrode in harsh environments toward efficient electrocatalytic hydrogen evolution reactions. J. Mater. Chem. A 2017, 5, 13320–13328. [Google Scholar] [CrossRef]
- Noh, S.H.; Seo, M.H.; Kang, J.; Takeyoshi, O.; Byungchan, H.; Takeo, O. Towards a comprehensive understanding of FeCo coated with N-doped carbon as a stable bi-functional catalyst in acidic media. NPG Asia Mater. 2016, 8, e312. [Google Scholar] [CrossRef]
- Ai, L.H.; Tian, T.; Jiang, J. Ultrathin graphene layers encapsulating nickel nanoparticles derived metal-organic frameworks for highly efficient electrocatalytic hydrogen and oxygen evolution reactions. ACS Sustain. Chem. Eng. 2017, 5, 4771–4777. [Google Scholar] [CrossRef]
- Yanyan, L.; Hongliang, J.; Yihua, Z.; Xiaoling, Y.; Chunzhong, L. Transition metals (Fe, Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions. J. Mater. Chem. A 2016, 4, 1694–1701. [Google Scholar]
- Dong, U.L.; Bae, J.K.; Zhongwei, C. One-pot synthesis of a mesoporous NiCo2O4 nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J. Mater. Chem. A 2013, 1, 4754–4762. [Google Scholar]
- Santosh, K.B.; Pagona, P. CuCo2O4 nanoparticles on nitrogenated graphene as highly efficient oxygen evolution catalyst. J. Power Sources 2015, 281, 243–251. [Google Scholar]
- Mian, L.; Yueping, X.; Xiaotian, L.; Xiangjie, B.; Yufan, Z.; Ce, H.; Liping, G. Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale 2015, 7, 8920–8930. [Google Scholar]
- Kun, X.; Pengzuo, C.; Xiuling, L.; Yun, T.; Hui, D.; Xiaojun, W.; Wangsheng, C.; Zhenmeng, P.; Changzheng, W.; Yi, X. Metallic Nickel Nitride Nanosheets Realizing Enhanced Electrochemical Water Oxidation. J. Am. Chem. Soc. 2015, 137, 4119–4125. [Google Scholar]
- Swierk, J.R.; Klaus, S.; Trotochaud, L.; Bell, A.T.; Tilley, T.D. Electrochemical Study of the Energetics of the Oxygen Evolution Reaction at Nickel Iron (Oxy)Hydroxide Catalysts. J. Phys. Chem. C 2015, 119, 19022–19029. [Google Scholar] [CrossRef]
- Li, F.; Bu, Y.; Lv, Z.; Mahmood, J.; Han, G.-F.; Ahmad, I.; Kim, G.; Zhong, Q.; Baek, J.-B. Porous Cobalt Phosphide Polyhedrons with Iron Doping as an Efficient Bifunctional Electrocatalyst. Small 2017, 13, 1701167. [Google Scholar] [CrossRef] [PubMed]





| Catalyst | Electrolyte | Overpotential @10 mA∙cm−2 (mV) | Ref |
|---|---|---|---|
| Hydrogen evolution reaction | |||
| Co2B/CoSe2 | 1 M KOH | 300 | [33] |
| Cubic CoSe2/GD | 0.5 M H2SO4 | 200 | [34] |
| Interwoven CoSe2/CNT | 0.5 M H2SO4 | 186 | [35] |
| CoSe2/CNT | 0.5 M H2SO4 | 174 | [36] |
| CoSe2 hollow microsphere/rGo | 0.5 M H2SO4 | 250 | [37] |
| CoS/CC | 1 M KOH | 197 | [38] |
| Co9S8/NC@MoS2 | 0.5 M H2SO4 | 217 | [38] |
| Co@BCN | 1.M KOH | 183 | [39] |
| Co-NCNTs-10 | 1.M KOH | 204 | [40] |
| N-Co@G | 0.1 M NaOH | 337 | [41] |
| Co0.75Fe0.25-NC | 1.0 M KOH | 202 | [42] |
| G-Coated Cu NWs | 0.5 M H2SO4 | 252 | [43] |
| FeCo@N-C | 0.5 M H2SO4 | 230 | [44] |
| Ni@graphene | 1.0 M KOH | 240 | [45] |
| Co@NC/GC | 1.0 M KOH | 243 @ 10 mA cm−2 | This work |
| Co@NC/Ni | 1.0 M KOH | 170 @ 10 mA cm−2 | This work |
| Oxygen Evolution Reaction | |||
| Fe/N-CNTs | 1.0 M KOH | 520 | [46] |
| Ni/N-CNTs | 1.0 M KOH | 590 | [46] |
| NiCo2O4 | 1.0 M KOH | 490 | [47] |
| CuCo2O4/NrGO | 1.0 M PBS | 1150 | [48] |
| MnFe2O4 | 1.0 M KOH | 470 | [49] |
| Ni Fe2O4 | 1.0 M KOH | 440 | [49] |
| Ni3N bulk | 1.0 M KOH | 490 | [50] |
| FeOOH | 1.0 M KOH | 533 | [51] |
| IrO2 | 1.0 M KOH | 587 | [52] |
| Co@NC/GC | 1.0 M KOH | 450 @ 10 mA cm−2 | This work |
| Co@NC/Ni | 1.0 M KOH | 452 @ 50 mA cm−2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peera, S.G.; Koutavarapu, R.; Liu, C.; Rajeshkhanna, G.; Asokan, A.; Reddy, C.V. Cobalt Nanoparticle-Embedded Nitrogen-Doped Carbon Catalyst Derived from a Solid-State Metal-Organic Framework Complex for OER and HER Electrocatalysis. Energies 2021, 14, 1320. https://doi.org/10.3390/en14051320
Peera SG, Koutavarapu R, Liu C, Rajeshkhanna G, Asokan A, Reddy CV. Cobalt Nanoparticle-Embedded Nitrogen-Doped Carbon Catalyst Derived from a Solid-State Metal-Organic Framework Complex for OER and HER Electrocatalysis. Energies. 2021; 14(5):1320. https://doi.org/10.3390/en14051320
Chicago/Turabian StylePeera, Shaik Gouse, Ravindranadh Koutavarapu, Chao Liu, Gaddam Rajeshkhanna, Arunchander Asokan, and Ch. Venkata Reddy. 2021. "Cobalt Nanoparticle-Embedded Nitrogen-Doped Carbon Catalyst Derived from a Solid-State Metal-Organic Framework Complex for OER and HER Electrocatalysis" Energies 14, no. 5: 1320. https://doi.org/10.3390/en14051320
APA StylePeera, S. G., Koutavarapu, R., Liu, C., Rajeshkhanna, G., Asokan, A., & Reddy, C. V. (2021). Cobalt Nanoparticle-Embedded Nitrogen-Doped Carbon Catalyst Derived from a Solid-State Metal-Organic Framework Complex for OER and HER Electrocatalysis. Energies, 14(5), 1320. https://doi.org/10.3390/en14051320








