Design of Cylindrical Thermal Dummy Cell for Development of Lithium-Ion Battery Thermal Management System
Abstract
:1. Introduction
- Energy-intensive. Since the battery is designed as an energy storage element, most of the electric energy required by the experiment is stored in the battery or consumed by the load. Only a small fraction of the electric energy is converted to heat, wasting energy during the experiments of thermal management systems.
- Time-intensive. The charging and discharging of the battery pack takes quite a long time, resulting in the test cycle being extended. For example, a battery pack with a capacity of 39 Ah takes 50 min to get fully charged in fast charging with negative pulse mode [9].
- Impossible and unsafe to test extreme heating conditions. To gain the overheating condition of the battery, high-power loads are used to discharge the battery pack with high current. However, restricted by the capacity of the tested battery, the time during discharge with high current and the heating power of the battery are limited. For example, under NEDC operating conditions, the maximum heating power of a 26,650-type cylindrical lithium-ion cell is less than 10 W [10]. Therefore, the performance of the thermal management system cannot be adequately evaluated using real cells.
- It is difficult to simulate the local thermal imbalance when the overheating problem exists in one or more cells.
- High equipment cost. Costly equipment is required: battery pack, battery cycler, thermal chamber, and other accessories related to health and safety.
- All the electric energy consumed is used for battery heating, which significantly reduces the experiment energy consumption.
- TDCs can be heated any time, and there is no need for charging, which quietly shortens the test time.
- The heating power of TDCs is much larger than that of the real cell, so it is easy to simulate the bad working conditions of battery packs, such as fast heating and high temperature conditions.
- With the feedback of its built-in temperature sensor, the accurate control of core temperature can be achieved.
- The working power of each TDC in the pack can be adjusted separately, so it is easy to simulate thermal imbalance among cells in one battery pack.
2. Material and Methods
2.1. Structure and Thermal Properties of Cylindrical Cells
2.1.1. Structural Characteristics
2.1.2. Thermal Model
2.2. TDC Model Design
2.3. Thermal Simulation of TDC
2.3.1. Initial and Boundary Conditions
2.3.2. Governing Equations
2.3.3. TDC Modeling
2.3.4. Meshing Independence Validation
2.4. Fabrication of TDC Samples
2.5. Experimental Setup
3. Results and Discussion
3.1. Temperature Drift of Resistance of Heating Unit
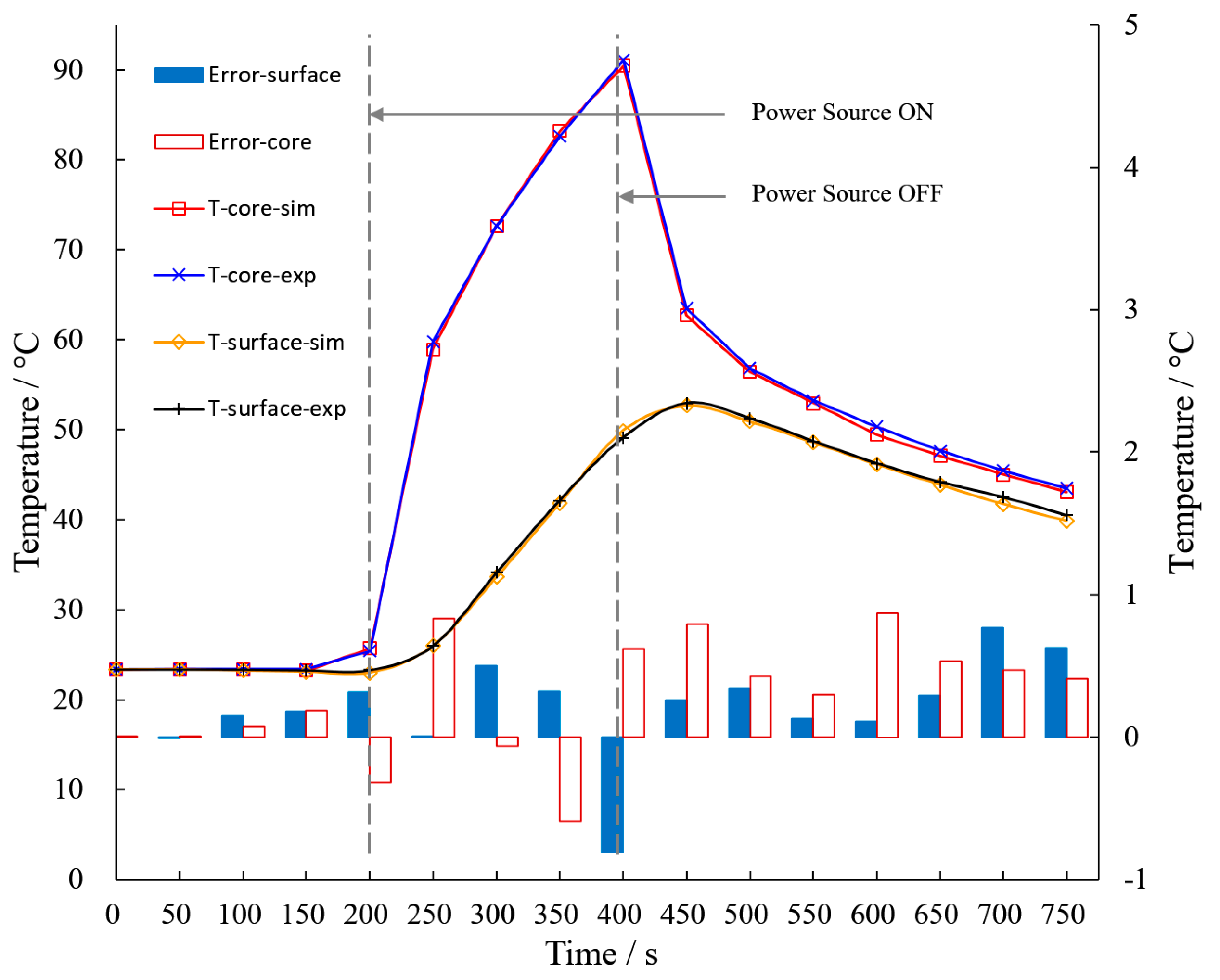
3.2. Validation of TDC Model
3.3. Experiment Results of Temperature Distribution on the Surface
3.3.1. Temperature Distribution along the Circumference
3.3.2. Temperature Distribution along the Axial Direction
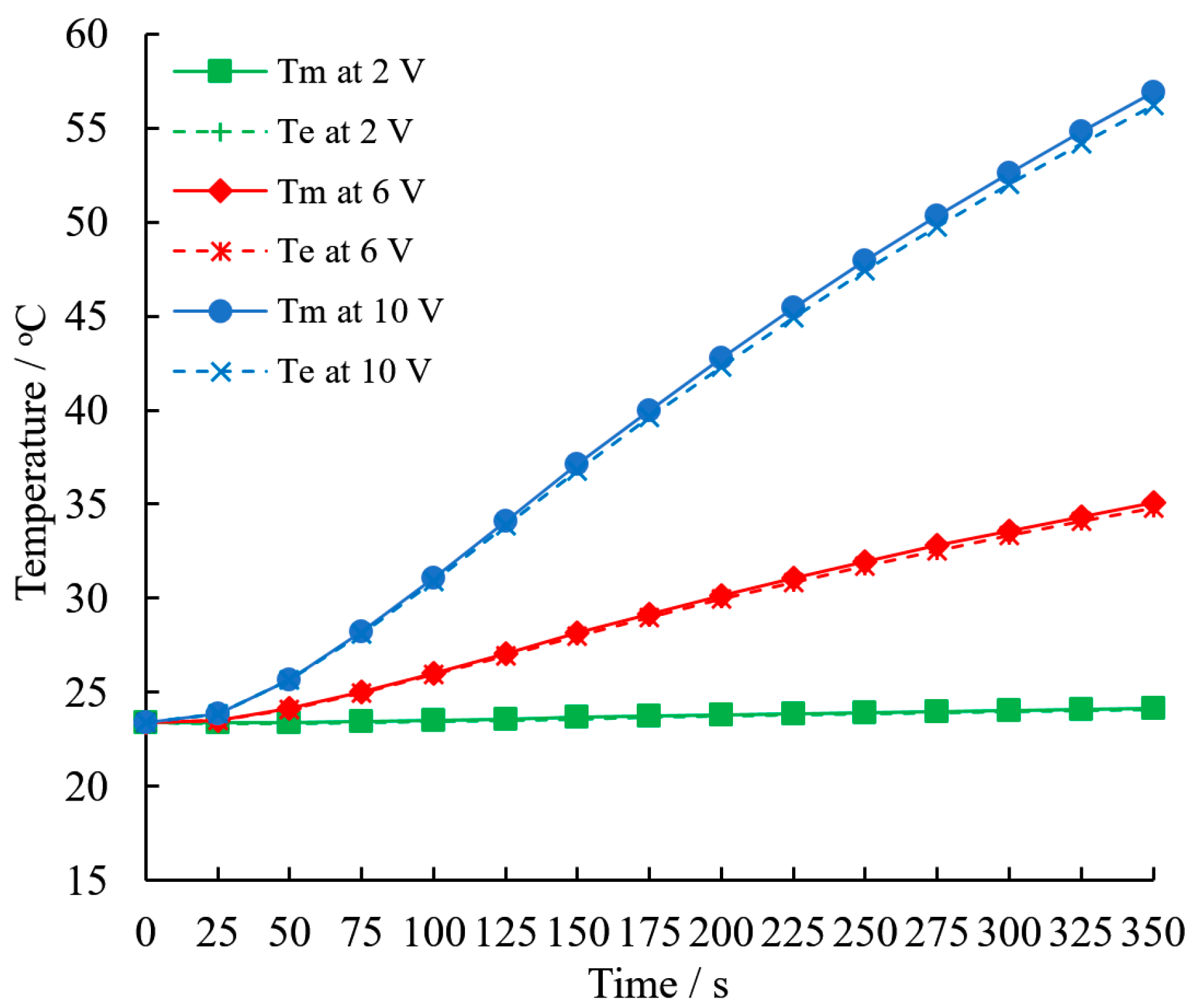
3.4. Temperature Rising Rate
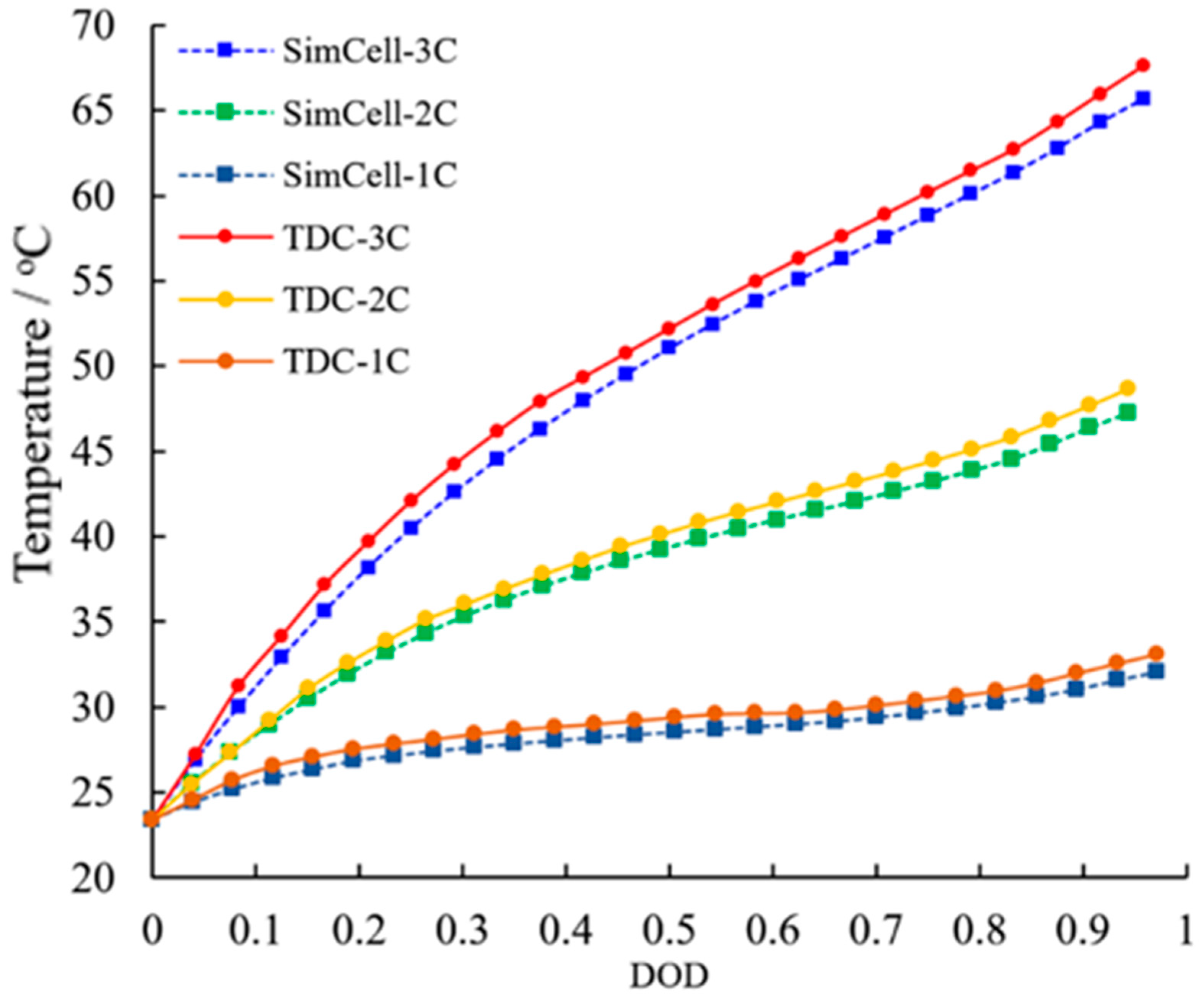
3.5. Surface Temperature Compared with a Simulation Cell
3.5.1. Temperature Rising at Constant Discharge Current
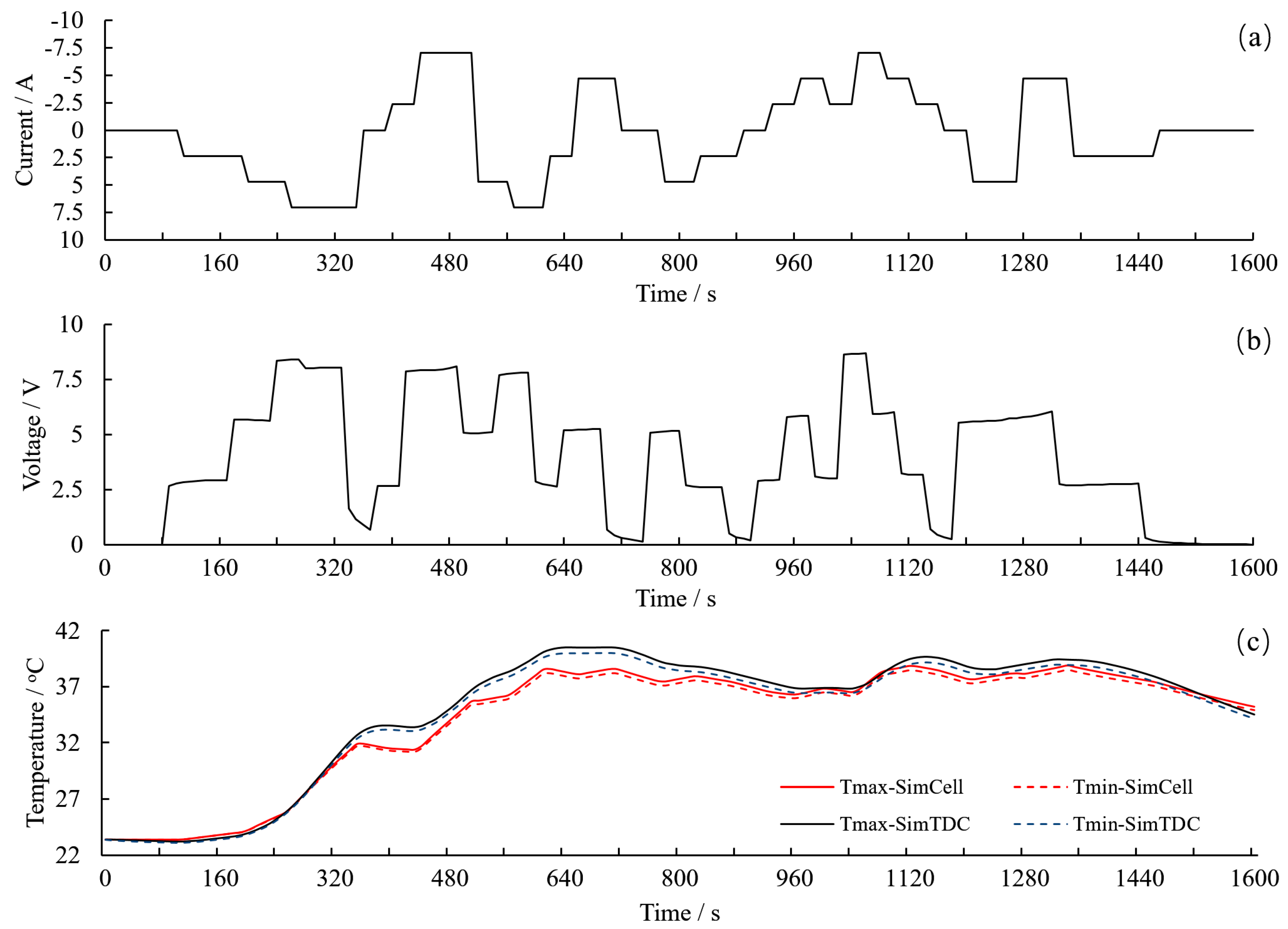
3.5.2. Temperature Change at Dynamic Current Load
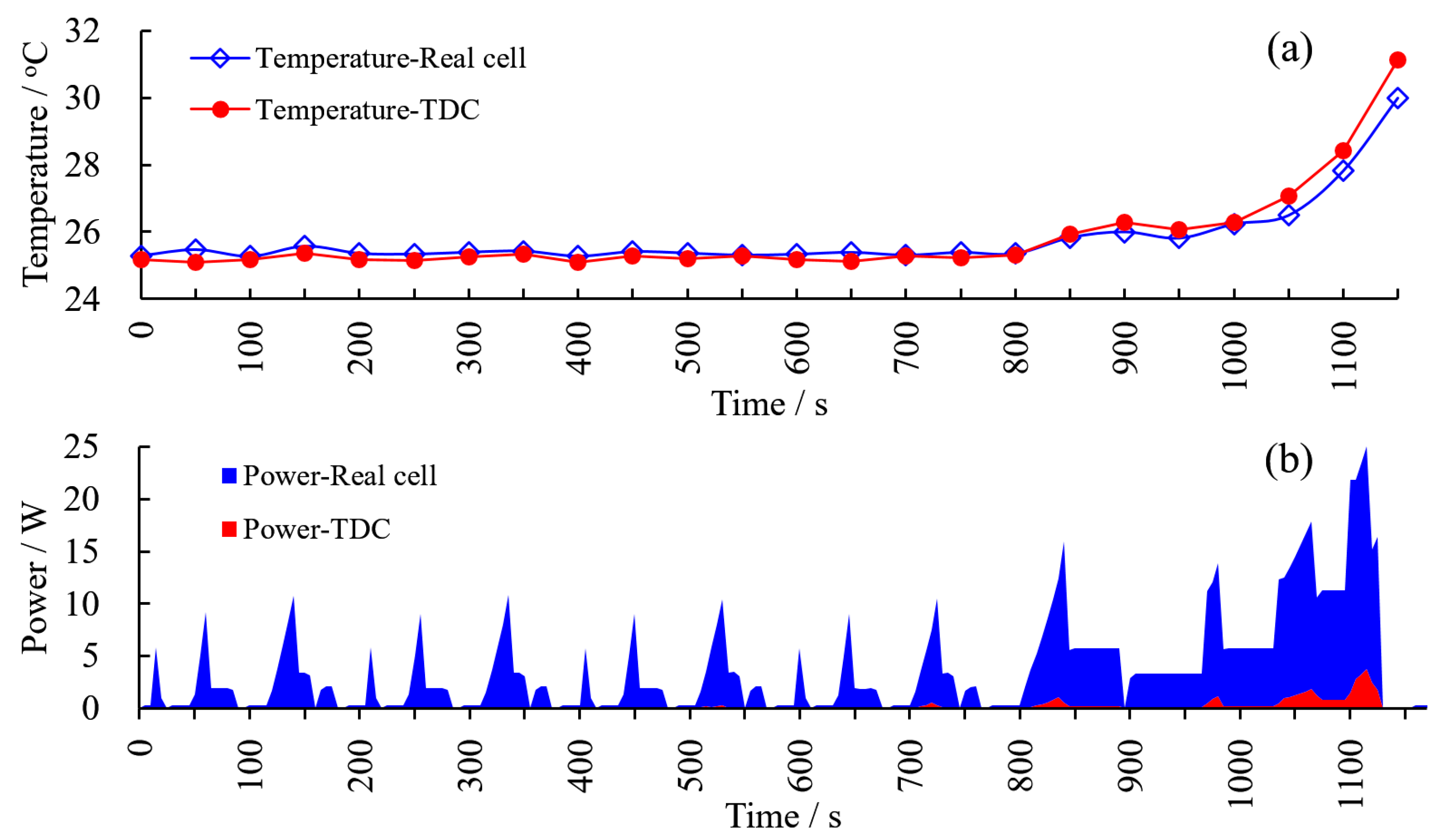
3.6. Contrast Test with Real Cell under NEDC Operating Condition
4. Conclusions
- The proposed electrothermal material can provide stable heating powers (0.025 W variation at 12 V voltage) in the temperature of 27.5–90.5 °C, which is beneficial to precisely control TDC heat generation.
- The surface temperature distribution of the sample is similar to that of the real cell. Their axial temperature difference does not exceed 1.6 °C.
- The temperature rising rate of the sample reaches 1.9 °C/min powered at a 6 V constant voltage, which exceeds the upper safety limit in the condition of normal high-power discharge. The value reaches 5.7 °C/min powered by a 10 V constant voltage, which can simulate battery thermal failure.
- Both in static and dynamic load, the TDC has the ability to mimic the temperature change on the surface of a real cell, and the deviation of the temperature can be controlled by adjustment of the voltage source.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Cc | equivalent specific heat capacity of active battery material (J/kg·K) |
| Cs | specific heat capacity of the can (J/kg·K) |
| Tc | core temperature of the cell (K) |
| Ts | surface temperature of the cell (K) |
| Tf | air temperature (K) |
| Re | battery internal electrical resistance (Ω) |
| Ru | thermal resistance between the can and the environment (K/W) |
| Rc | thermal resistance between the equivalent core and the shell (K/W) |
| I | working current (A) |
| Tinit | initial temperature (K) |
| hs | heat transfer coefficient on the side face (W/m2·K) |
| he | heat transfer coefficient on the end face (W/m2·K) |
| qs | initial heat flux perpendicular to the side face (W) |
| qe | initial heat flux perpendicular to the end face (W) |
| Tws | temperature on the side face (K) |
| Twe | temperature on the end face (K) |
| Rt | thermal contact resistance (m2·K/W) |
| cp | specific heat capacity at constant pressure (J/kg·K) |
| T | temperature (K) |
| t | time (s) |
| q | heat flux vector (W) |
| Q | heat generation rate (W/m³) |
| k | thermal conductivity (W/m·K) |
| J | current density (A/m²) |
| E | electric field (V/m) |
| P | heating power (W) |
| U | voltage of the source (V) |
| R | electrical resistance of the heating unit (Ω) |
| UOCV | open circuit voltage of the battery (V) |
| UT | terminal voltage of the battery (V) |
| ρ | density (kg/m3) |
| σ | electrical conductivity (S/m) |
References
- Cao, W.; Zhao, C.; Wang, Y.; Dong, T.; Jiang, F. Thermal modeling of full-size-scale cylindrical battery pack cooled by channeled liquid flow. Int. J. Heat Mass Transf. 2019, 138, 1178–1187. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Z.; He, W.; Zhao, J. Thermal issues about Li-ion batteries and recent progress in battery thermal management systems: A review. Energy Convers. Manag. 2017, 150, 304–330. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Peng, H. A comparative study of equivalent circuit models for Li-ion batteries. J. Power Sources 2012, 198, 359–367. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, J.; Liang, J.; Huang, Z.; Hu, M. Development of thermal equivalent circuit model of heat pipe-based thermal management system for a battery module with cylindrical cells. Appl. Therm. Eng. 2020, 164, 114523. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Behi, M.; Ghanbarpour, M.; Jaguemont, J.; Sokkeh, M.A.; Gandoman, F.H.; Berecibar, M.; Van Mierlo, J. A new concept of thermal management system in Li-ion battery using air cooling and heat pipe for electric vehicles. Appl. Therm. Eng. 2020, 174, 115280. [Google Scholar] [CrossRef]
- Zhang, K.; Li, M.; Yang, C.; Shao, Z.; Wang, L. Exergy Analysis of Electric Vehicle Heat Pump Air Conditioning System with Battery Thermal Management System. J. Therm. Sci. 2019, 29, 408–422. [Google Scholar] [CrossRef]
- Al-Zareer, M.; Dincer, I.; Rosen, M.A. A thermal performance management system for lithium-ion battery packs. Appl. Therm. Eng. 2020, 165, 114378. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, Z.; Wang, B.; Gao, H.; Chen, S.; Zong, S.; Luo, K. A Li-Ion Battery Thermal Management System Combining a Heat Pipe and Thermoelectric Cooler. Energies 2020, 13, 841. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Choe, S.-Y. Fast and safe charging method suppressing side reaction and lithium deposition reaction in lithium ion battery. J. Power Sources 2019, 436, 226835. [Google Scholar] [CrossRef]
- Hu, X.; Liu, W.; Lin, X.; Xie, Y. A Comparative Study of Control-Oriented Thermal Models for Cylindrical Li-Ion Batteries. IEEE Trans. Transp. Electrif. 2019, 5, 1237–1253. [Google Scholar] [CrossRef]
- Saw, L.H.; King, Y.J.; Yew, M.C.; Ng, T.C.; Chong, W.T.; Pambudi, N.A. Feasibility study of mist cooling for lithium-ion battery. Energy Procedia 2017, 142, 2592–2597. [Google Scholar] [CrossRef]
- Saw, L.H.; Poon, H.M.; Thiam, H.S.; Cai, Z.; Chong, W.T.; Pambudi, N.A.; King, Y.J. Novel thermal management system using mist cooling for lithium-ion battery packs. Appl. Energy 2018, 223, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Volck, T.; Sinz, W.; Gstrein, G.; Breitfuss, C.; Heindl, S.F.; Steffan, H.; Freunberger, S.; Wilkening, M.; Uitz, M.; Fink, C. Method for De-termination of the Internal Short Resistance and Heat Evolution at Different Mechanical Loads of a Lithium Ion Battery Cell Based on Dummy Pouch Cells. Batteries 2016, 2, 8. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.; Ma, L. Actively controlled thermal management of prismatic Li-ion cells under elevated temperatures. Int. J. Heat Mass Transf. 2016, 102, 315–322. [Google Scholar] [CrossRef]
- Zhu, Y.; Fang, Y.; Su, L.; Fei, Y. Experimental study on thermal performance of a pumped two-phase battery thermal management system. Int. J. Energy Res. 2020, 44, 4664–4676. [Google Scholar] [CrossRef]
- Joshua, S.; Randeep, S.; Michael, H. Battery thermal management system for electric vehicle using heat pipes. Int. J. Therm. Sci. 2018, 134, 517–529. [Google Scholar]
- Kang, D.; Lee, P.-Y.; Yoo, K.; Kim, J. Internal thermal network model-based inner temperature distribution of high-power lithium-ion battery packs with different shapes for thermal management. J. Energy Storage 2020, 27, 101017. [Google Scholar] [CrossRef]
- Du, S.L.; Lai, Y.Q.; Jia, M. Electrothermal characteristics simulation of cylindrical automotive lithium-ion battery. Chin. J. Nonferrous Met. 2014, 24, 1823–1830. [Google Scholar]
- Lin, X.; Fu, H.; Perez, H.E.; Siege, J.B.; Stefanopoulou, A.G.; Ding, Y.; Castanier, M.P. Parameterization and Observability Analysis of Scalable Battery Clusters for Onboard Thermal Management. Oil Gas Sci. Technol. 2013, 68, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Perez, H.E.; Mohan, S.; Siegel, J.B.; Stefanopoulou, A.G.; Ding, Y.; Castanier, M.P. A lumped-parameter electro-thermal model for cylindrical batteries. J. Power Source 2014, 257, 1–11. [Google Scholar] [CrossRef]
- Lin, X.; Perez, H.E.; Siegel, J.B.; Stefanopoulou, A.G.; Li, Y.; Anderson, R.D.; Ding, Y.; Castanier, M.P. Online Parameterization of Lumped Thermal Dynamics in Cylindrical Lithium Ion Batteries for Core Temperature Estimation and Health Monitoring. IEEE Trans. Control. Syst. Technol. 2012, 21, 1745–1755. [Google Scholar] [CrossRef]
- Xiong, S.; Xuan, D.; Zhang, Y.; Wu, Z.; Li, W.; Hu, Z.; Xu, J. Study on the Online Reforming of Low Concentration Alcohol as Vehicle Fuel. Energy Eng. 2020, 117, 1–14. [Google Scholar] [CrossRef]
- Li, J.Q.; Wu, P.; Zhang, C.N. Study and Implementation of Thermal Management Technology for the Power Batteries of Electric Vehicles. Automot. Eng. 2016, 38, 22–27. [Google Scholar]
- Chanwoo, P.; Arun, K.J. Dynamic Thermal Model of Li-Ion Battery for Predictive Behavior in Hybrid and Fuel Cell Vehicles. SAE Trans. 2003, 112, 1835–1842. [Google Scholar]
- Ouyang, D.; Weng, J.; Hu, J.; Chen, M.; Huang, Q.; Wang, J. Experimental investigation of thermal failure propagation in typical lithium-ion battery modules. Thermochim. Acta 2019, 676, 205–213. [Google Scholar] [CrossRef]
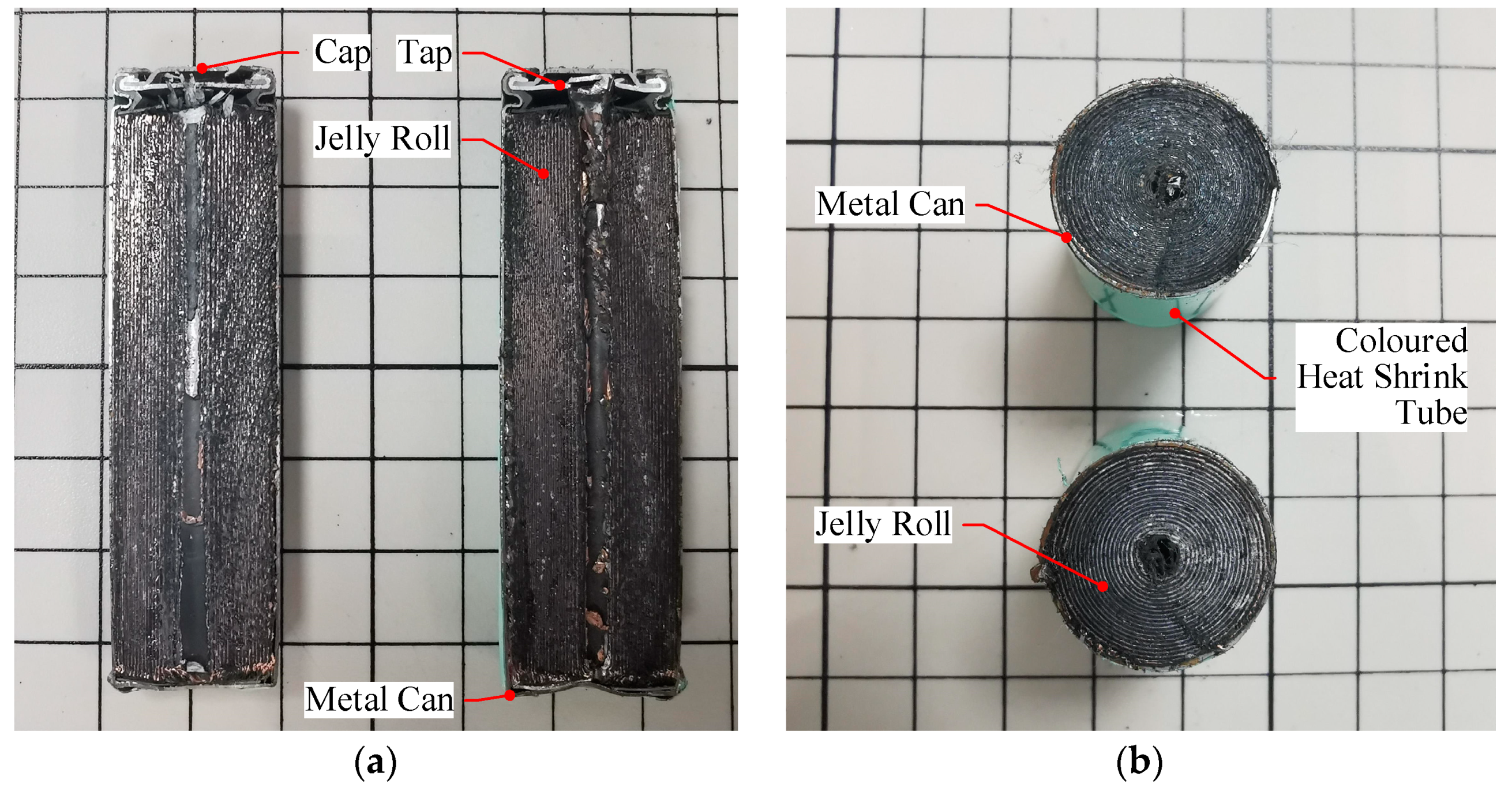
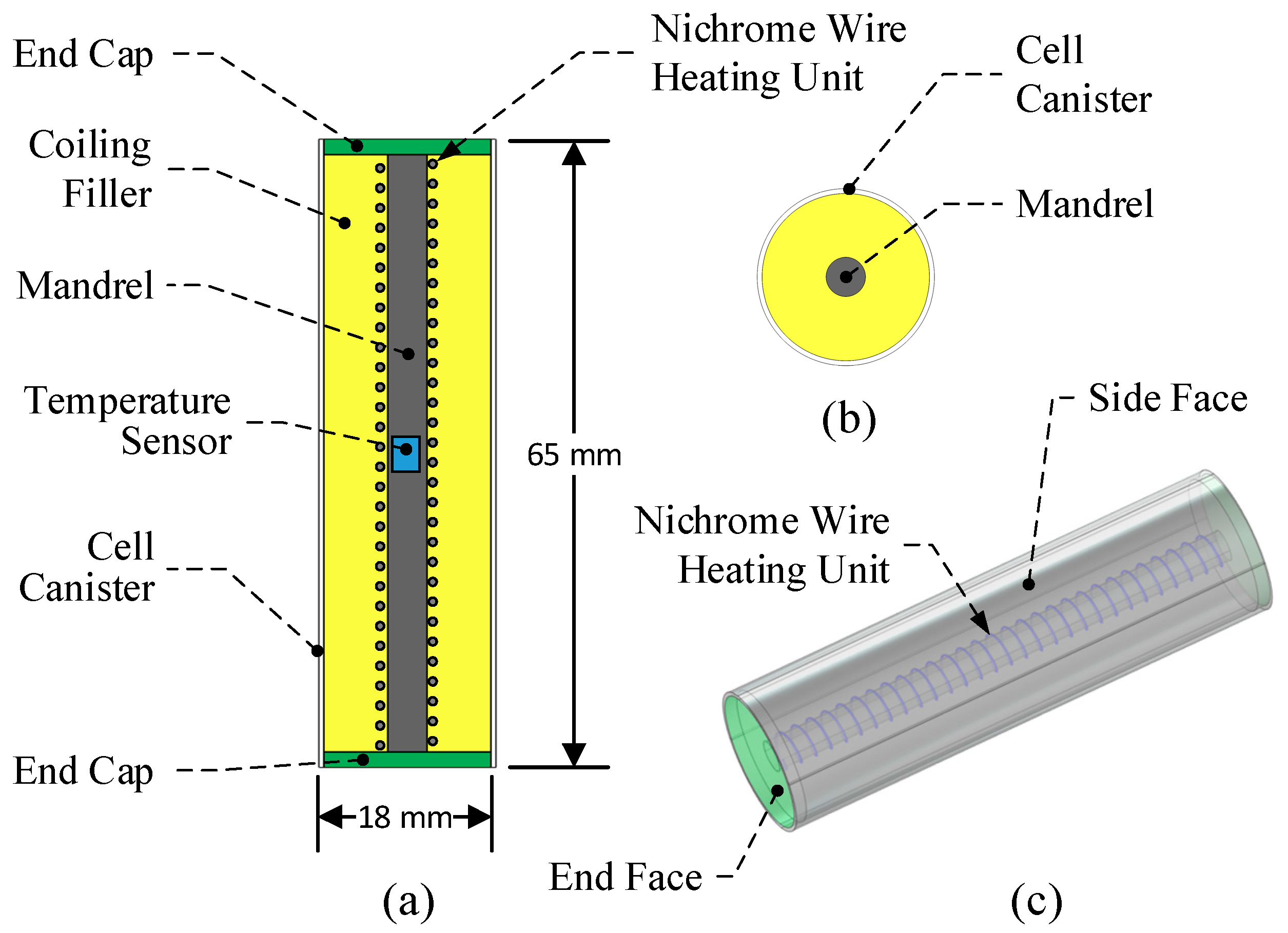

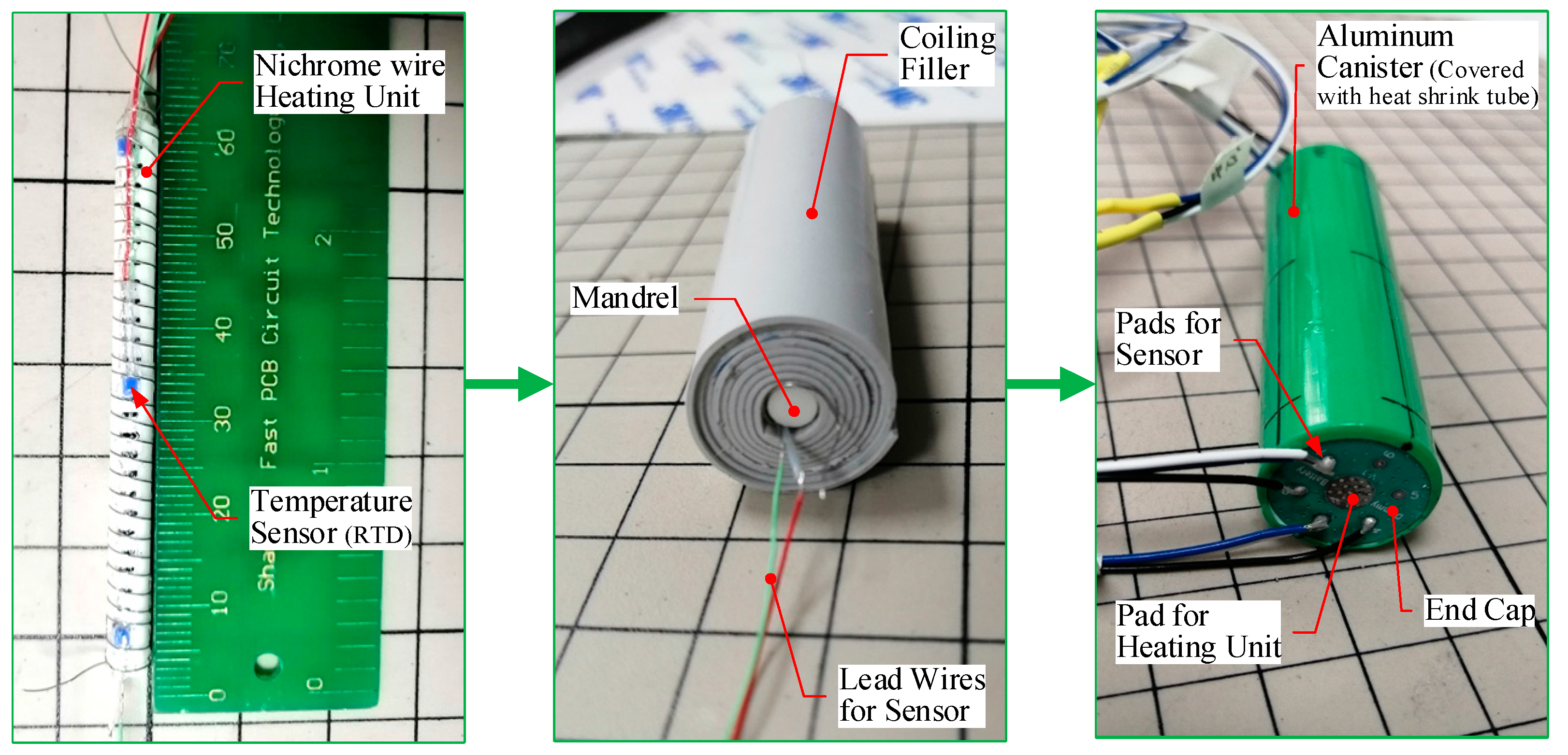
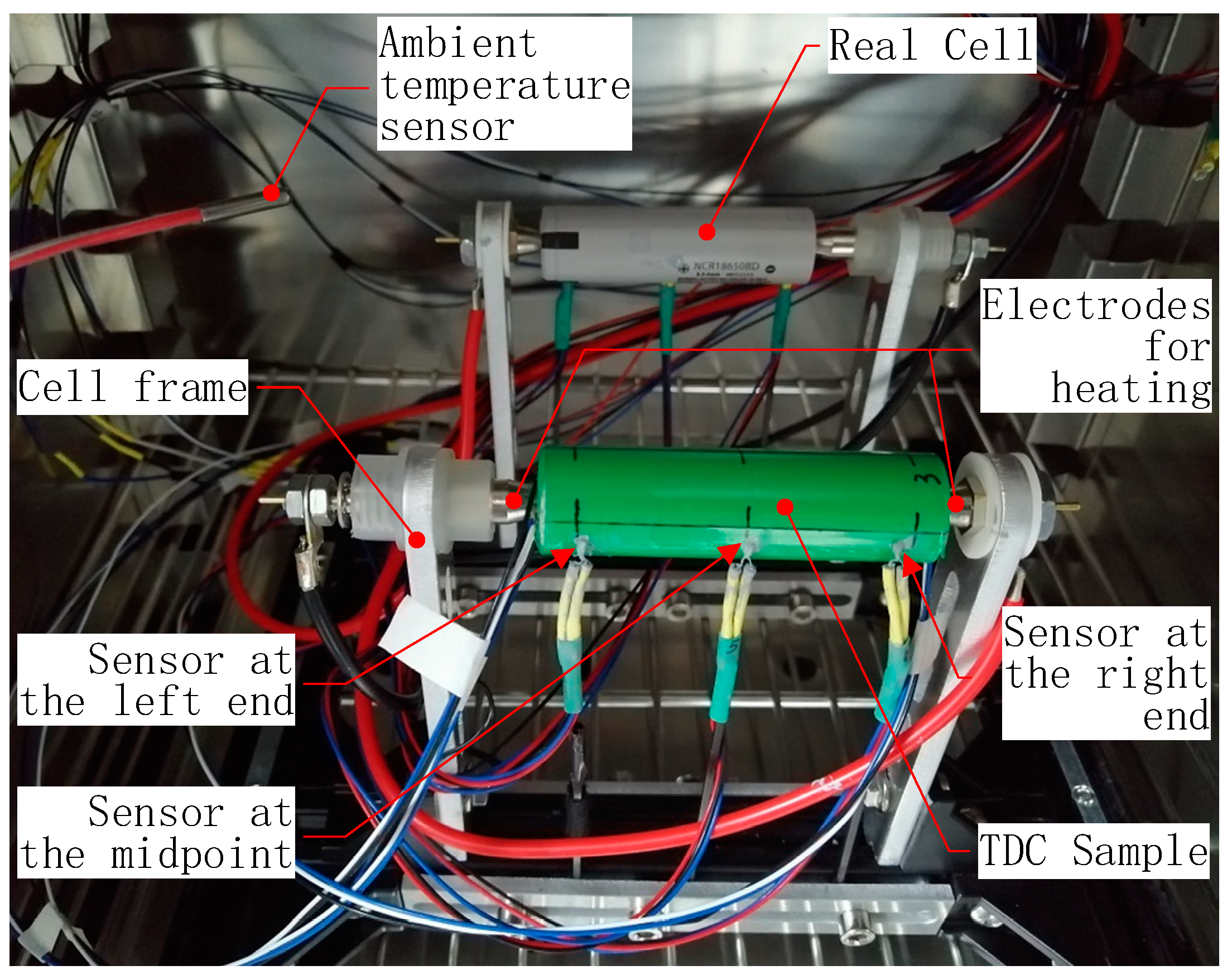


| Part | Material | Density (kg·m−3) | Specific Heat Capacity (J·kg−1·K−1) | Thermal Resistance (W·m−1·K−1) | Maximum Allowable Temperature (°C) |
|---|---|---|---|---|---|
| Mandrel | Zirconia ceramic | 2650 | 720 | 1.1 | >1000 |
| Cell Canister | Aluminum | 2700 | 900 | 201 | >500 |
| Coiling Filler | Silica gel | 2850 | 920 | 4 | 220 |
| End Cap | FR4 | 1900 | 1365 | 1.3 | 180 |
| Heating Unit | Nichrome | 8400 | 20 | 15 | >1000 |
| NO. | Initial and Boundary Condition | Equation |
|---|---|---|
| (1) | the initial temperature of TDC and surrounding air is 23.4 °C | Tinit = 23.4 1 |
| (2) | the mandrel and the filler shown in Figure 2 are of complete electrical insulation | n·J = 0 2 |
| (3) | the convection of the air on the side of the cylinder as shown in Figure 2. is regarded as one fix heat flux | hs = qs/(Tws-Tf) = A 3 |
| (4) | the convection of the air and the conduction of the cable on the end face as shown in Figure 2. are regarded as another fix heat flux | he = qe/(Twe-Tf) = B 4 |
| (5) | thermal contact resistance among the heating unit, the filler, the end caps, and the cell canister are not taken into consideration | Rt = 0 5 |
| (6) | all the parameters of material property are considered to be constant |
| Core Temperature °C | 27.5 | 34.5 | 41.5 | 48.5 | 55.5 | 62.5 | 69.5 | 76.5 | 83.5 | 90.5 |
| Resistance Ω | 16.392 | 16.387 | 16.381 | 16.375 | 16.371 | 16.365 | 16.360 | 16.355 | 16.349 | 16.346 |
| Conditions | Value | Unit |
|---|---|---|
| Initial temperature | 23.4 | °C |
| Voltage when power on | 12 | V |
| Equivalent heat flux on the side | 14 | W/(m2·K) |
| Equivalent heat flux on the ends | 91 | W/(m2·K) |
| Thermal conductivity along the radial direction | 0.5 | W/(m·K) |
| Thermal conductivity along the axial direction | 4.0 | W/(m·K) |
| Properties | Value | Unit |
|---|---|---|
| Initial temperature | 23.4 | °C |
| Capacity | 2.35 | Ah |
| Specific Heat Capacity | 1250 | J/(kg·K) |
| Density | 2750 | kg/m3 |
| Thermal conductivity along the radial direction | 0.3 | W/(m·K) |
| Thermal conductivity along the axial direction | 30 | W/(m·K) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xiong, S.; Zhou, X.; Shi, W.; Wang, C.; Lin, X.; Cheng, J. Design of Cylindrical Thermal Dummy Cell for Development of Lithium-Ion Battery Thermal Management System. Energies 2021, 14, 1357. https://doi.org/10.3390/en14051357
Li W, Xiong S, Zhou X, Shi W, Wang C, Lin X, Cheng J. Design of Cylindrical Thermal Dummy Cell for Development of Lithium-Ion Battery Thermal Management System. Energies. 2021; 14(5):1357. https://doi.org/10.3390/en14051357
Chicago/Turabian StyleLi, Wei, Shusheng Xiong, Xiaojun Zhou, Wei Shi, Chongming Wang, Xianke Lin, and Junjie Cheng. 2021. "Design of Cylindrical Thermal Dummy Cell for Development of Lithium-Ion Battery Thermal Management System" Energies 14, no. 5: 1357. https://doi.org/10.3390/en14051357






