Sodium Tungsten Oxide Bronze Nanowires Bundles in Adsorption of Methylene Blue Dye under UV and Visible Light Exposure
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.4. Adsorption Performance
3. Results and Discussion
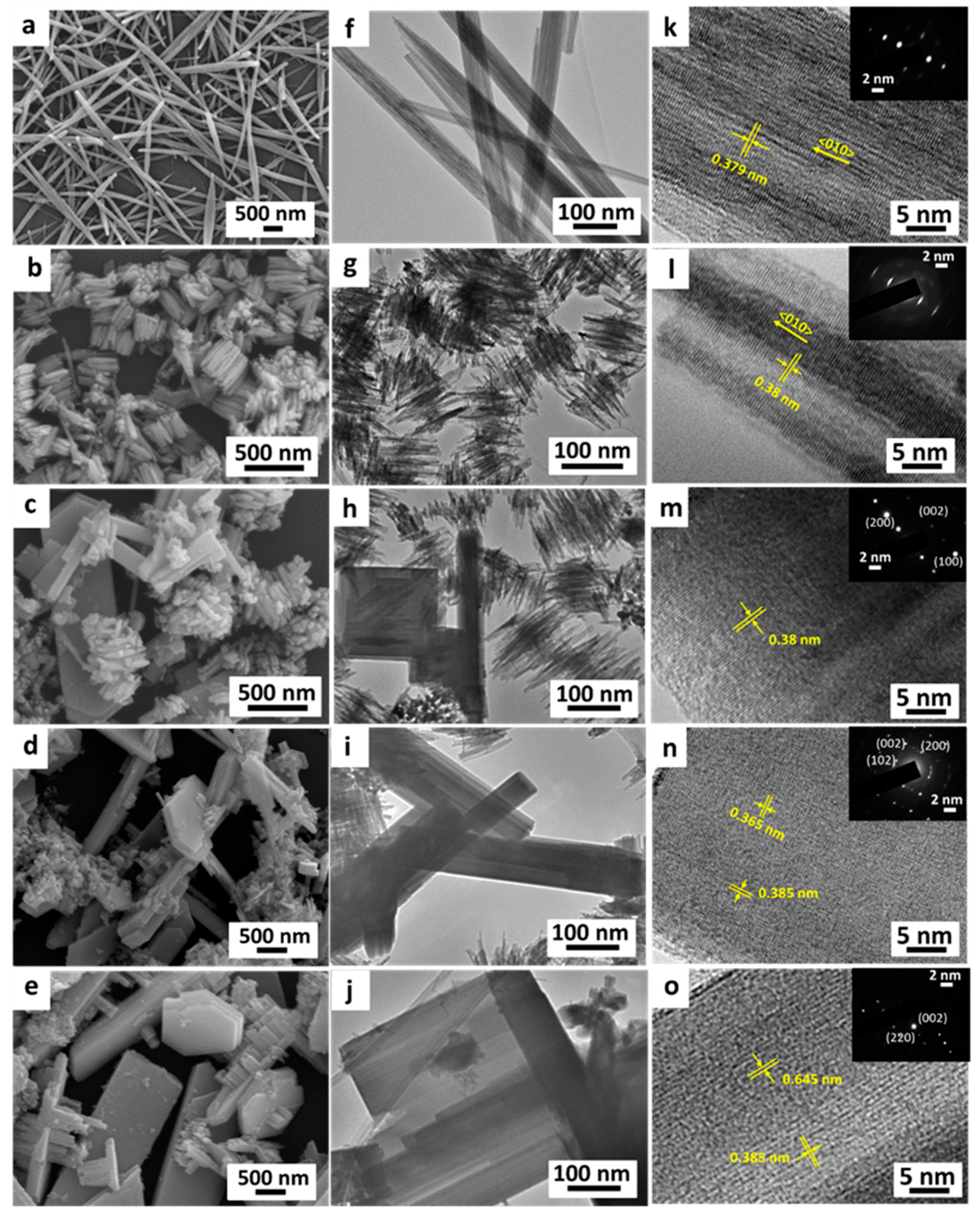
3.1. Structure and Morphology
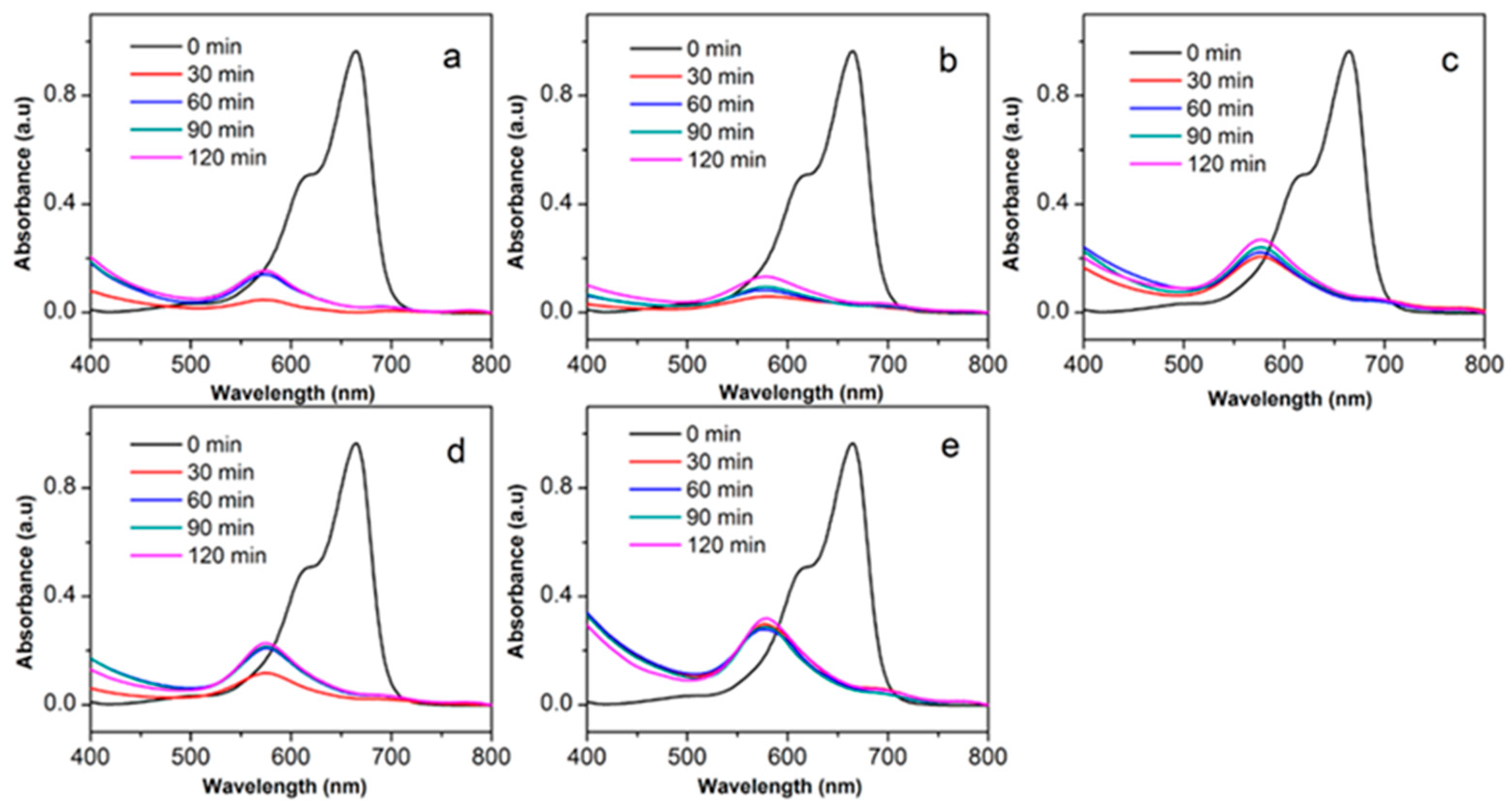
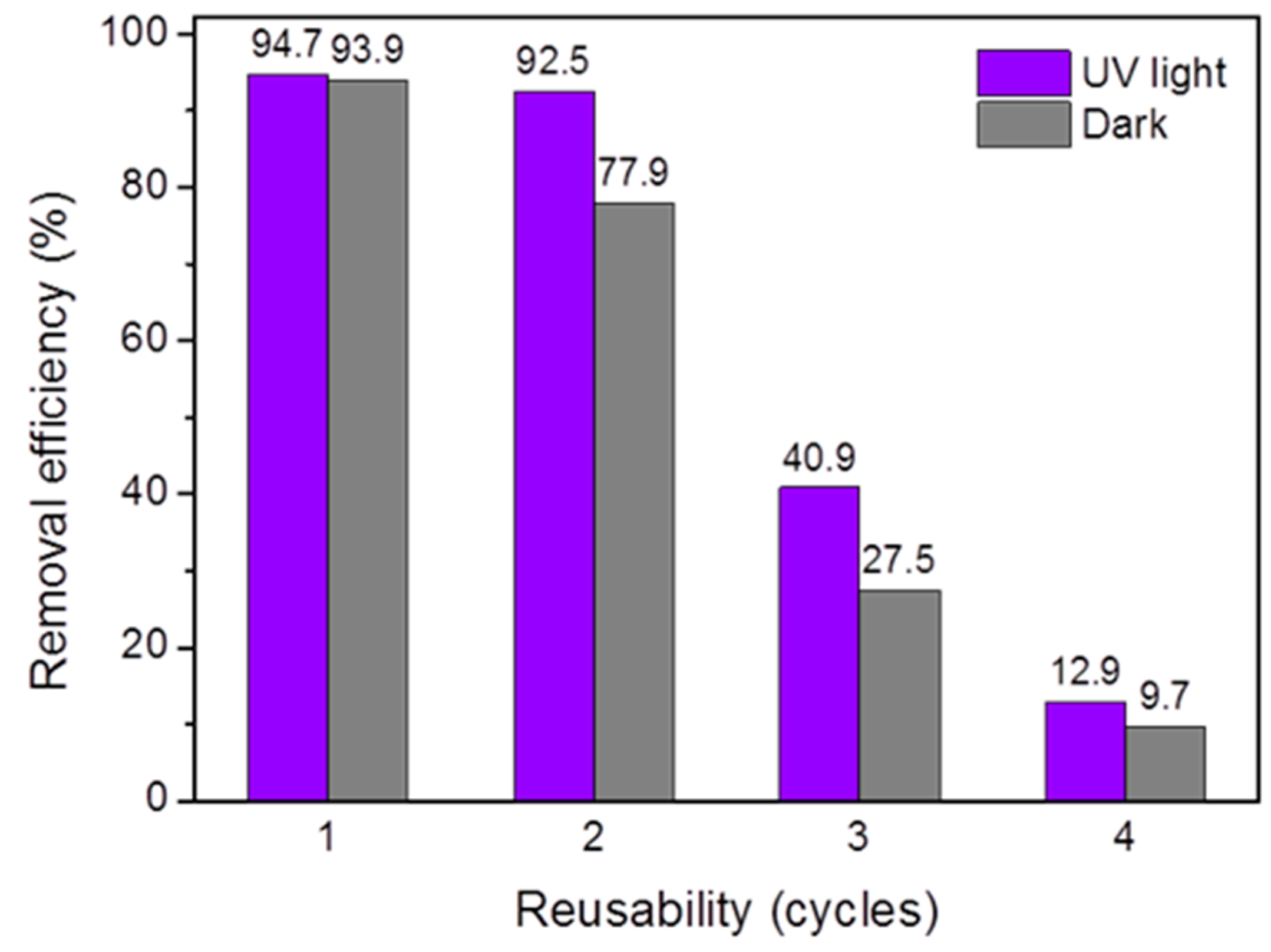
3.2. Adsorption Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ying, Y.L.; Pung, S.Y.; Ong, M.T.; Pung, Y.F. Photocatalytic activity of ZnO nanodisks in degradation of Rhodamine B and Bromocresol Green under UV light exposure. J. Phys. Conf. Ser. 2018, 1082, 012085. [Google Scholar] [CrossRef]
- Odling, G.; Robertson, N. Bridging the gap between laboratory and application in photocatalytic water purification. Catal. Sci. Technol. 2019, 9, 533–545. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Mills, A. Detoxification of water by semiconductor photocatalysis. J. Ind. Eng. Chem. 2004, 10, 173–187. [Google Scholar]
- Doma, A.; Hassan, N.; Abd-Elhamid, A.I.; Soliman, H. Adsorption of Methylene Blue Dye on Hydrothermally Prepared Tungsten Oxide Nanosheets. Egypt. J. Chem. 2020, 63, 483–498. [Google Scholar] [CrossRef]
- Hickman, R.; Walker, E.; Chowdhury, S. TiO2 -PDMS composite sponge for adsorption and solar mediated photodegradation of dye pollutants. J. Water Process. Eng. 2018, 24, 74–82. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Aziz, S.N.Q.A.A.; Pung, S.-Y.; Ramli, N.N.; Lockman, Z. Growth of ZnO Nanorods on Stainless Steel Wire Using Chemical Vapour Deposition and Their Photocatalytic Activity. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Pung, S.-Y.; Sreekantan, S. Degradation of organic dye using ZnO nanorods based continuous flow water purifier. J. Sol-Gel. Sci. Technol. 2013, 66, 399–405. [Google Scholar] [CrossRef]
- Ng, H.M.; Leo, C. Translucent and adsorptive PVA thin film containing microfibrillated cellulose intercalated with TiO2 nanoparticles for dye removal. Colloids Surfaces A: Physicochem. Eng. Asp. 2019, 578, 123590. [Google Scholar] [CrossRef]
- Poulopoulos, S.G.; Yerkinova, A.; Ulykbanova, G.; Inglezakis, V.J. Photocatalytic treatment of organic pollutants in a synthetic wastewater using UV light and combinations of TiO2, H2O2 and Fe(III). PLoS ONE 2019, 14, e0216745. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Liu, T.; Zhang, C.; Geng, X.; Meng, Y.; Li, X. Fluorescent aliphatic hyperbranched polyether: Chromophore-free and without any N and P atoms. Phys. Chem. Chem. Phys. 2016, 18, 4295–4299. [Google Scholar] [CrossRef]
- Kasinathan, K.; Kennedy, J.; Elayaperumal, M.; Henini, M.; Malik, M. Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Abou-Gamra, Z.; Medien, H.; Hamza, M. Effect of porphyrin on photocatalytic activity of TiO2 nanoparticles toward Rhodamine B photodegradation. J. Photochem. Photobiol. B Biol. 2017, 176, 25–35. [Google Scholar] [CrossRef]
- Samsuddin, A.F.; Aziz, S.N.Q.A.A.; Pung, S.-Y. Loading effect of Ag/AgO on the photocatalytic performance of ZnO rods. Appl. Phys. A 2016, 123, 1–12. [Google Scholar] [CrossRef]
- Yin, X.-L.; Li, L.-L.; Liu, M.-L.; Li, D.-C.; Shang, L.; Dou, J.-M. MoSx/CdS nano-heterostructures accurately constructed on the defects of CdS for efficient photocatalytic H2 evolution under visible light irradiation. Chem. Eng. J. 2019, 370, 305–313. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, H.-Q.; Zhang, W.; Yin, J.; Cao, F.-H.; Pan, Y.-X. Noble-metal-free CuS/CdS photocatalyst for efficient visible-light-driven photocatalytic H2 production from water. Catal. Today 2019, 330, 203–208. [Google Scholar] [CrossRef]
- Wicaksana, Y.; Liu, S.; Scott, J.; Amal, R. Tungsten Trioxide as a Visible Light Photocatalyst for Volatile Organic Carbon Removal. Molecules 2014, 19, 17747–17762. [Google Scholar] [CrossRef] [PubMed]
- Widiyandari, H.; Firdaus, I.; Kadarisman, V.G.S.; Purwanto, A. Optical properties and photocatalytic activities of tungsten oxide (WO3) with platinum co-catalyst addition. AIP Conf. Proc. 2016, 1712, 50027. [Google Scholar] [CrossRef]
- Tahir, M.B.; Sagir, M. Carbon nanodots and rare metals (RM = La, Gd, Er) doped tungsten oxide nanostructures for photocatalytic dyes degradation and hydrogen production. Sep. Purif. Technol. 2019, 209, 94–102. [Google Scholar] [CrossRef]
- Lubis, S. Murisna Synthesis, Characterization and Photocatalytic Activity of α-Fe2O3/Bentonite Composite Prepared by Mechanical Milling. J. Physics: Conf. Ser. 2018, 1116, 042016. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; Gadri, A. Retracted Article: Photocatalytic degradation of methylene blue dye by iron oxide (α-Fe2O3) nanoparticles under visible irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 8142–8152. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Pung, S.-Y.; Sreekantan, S.; Yeoh, F.-Y. Photocatalytic activity of β-MnO2 nanotubes grown on PET fibre under visible light irradiation. J. Exp. Nanosci. 2015, 11, 1–16. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Bazarjani, M.S.; Hojamberdiev, M.; Morita, K.; Zhu, G.; Cherkashinin, G.; Fasel, C.; Herrmann, T.; Breitzke, H.; Gurlo, A.; Riedel, R. Visible Light Photocatalysis with c-WO3–x/WO3×H2O Nanoheterostructures In Situ Formed in Mesoporous Polycarbosilane-Siloxane Polymer. J. Am. Chem. Soc. 2013, 135, 4467–4475. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, J.; Fan, W.; Cui, G.; Sun, H.; Zhuo, L.; Zhao, X.; Tang, B. Microcrystalline sodium tungsten bronze nanowire bundles as efficient visible light-responsive photocatalysts. Chem. Commun. 2009, 46, 8833–8835. [Google Scholar] [CrossRef]
- Murillo-Sierra, J.; Hernández-Ramírez, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J. A review on the development of visible light-responsive WO3-based photocatalysts for environmental applications. Chem. Eng. J. Adv. 2021, 5, 100070. [Google Scholar] [CrossRef]
- Ronconi, F.; Syrgiannis, Z.; Bonasera, A.; Prato, M.; Argazzi, R.; Caramori, S.; Cristino, V.; Bignozzi, C.A. Modification of Nanocrystalline WO3 with a Dicationic Perylene Bisimide: Applications to Molecular Level Solar Water Splitting. J. Am. Chem. Soc. 2015, 137, 4630–4633. [Google Scholar] [CrossRef] [PubMed]
- Shabdan, Y.; Markhabayeva, A.; Bakranov, N.; Nuraje, N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials 2020, 10, 1871. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Wu, C.-M.; Naseem, S.; Chou, M.-H.; Wang, J.-H.; Jian, Y.-Q. Recent Advances in Tungsten-Oxide-Based Materials and Their Applications. Front. Mater. 2019, 6, 1–17. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Schneemann, A.; Fischer, R.A.; Zhu, Y.; Xia, Y. MOF Derived Porous ZnO/C Nanocomposites for Efficient Dye Photodegradation. ACS Appl. Energy Mater. 2018, 1, 4695–4707. [Google Scholar] [CrossRef]
- Shirke, Y.M.; Mukherjee, S.P. Selective synthesis of WO3and W18O49nanostructures: Ligand-free pH-dependent morphology-controlled self-assembly of hierarchical architectures from 1D nanostructure and sunlight-driven photocatalytic degradation. CrystEngComm 2017, 19, 2096–2105. [Google Scholar] [CrossRef]
- Mohamedkhair, A.K.; Drmosh, Q.A.; Qamar, M.; Yamani, Z.H. Nanostructured Magnéli-Phase W18O49 Thin Films for Photoelectrochemical Water Splitting. Catalyst 2020, 10, 526. [Google Scholar] [CrossRef]
- Wang, F.F.; Chen, C.; Wang, W.; Kang, M.; Gao, Y.; Chen, X.B.; Zhang, J. Internal field engineering of WO3 by ion channel migration with enhanced photocatalytic oxygen evolution ability. J. Mater. Chem. A 2021, 9, 1678–1691. [Google Scholar] [CrossRef]
- Shankar, R.; Kolandaivel, P.; Senthilkumar, L.; Lakshmipathi, S. Interaction studies of cysteine with Li+, Na+, K+, Be2+, Mg2+, and Ca2+ metal cation complexes. J. Phys. Org. Chem. 2010, 24, 553–567. [Google Scholar] [CrossRef]
- Kunyapat, T.; Xu, F.; Neate, N.; Wang, N.; De Sanctis, A.; Russo, S.; Zhang, S.; Xia, Y.; Zhu, Y. Ce-Doped bundled ultrafine diameter tungsten oxide nanowires with enhanced electrochromic performance. Nanoscale 2018, 10, 4718–4726. [Google Scholar] [CrossRef] [PubMed]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Da Conceição, L.R.V.; Carneiro, L.M.; Rivaldi, J.D.; De Castro, H.F. Solid acid as catalyst for biodiesel production via simultaneous esterification and transesterification of macaw palm oil. Ind. Crop. Prod. 2016, 89, 416–424. [Google Scholar] [CrossRef]
- Liu, B.; Wen, L.; Nakata, K.; Zhao, X.; Liu, S.; Ochiai, T.; Murakami, T.; Fujishima, A. Polymeric Adsorption of Methylene Blue in TiO2 Colloids-Highly Sensitive Thermochromism and Selective Photocatalysis. Chem.-A Eur. J. 2012, 18, 12705–12711. [Google Scholar] [CrossRef]
- Xia, Y.; Yao, Q.; Zhang, W.; Zhang, Y.; Zhao, M. Comparative adsorption of methylene blue by magnetic baker’s yeast and EDTAD-modified magnetic baker’s yeast: Equilibrium and kinetic study. Arab. J. Chem. 2019, 12, 2448–2456. [Google Scholar] [CrossRef]
- Kumar, V.B.; Mohanta, D. Formation of nanoscale tungsten oxide structures and colouration characteristics. Bull. Mater. Sci. 2011, 34, 435–442. [Google Scholar] [CrossRef]
- Gao, T.; Jelle, B.P. Electrochromism of hexagonal sodium tungsten bronze nanorods. Sol. Energy Mater. Sol. Cells 2018, 177, 3–8. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Botcha, N.K.; Zarie, E.S.; Elbahri, M. Ups and Downs of Water Photodecolorization by Nanocomposite Polymer Nanofibers. Nanomaterials 2019, 9, 250. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A Novel Nanohybrid Nanofibrous Adsorbent for Water Purification from Dye Pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Lin, H.; Xie, Y.; Tong, N.; Lin, J.; Zhang, X.; Zhang, Z.; Wang, X. Surface oxygen vacancy and defect engineering of WO3 for improved visible light photocatalytic performance. Catal. Sci. Technol. 2018, 8, 4399–4406. [Google Scholar] [CrossRef]
- Hidayat, D.; Purwanto, A.; Wang, W.-N.; Okuyama, K. Preparation of size-controlled tungsten oxide nanoparticles and evaluation of their adsorption performance. Mater. Res. Bull. 2010, 45, 165–173. [Google Scholar] [CrossRef]
- Meng, J.; Lin, Q.; Chen, T.; Wei, X.; Li, J.; Zhang, Z. Oxygen vacancy regulation on tungsten oxides with specific exposed facets for enhanced visible-light-driven photocatalytic oxidation. Nanoscale 2018, 10, 2908–2915. [Google Scholar] [CrossRef]







| Samples | Height | Intensity Ratio (002)/(200) Peak | FWHM 1 | ||
|---|---|---|---|---|---|
| Plane (002) | Plane (200) | Plane (002) | Plane (200) | ||
| Na:W = 1:16 | 26,995 | 10,834 | 2.49 | 0.28 | 1.105 |
| Na:W = 1:12 | 20,650 | 12,791 | 1.61 | 0.31 | 0.63 |
| Na:W = 1:8 | 15,389 | 13,922 | 1.11 | 0.46 | 0.58 |
| Na:W = 1:4 | 13,549 | 15,262 | 0.88 | 0.53 | 0.50 |
| Samples | Surface Composition (Atomic Ratio) | ||||
|---|---|---|---|---|---|
| W5+/W6+ | O 1s Peak2/Peak 1 | Ototal/ (O + Na + W) | Wtotal/ (O + Na + W) | Natotal/ (O + Na + W) | |
| Pure W18O49 | 0.102 | 0.19 | 0.790 | 0.21 | - |
| Na:W = 1:16 | 0.160 | 0.22 | 0.747 | 0.221 | 0.032 |
| Na:W = 1:12 | 0.182 | 0.24 | 0.723 | 0.237 | 0.04 |
| Na:W = 1:8 | 0.241 | 0.25 | 0.699 | 0.24 | 0.061 |
| Na:W = 1:4 | 0.227 | 0.21 | 0.681 | 0.251 | 0.068 |
| Samples | Bandgap Energy (eV) | BET Surface Area (m2/g) | Pore Volume of Sample (cm3/g) |
|---|---|---|---|
| W18O49 | 2.98 | 124 ± 3 | 0.23 ± 0.06 |
| 1:16 | 2.89 | 48.3 ± 1.5 | 0.22 ± 0.05 |
| 1:12 | 2.50 | 75.7 ± 2.0 | 0.38 ± 0.09 |
| 1:8 | 2.43 | 117.8 ± 2.9 | 0.29 ± 0.07 |
| 1:4 | 2.39 | 126.9 ± 3.4 | 0.36 ± 0.08 |
| N | Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference | T-Value | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| UV-Visible | UV | 6 | 95.274 | 1.285 | 0.525 | −1.582 | 2.583 | 0.62 | 0.564 |
| Visible | 6 | 94.773 | 1.985 | 0.81 | |||||
| Difference | 6 | 0.5 | 1.984 | 0.81 | |||||
| Visible-Dark | Visible | 6 | 94.773 | 1.985 | 0.81 | −0.721 | 4.231 | 1.82 | 0.128 |
| Dark | 6 | 93.019 | 2.131 | 0.87 | |||||
| Difference | 6 | 1.755 | 2.359 | 0.963 | |||||
| UV-Dark | UV | 6 | 95.274 | 1.285 | 0.525 | 0.416 | 4.095 | 3.15 | 0.025 |
| Dark | 6 | 93.019 | 2.131 | 0.87 | |||||
| Different | 6 | 2.255 | 1.753 | 0.715 | |||||
| 2nd Order | Unit | W18O49 | 1:4 | 1:8 | 1:12 | 1:16 | |
|---|---|---|---|---|---|---|---|
| Slope | 1/qe | g/mg | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Intercept | 1/(Kqe2) | min (mg/g)−2 | 0.071 | 0.049 | 0.059 | 0.016 | 0.023 |
| Rate constant | K | min−1 | 0.013 | 0.018 | 0.015 | 0.056 | 0.039 |
| Correlation coefficients | R2 | - | 0.99 | 0.99 | 1.00 | 0.99 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thummavichai, K.; Thi, L.A.; Pung, S.-Y.; Ola, O.; Hussain, M.Z.; Chen, Y.; Xu, F.; Chen, W.; Wang, N.; Zhu, Y. Sodium Tungsten Oxide Bronze Nanowires Bundles in Adsorption of Methylene Blue Dye under UV and Visible Light Exposure. Energies 2021, 14, 1322. https://doi.org/10.3390/en14051322
Thummavichai K, Thi LA, Pung S-Y, Ola O, Hussain MZ, Chen Y, Xu F, Chen W, Wang N, Zhu Y. Sodium Tungsten Oxide Bronze Nanowires Bundles in Adsorption of Methylene Blue Dye under UV and Visible Light Exposure. Energies. 2021; 14(5):1322. https://doi.org/10.3390/en14051322
Chicago/Turabian StyleThummavichai, Kunyapat, Le Anh Thi, Swee-Yong Pung, Oluwafunmilola Ola, Mian Zahid Hussain, Yu Chen, Fang Xu, Wenting Chen, Nannan Wang, and Yanqiu Zhu. 2021. "Sodium Tungsten Oxide Bronze Nanowires Bundles in Adsorption of Methylene Blue Dye under UV and Visible Light Exposure" Energies 14, no. 5: 1322. https://doi.org/10.3390/en14051322
APA StyleThummavichai, K., Thi, L. A., Pung, S.-Y., Ola, O., Hussain, M. Z., Chen, Y., Xu, F., Chen, W., Wang, N., & Zhu, Y. (2021). Sodium Tungsten Oxide Bronze Nanowires Bundles in Adsorption of Methylene Blue Dye under UV and Visible Light Exposure. Energies, 14(5), 1322. https://doi.org/10.3390/en14051322







