The Effect of Hydroxylated Multi-Walled Carbon Nanotubes on the Properties of Peg-Cacl2 Form-Stable Phase Change Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of the PEG1500·CaCl2
2.3. Preparation of the PEG1500·CaCl2(1:3)/MWCNTs
2.4. Characterization
3. Results and Discussion
3.1. Thermal Characterization
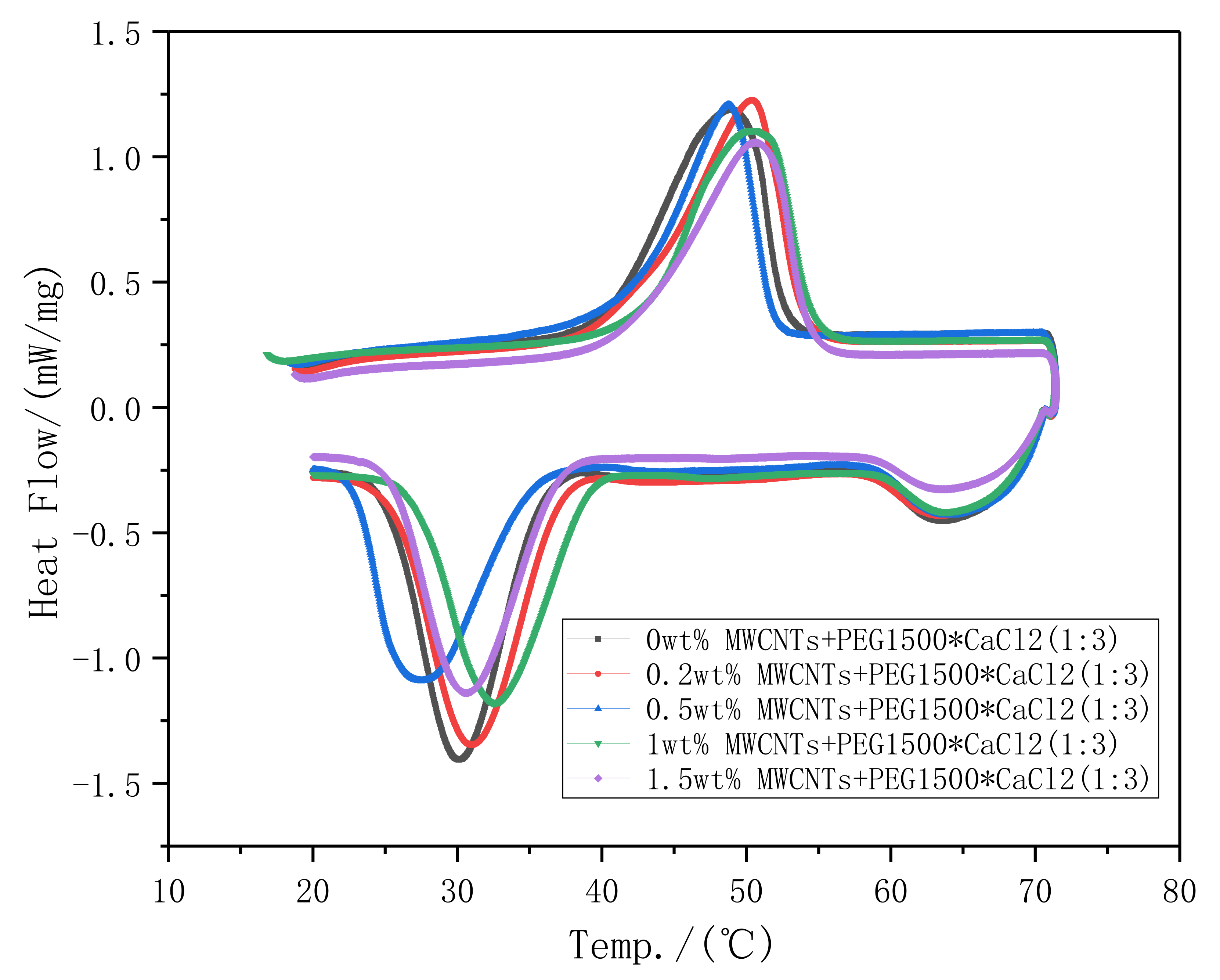
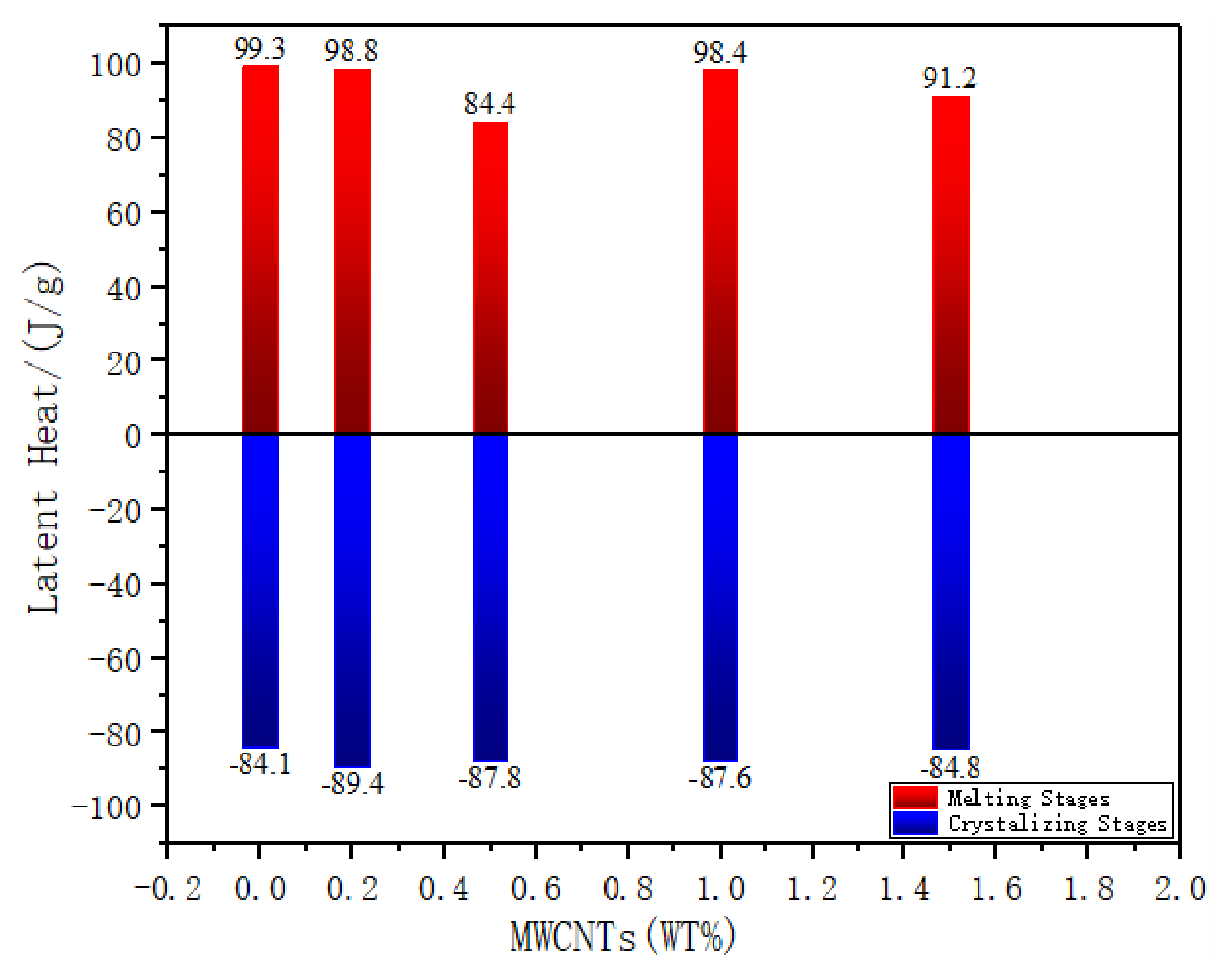
3.1.1. Latent Heat
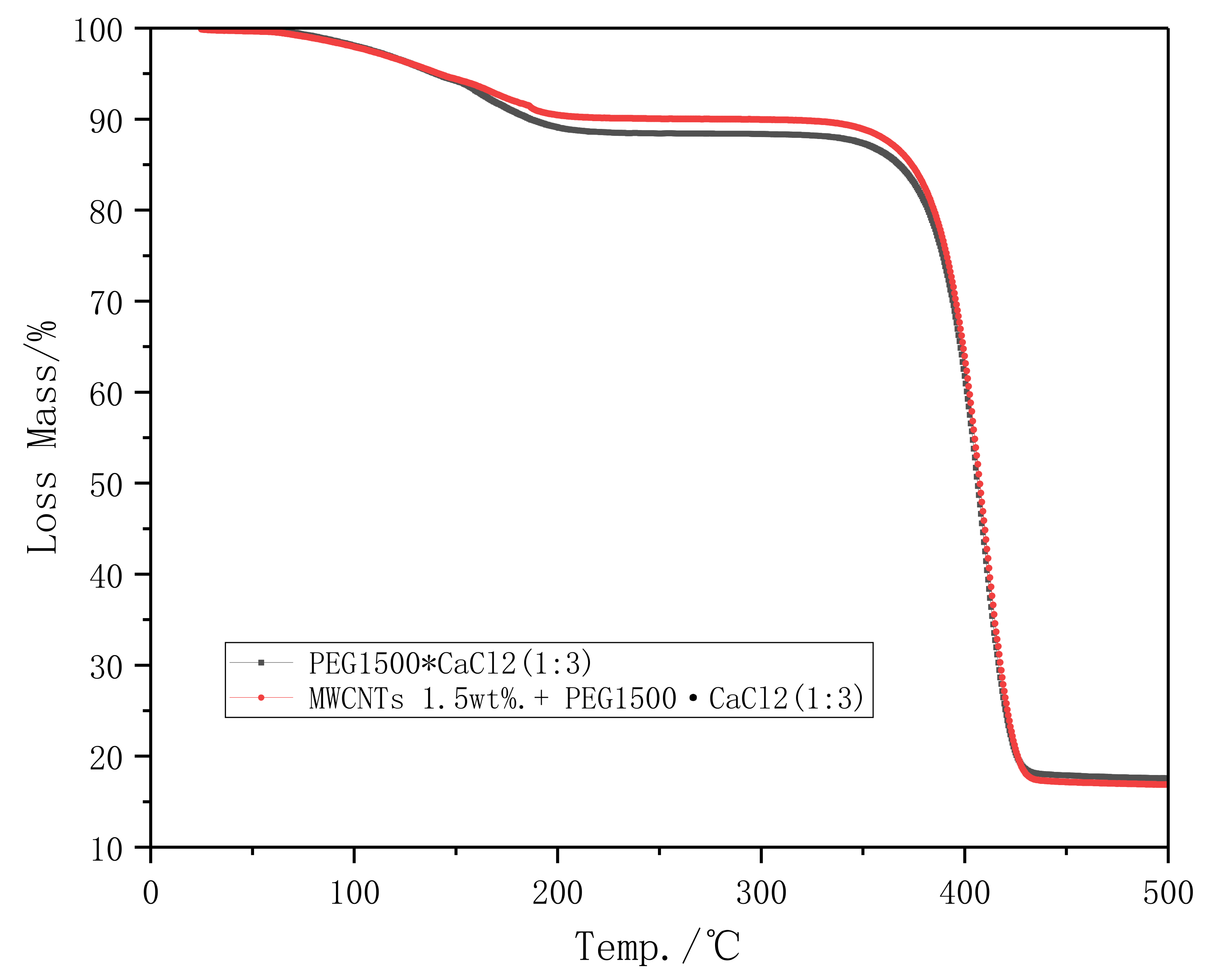
3.1.2. Thermal Stability Analysis
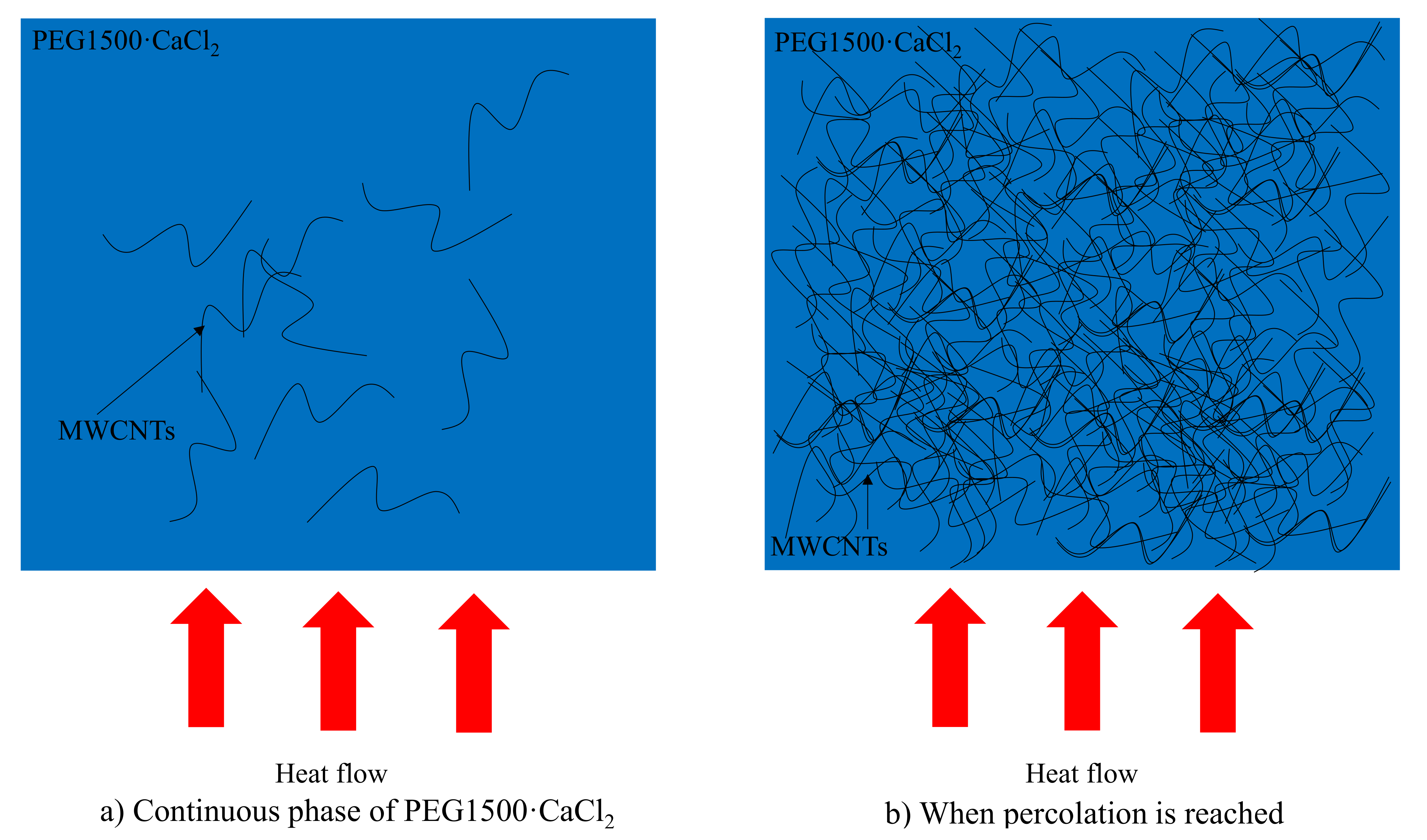
3.1.3. Thermal Conductivity
3.2. Shape Stability of PEG1500·CaCl2/MWCNTs Composites
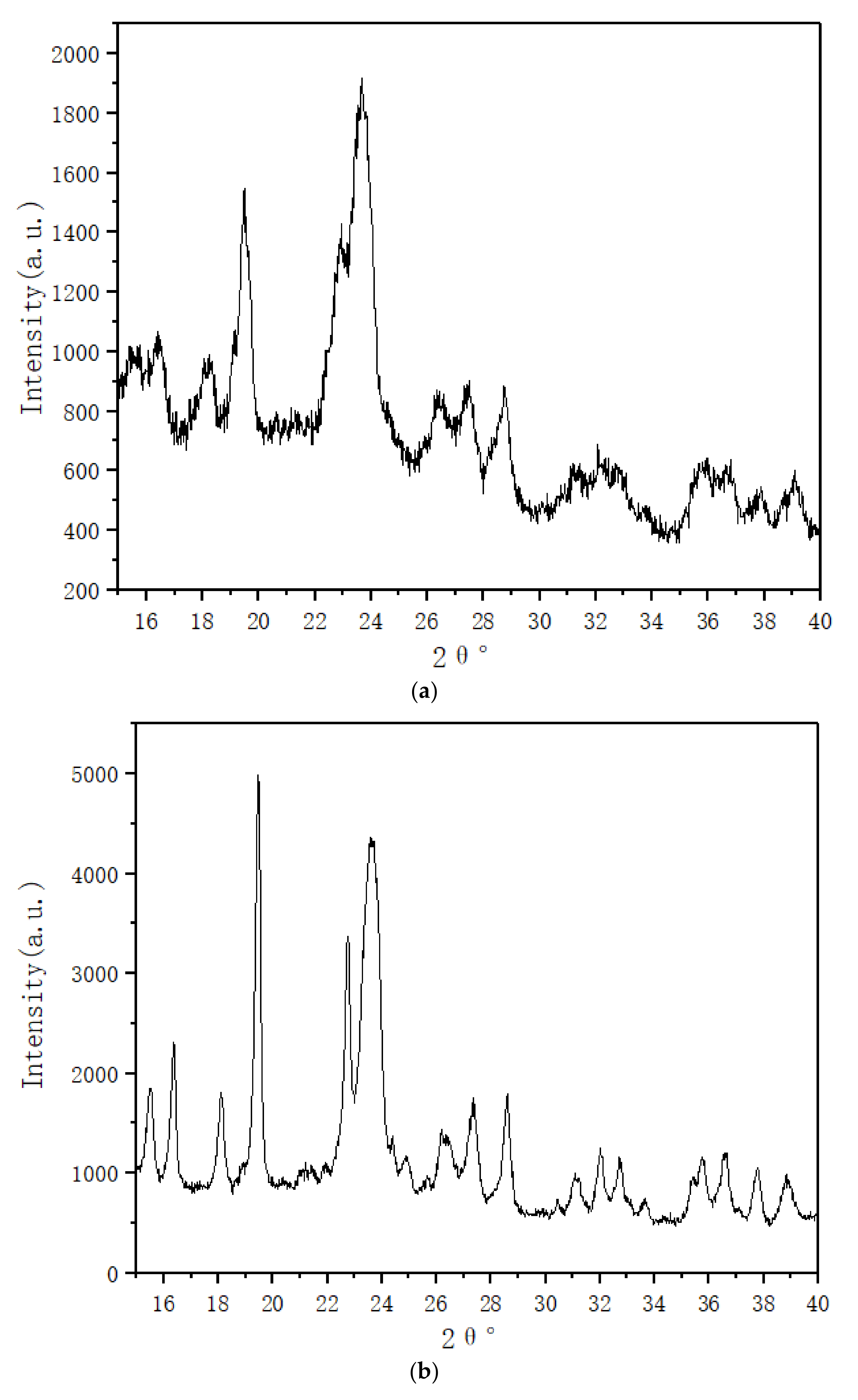
3.3. XRD and FT-IR Characterization
4. Conclusions
- (1)
- Phase change temperature increases with the increasing of MWCNTs mass fraction, while the latent heat decreasing. non MWCNTs material’s phase change temperature is 40.3 °C, and its latent heat is 99.3 J/g, compared to PEG1500·CaCl2(1:3)/MWCNTs 1.5 wt% that has the highest phase change temperature (42 °C). An obvious phase change hysteresis is found, which due to the uneven size of polymer crystals, and the speed of the recrystallization process is different. The sample in this study will not melt into a liquid at the target temperature, which makes the internal heat transfer mechanism and rate of the sample inconsistent. During the heating process, the internal sample’s temperature the is lower than the temperature of the surface, and during the cooling process the sample temperature is lower than estimated, so the peak will shift to a lower temperature, aggravating the hysteresis of the DSC curve.
- (2)
- TG curve indicating that MWCNTs does not help to improve the thermal stability of PEG1500·CaCl2 (1:3), which may be due to the low content.
- (3)
- The thermal conductivity of pure PEG1500·CaCl2 is 0.23 W/(m·K). When 0.2, 0.5, 1, and 1.5 wt% MWCNTs are added, their thermal conductivities increase to 0.41, 0.64, 0.78, and 0.90 W/(m·K). This is due to the large average phonon free path of MWCNTs, which can improve the mean free path of phonons effectively, and the added MWCNTs effectively reduce the interface thermal resistance between PCM molecules. The results of this study also showed the thermal conductivity double percolation mechanism of PEG1500·CaCl2(1:3)/MWCNTs.
- (4)
- XRD patterns indicating that additional MWCNTs do not affect the crystal structure of PEG1500·CaCl2, but they increase the polymer interplanar spacing. FT-IR analyses shows that the complex has an association reaction.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pineda, I. Challenges and Opportunities in Renewable Energy Developments; Palgrave Macmillan: Cham, Switzerland, 2021. [Google Scholar]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados-Focil, S.; Van Dessel, S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- Rostami, S.; Afrand, M.; Shahsavar, A.; Sheikholeslami, M.; Kalbasi, R.; Aghakhani, S.; Shadloo, M.S.; Oztop, H.F. A review of melting and freezing processes of PCM/nano-PCM and their application in energy storage. Energy 2020, 211. [Google Scholar] [CrossRef]
- Mao, Q.; Zhang, Y. Thermal energy storage performance of a three-PCM cascade tank in a high-temperature packed bed system. Renew. Energy 2020, 152, 110–119. [Google Scholar] [CrossRef]
- Manoj Kumar, P.; Mylsamy, K.; Saravanakumar, P.T. Experimental investigations on thermal properties of nano-SiO2/paraffin phase change material (PCM) for solar thermal energy storage applications. Energy Sourcespart A Recovery Util. Environ. Eff. 2019, 42, 2420–2433. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Ji, J.; Hua, W.; Mao, F. Preparation and characterization of urea/ammonia bromide composite phase change material. Int. J. Energy Res. 2020, 44, 3626–3639. [Google Scholar] [CrossRef]
- Gholamibozanjani, G.; Farid, M. A comparison between passive and active PCM systems applied to buildings. Renew. Energy 2020, 162, 112–123. [Google Scholar] [CrossRef]
- Beemkumar, N.; Yuvarajan, D.; Arulprakasajothi, M.; Elangovan, K.; Arunkumar, T. Control of room temperature fluctuations in the building by incorporating PCM in the roof. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Gholamibozanjani, G.; Farid, M. Application of an active PCM storage system into a building for heating/cooling load reduction. Energy 2020, 210, 118572. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Sardari, P.T.; Bashir, M.A.; Faraji, H.; Yan, Y. Transient simulation of finned heat sinks embedded with PCM for electronics cooling. Therm. Sci. Eng. Prog. 2020, 18, 100520. [Google Scholar] [CrossRef]
- Reuben Raj, C.; Suresh, S.; Vasudevan, S.; Chandrasekar, M.; Kumar Singh, V.; Bhavsar, R.R. Thermal performance of nano-enriched form-stable PCM implanted in a pin finned wall-less heat sink for thermal management application. Energy Convers. Manag. 2020, 226, 113466. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, P.; Zhu, J. Thermal management of electronic devices using pin-fin based cascade microencapsulated PCM/expanded graphite composite. Int. J. Heat Mass Transf. 2020, 149, 119119. [Google Scholar] [CrossRef]
- McCarthy, L.K.; di Marzo, M. The Application of Phase Change Material in Fire Fighter Protective Clothing. Fire Technol. 2011, 48, 841–864. [Google Scholar] [CrossRef]
- Du, J.; Nie, B.; Zhang, Y.; Du, Z.; Wang, l.; Ding, Y. Cooling performance of a thermal energy storage-based portable box for cold chain applications. J. Energy Storage 2020, 28, 101238. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Xu, X.; Zhang, S. Research progress of phase change cold storage materials used in cold chain transportation and their different cold storage packaging structures. J. Mol. Liq. 2020, 319, 114360. [Google Scholar] [CrossRef]
- Wan, X.; Chen, C.; Tian, S.; Guo, B. Thermal characterization of net-like and form-stable ML/SiO2 composite as novel PCM for cold energy storage. J. Energy Storage 2020, 28, 101276. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, J.; Xu, T.; Fang, X.; Gao, X.; Zhang, Z. Thermal conductivity of an organic phase change material/expanded graphite composite across the phase change temperature range and a novel thermal conductivity model. Energy Convers. Manag. 2015, 102, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Wang, G.; Xu, C.; Ju, X.; Xing, L.; Du, X.; Yang, Y. Selection principles and thermophysical properties of high temperature phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2018, 81, 1771–1786. [Google Scholar] [CrossRef]
- Yu, Q.; Tchuenbou-Magaia, F.; Al-Duri, B.; Zhang, Z.; Ding, Y.; Li, Y. Thermo-mechanical analysis of microcapsules containing phase change materials for cold storage. Appl. Energy 2018, 211, 1190–1202. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.; Ouyang, D.; Yang, X.; Chen, M.; Zhang, G.; Wang, J. Alleviation of thermal runaway propagation in thermal management modules using aerogel felt coupled with flame-retarded phase change material. Energy Convers. Manag. 2019, 200, 112071. [Google Scholar] [CrossRef]
- Kumar, U.A.; Satyanarayana PV, V.; Srikanth, T. Influence of Polyethylene Glycol on Asphaltic Concrete for Cubical and Rod shaped Aggregates. J. Eng. Res. Appl. 2014, 4, 6. [Google Scholar]
- Jiang, Y.; Ding, E.; Li, G. Study on transition characteristics of PEG/CDA solid–solid phase change materials. Polymer 2002, 43, 5. [Google Scholar] [CrossRef]
- Paberit, R.; Rilby, E.; Göhl, J.; Swenson, J.; Refaa, Z.; Johansson, P.; Jansson, H. Cycling Stability of Poly(ethylene glycol) of Six Molecular Weights: Influence of Thermal Conditions for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 10578–10589. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Tas, C.E.; Unal, H. Thermally buffering polyethylene/halloysite/phase change material nanocomposite packaging films for cold storage of foods. J. Food Eng. 2021, 292. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Chen, K.; Wang, C.; Wang, T.; Zhu, Z.; Ma, R.; Jiang, H. Preparation and performances of form-stable polyethylene glycol/methylcellulose composite phase change materials. J. Polym. Res. 2020, 27. [Google Scholar] [CrossRef]
- Meng, Y.; Majoinen, J.; Zhao, B.; Rojas, O.J. Form-stable phase change materials from mesoporous balsa after selective removal of lignin. Compos. Part B Eng. 2020, 199. [Google Scholar] [CrossRef]
- Fang, H.; Lin, J.; Zhang, L.; Chen, A.; Wu, F.; Geng, L.; Peng, X. Fibrous form-stable phase change materials with high thermal conductivity fabricated by interfacial polyelectrolyte complex spinning. Carbohydr. Polym. 2020, 249, 116836. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Q.; Yuan, Y.; Zhang, Z.; Cao, X. A novel form-stable phase change composite with excellent thermal and electrical conductivities. Chem. Eng. J. 2018, 336, 342–351. [Google Scholar] [CrossRef]
- Heyhat, M.M.; Mousavi, S.; Siavashi, M. Battery thermal management with thermal energy storage composites of PCM, metal foam, fin and nanoparticle. J. Energy Storage 2020, 28. [Google Scholar] [CrossRef]
- Sardari, P.T.; Babaei-Mahani, R.; Giddings, D.; Yasseri, S.; Moghimi, M.A.; Bahai, H. Energy recovery from domestic radiators using a compact composite metal Foam/PCM latent heat storage. J. Clean. Prod. 2020, 257. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, L.; Zou, B.; Qiao, G.; Zhang, T.; Cong, L.; Jiang, F.; Li, C.; Huang, Y.; Ding, Y. Expanded graphite—Paraffin composite phase change materials: Effect of particle size on the composite structure and properties. Appl. Therm. Eng. 2020, 171. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, Y.; Liu, Y.; Guo, H. Preparation and thermal properties of polyethylene glycol/expanded graphite as novel form-stable phase change material for indoor energy saving. Mater. Lett. 2018, 216, 220–223. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Xu, X.; Zhao, Y. Experimental study on the storage and release characteristics of phase change materials with different nanomaterials as addictives. Heat Mass Transf. 2020, 56, 2769–2777. [Google Scholar] [CrossRef]
- Bandini, M.; Benaglia, M.; Sinisi, R.; Tommasi, S.; Umani-Ronchi, A. Recoverable PEG-supported copper catalyst for highly stereocontrolled nitroaldol condensation. Org. Lett. 2010, 9, 2151–2153. [Google Scholar] [CrossRef]
- Zheng, J.; Smith Callahan, L.A.; Hao, J.; Guo, K.; Wesdemiotis, C.; Weiss, R.A.; Becker, M.L. Strain-Promoted Cross-Linking of PEG-Based Hydrogels via Copper-Free Cycloaddition. ACS Macro Lett. 2012, 1, 1071. [Google Scholar] [CrossRef] [Green Version]
- Qian, T.; Li, J.; Min, X.; Guan, W.; Deng, Y.; Ning, L. Enhanced thermal conductivity of PEG/diatomite shape-stabilized phase change materials with Ag nanoparticles for thermal energy storage. J. Mater. Chem. A 2015, 3, 8526–8536. [Google Scholar] [CrossRef]
- Pitie, F.; Zhao, C.Y.; Caceres, G. Thermo-mechanical analysis of ceramic encapsulated phase-change-material (PCM) particles. Energy Environ. Ence 2011, 4, 2117–2124. [Google Scholar] [CrossRef]
- Fukahori, R.; Nomura, T.; Zhu, C.; Sheng, N.; Okinaka, N.; Akiyama, T. Thermal analysis of Al–Si alloys as high-temperature phase-change material and their corrosion properties with ceramic materials. Appl. Energy 2016, 163, 1–8. [Google Scholar] [CrossRef]
- Koo, K.; Choe, J.; Park, Y. The application of PCMMcs and SiC by commercially direct dual-complex coating on textile polymer. Appl. Surf. Sci. 2009, 255, 8313–8318. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lahiri, B.B.; Philip, J. Carbon black nano particle loaded lauric acid-based form-stable phase change material with enhanced thermal conductivity and photo-thermal conversion for thermal energy storage. Energy 2020, 191, 116572. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lahiri, B.B.; Philip, J. Superior thermal conductivity and photo-thermal conversion efficiency of carbon black loaded organic phase change material—ScienceDirect. J. Mol. Liq. 2019, 285, 640–657. [Google Scholar] [CrossRef]
- Kumaresan, V.; Velraj, R.; Das, S.K. The effect of carbon nanotubes in enhancing the thermal transport properties of PCM during solidification. Heat Mass Transf. 2012, 48, 1345–1355. [Google Scholar] [CrossRef]
- Lian, C.; Wang, B.; Chen, J.; Yong, Q.; Huang, Y.; Liao, B. Dispersion of Multi-Walled Carbon Nanotubes by Polymers with Carbazole Pendants. J. Phys. Chem. B 2017, 121, 8408–8416. [Google Scholar] [CrossRef]
- Liang, J.; Jeyasingh, R.G.D.; Chen, H.Y.; Wong, H.S.P. An Ultra-Low Reset Current Cross-Point Phase Change Memory With Carbon Nanotube Electrodes. IEEE Trans. Electron Devices 2012, 59, 1155–1163. [Google Scholar] [CrossRef]
- Frusteri, F.; Leonardi, V.; Vasta, S.; Restuccia, G. Thermal conductivity measurement of a PCM based storage system containing carbon fibers. Appl. Therm. Eng. 2005, 25, 1623–1633. [Google Scholar] [CrossRef]
- Mayani, S.V.; Mayani, V.J.; Kim, S.W. Development of novel porous carbon frameworks through hydrogen-bonding interaction and its ethylene adsorption activity. J. Porous Mater. 2012, 19, 519–527. [Google Scholar] [CrossRef]
- Guangyu, C.; Quanfang, C. Characterization Study of the Thermal Conductivity of Carbon Nanotube Copper Nanocomposites. J. Compos. Mater. 2010, 44, 2863–2873. [Google Scholar] [CrossRef]
- Magendran, S.S.; Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.; Karri, R.R. Synthesis of organic phase change materials by using carbon nanotubes as filler material. Nano Struct. Nano Objects 2019, 19. [Google Scholar] [CrossRef]
- Cao, R.; Chen, S.; Wang, Y.; Han, N.; Liu, H.; Zhang, X. Functionalized carbon nanotubes as phase change materials with enhanced thermal, electrical conductivity, light-to-thermal, and electro-to-thermal performances. Carbon 2019, 149, 263–272. [Google Scholar] [CrossRef]
- Sagar, S. MWCNTS Incorporated Natural Rubber Composites: Thermal Insulation, Phase Transition and Mechanical Properties. Int. J. Eng. Technol. 2014, 6, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Barz, T.; Sommer, A. Modeling hysteresis in the phase transition of industrial-grade solid/liquid PCM for thermal energy storages. Int. J. Heat Mass Transf. 2018, 127, 701–713. [Google Scholar] [CrossRef]
- Gomez-Heredia, C.L.; Ramirez-Rincon, J.A.; Bhardwaj, D.; Rajasekar, P.; Tadeo, I.J.; Cervantes-Lopez, J.L.; Ordonez-Miranda, J.; Ares, O.; Umarji, A.M.; Drevillon, J.; et al. Measurement of the hysteretic thermal properties of W-doped and undoped nanocrystalline powders of VO2. Sci. Rep. 2019, 9, 14687. [Google Scholar] [CrossRef] [Green Version]
- Tariq, M.; Hameed, S.; Bechtold, I.H.; Bortoluzzi, A.J.; Merlo, A.A. Synthesis and characterization of some novel tetrazole liquid crystals. J. Mater. Chem. C 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Xu, X.; Zhang, X.; Yin, Y. Determination of the PCM melting temperature range using DSC. Thermochim. Acta 2014, 595, 17–21. [Google Scholar] [CrossRef]
- Ivanovic, M.; Svicevic, M.; Savovic, S. Numerical solution of Stefan problem with variable space grid method based on mixed finite element/finite difference approach. Int. J. Numer. Methods Heat Fluid Flow 2017, 27, 2682–2695. [Google Scholar] [CrossRef]
- Savović, S.; Caldwell, J. Finite difference solution of one-dimensional Stefan problem with periodic boundary conditions. Int. J. Heat Mass Transf. 2003, 46, 2911–2916. [Google Scholar] [CrossRef]
- Li, R.; Shan, Z. Enhancement of thermal conductivity of PEG-PPG-based waterborne polyurethane coating by incorporating ordered polyethylene glycol fragment. Polym. Test. 2018, S0142941818301247. [Google Scholar] [CrossRef]
- Han, W.; Ge, C.; Zhang, R.; Ma, Z.; Wang, L.; Zhang, X. Boron nitride foam as a polymer alternative in packaging phase change materials: Synthesis, thermal properties and shape stability. Appl. Energy 2019, 238, 942–951. [Google Scholar] [CrossRef]
- Bahrani, S.A.; Royon, L.; Abou, B.; Osipian, R.; Azzouz, K.; Bontemps, A. A phenomenological approach of solidification of polymeric phase change materials. J. Appl. Phys. 2017, 121, 035103. [Google Scholar] [CrossRef]
- Sääskilahti, K.; Oksanen, J.; Volz, S.; Tulkki, J. Frequency-dependent phonon mean free path in carbon nanotubes from nonequilibrium molecular dynamics. Phys. Rev. B 2015, 91. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Liu, C.; Hu, S.; Yu, X. The experimental exploration of carbon nanofiber and carbon nanotube additives on thermal behavior of phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 1208–1212. [Google Scholar] [CrossRef]
- Prasher, R.S.; Hu, X.J.; Chalopin, Y.; Mingo, N.; Lofgreen, K.; Volz, S.; Cleri, F.; Keblinski, P. Turning Carbon Nanotubes from Exceptional Heat Conductors into Insulators. Phys. Rev. Lett. 2009, 102, 105901. [Google Scholar] [CrossRef]
- Kalidoss, P.; Venkatachalapathy, S.; Suresh, S. Optical and Thermal Properties of Therminol 55-TiO2 Nanofluids for Solar Energy Storage. Int. J. Photoenergy 2020, 2020, 7085497. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, A.; Lorenz, T.; Breitkopf, C. Thermal Conductivity of Polyisoprene and Polybutadiene from Molecular Dynamics Simulations and Transient Measurements. Polymers 2020, 12, 1081. [Google Scholar] [CrossRef]
- Zhong, H.; Lukes, J.R. Interfacial thermal resistance between carbon nanotubes: Molecular dynamics simulations and analytical thermal modeling. Phys. Rev. B Condens. Matter 2006, 74, 125403. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xie, H.; Xin, Z.; Li, Y.; Chen, L. Enhancing thermal conductivity of palmitic acid based phase change materials with carbon nanotubes as fillers. Sol. Energy 2010, 84, 339–344. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Y.; Xu, L.; Chen, J.; Jiang, W.; Nie, X. Massive enhancement in the thermal conductivity of polymer composites by trapping graphene at the interface of a polymer blend. Compos. Sci. Technol. 2016, 129, 160–165. [Google Scholar] [CrossRef]
- Zhang, D.-L.; Zha, J.-W.; Li, C.-Q.; Li, W.-K.; Wang, S.-J.; Wen, Y.; Dang, Z.-M. High thermal conductivity and excellent electrical insulation performance in double-percolated three-phase polymer nanocomposites. Compos. Sci. Technol. 2017, 144, 36–42. [Google Scholar] [CrossRef]
- Wu, K.; Xue, Y.; Yang, W.; Chai, S.; Chen, F.; Fu, Q. Largely enhanced thermal and electrical conductivity via constructing double percolated filler network in polypropylene/expanded graphite—Multi-wall carbon nanotubes ternary composites. Compos. Sci. Technol. 2016, 130, 28–35. [Google Scholar] [CrossRef]
- Hawlader, M.N.A.; Uddin, M.S.; Khin, M.M. Microencapsulated PCM thermal-energy storage system. Appl. Energy 2003, 74, 195–202. [Google Scholar] [CrossRef]
- Shirin-Abadi, A.R.; Mahdavian, A.R.; Khoee, S. New Approach for the Elucidation of PCM Nanocapsules through Miniemulsion Polymerization with an Acrylic Shell. Macromolecules 2011, 44, 7405–7414. [Google Scholar] [CrossRef]
- Ma, X.H.; Xu, Z.L.; Ji, C.Q.; Wei, Y.M.; Yang, H. Characterization, separation performance, and model analysis of STPP-chitosan/PAN polyelectrolyte complex membranes. J. Appl. Polym. Sci. 2011, 120, 1017–1026. [Google Scholar] [CrossRef]
- Mu, S.; Guo, J.; Zhang, S.; An, Q.; Wang, D.; Liu, Y.; Guan, F. Preparation and thermal properties of cross-linked poly(acrylonitrile-co-itaconate)/polyethylene glycol as novel form-stable phase change material for thermal energy storage. Mater. Lett. 2016, 171, 23–26. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, N.; Jing, Y.; Cao, X.; Yuan, Y.; Haghighat, F. Experimental and numerical investigation on dodecane/expanded graphite shape-stabilized phase change material for cold energy storage. Energy 2019, 189, 116175. [Google Scholar] [CrossRef]
- Ren, X.; Shen, H.; Yang, Y.; Yang, J. Study on the properties of a novel shape-stable epoxy resin sealedexpanded graphite/paraffin composite PCM and itsapplication in buildings. Phase Transit. 2019, 92, 581–594. [Google Scholar] [CrossRef]
- Ng, D.Q.; Tseng, Y.L.; Shih, Y.F.; Lian, H.Y.; Yu, Y.H. Synthesis of novel phase change material microcapsule and its application. Polymer 2017, 133, 250–262. [Google Scholar] [CrossRef]
- Basal, G.; Deveci, S.S.; Yalcin, D.; Bayraktar, O. Properties of n-Eicosane-Loaded Silk Fibroin-Chitosan Microcapsules. J. Appl. Polym. Sci. 2011, 121, 1885–1889. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Zhang, W.; Wang, C. Covalent bonding and photochromic properties of double-shell polyurethane-chitosan microcapsules crosslinked onto cotton fabric. Cellulose 2015, 22, 1427–1438. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Zhang, X.; Hua, W.; Wu, X.; Mao, F. The Effect of Hydroxylated Multi-Walled Carbon Nanotubes on the Properties of Peg-Cacl2 Form-Stable Phase Change Materials. Energies 2021, 14, 1403. https://doi.org/10.3390/en14051403
Zheng L, Zhang X, Hua W, Wu X, Mao F. The Effect of Hydroxylated Multi-Walled Carbon Nanotubes on the Properties of Peg-Cacl2 Form-Stable Phase Change Materials. Energies. 2021; 14(5):1403. https://doi.org/10.3390/en14051403
Chicago/Turabian StyleZheng, Lingyu, Xuelai Zhang, Weisan Hua, Xinfeng Wu, and Fa Mao. 2021. "The Effect of Hydroxylated Multi-Walled Carbon Nanotubes on the Properties of Peg-Cacl2 Form-Stable Phase Change Materials" Energies 14, no. 5: 1403. https://doi.org/10.3390/en14051403




