Surface Roughness-Governed Shape Stability of the Coal Fly Ash-Based Phase Change Material: Molten Salt Processing and Thermal Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials and Chemical Reagents
2.2. Thermal Molten Salt Processing of Fly Ash
2.3. Preparation of Phase-Change Composite Material (PCCM)
2.4. Characterizations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhang, S.; Wang, Q.; Ni, W.; Li, K.; Fu, P.; Hu, W.; Li, Z. Feasibility of using fly ash-slag-based binder for mine backfilling and its associated leaching risks. J. Hazard. Mater. 2020, 400, 123191. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Suárez-Navarro, J.A.; Argiz, C.; Mora, P. Assessment of natural radioactivity and radiation hazards owing to coal fly ash and natural pozzolan Portland cements. J. Radioanal. Nucl. Chem. 2020, 325, 381–390. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, Y.; Yuan, K.; Tian, T.; Yi, Y. Mechanical properties of vertical vibration compacted lime–fly ash-stabilized macadam material. Constr. Build. Mater. 2020, 251, 119089. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Zeynali Famileh, I.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Hu, H. Recent advances of polymeric phase change composites for flexible electronics and thermal energy storage system. Compos. Part B Eng. 2020, 195, 108094. [Google Scholar] [CrossRef]
- Wen, R.; Zhang, X.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X.; Min, X.; Gao, W.; Huang, S. Preparation and thermal properties of fatty acid/diatomite form-stable composite phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 273–279. [Google Scholar] [CrossRef]
- Song, S.; Zhao, T.; Qiu, F.; Zhu, W.; Chen, T.; Guo, Y.; Zhang, Y.; Wang, Y.; Feng, R.; Liu, Y.; et al. Natural microtubule encapsulated phase change material with high thermal energy storage capacity. Energy 2019, 172, 1144–1150. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, X.; Ma, Z.W. A review of the composite phase change materials: Fabrication, characterization, mathematical modeling and application to performance enhancement. Appl. Energy 2016, 165, 472–510. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Kholmanov, I.; Kim, J.; Ou, E.; Ruoff, R.S.; Shi, L. Continuous Carbon Nanotube–Ultrathin Graphite Hybrid Foams for Increased Thermal Conductivity and Suppressed Subcooling in Composite Phase Change Materials. ACS Nano 2015, 9, 11699. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Experiment study on the thermal properties of paraffin/kaolin thermal energy storage form-stable phase change materials. Appl. Energy 2016, 182, 475–487. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of capric acid/expanded perlite composite for thermal energy storage. Mater. Chem. Phys. 2008, 109, 459–464. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Lu, Y.; Jin, Z.; Tan, L.; Gao, H.; Fan, S.; Dong, W.; Wang, G. Surface functionalization engineering driven crystallization behavior of polyethylene glycol confined in mesoporous silica for shape-stabilized phase change materials. Nano Energy 2016, 19, 78–87. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Thermal properties and heat storage analysis of palmitic acid-TiO 2 composite as nano-enhanced organic phase change material (NEOPCM). Appl. Therm. Eng. 2016, 99, 1254–1262. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.-Q.; Liu, Y.; Bao, R.-Y.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Hybrid graphene aerogels/phase change material composites: Thermal conductivity, shape-stabilization and light-to-thermal energy storage. Carbon 2016, 100, 693–702. [Google Scholar] [CrossRef]
- Ji, H.; Sellan, D.P.; Pettes, M.T.; Kong, X.; Ji, J.; Shi, L.; Ruoff, R.S. Enhanced thermal conductivity of phase change materials with ultrathin-graphite foams for thermal energy storage. Energy Environ. Sci. 2014, 7, 1185–1192. [Google Scholar] [CrossRef]
- Qiu, F.; Li, S.K.S.D.N.; Liu, Y.; Wang, Y.Q.; Dong, L.J. Experimental investigation on improvement of latent heat and thermal conductivity of shape-stable phase-change materials using modified fly ash. J. Clean. Prod. 2020, 246, 118952. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Yi, Z.; Yan, L.; Liu, S.; Yao, X.; Guo, A.; Liu, J.; Hou, F. Multiscale mullite fiber/whisker reinforced silica aerogel nanocomposites with enhanced compressive strength and thermal insulation performance. Ceram. Int. 2020, 46, 28561–28568. [Google Scholar] [CrossRef]
- Pu, D.M.; Xiao, P.J.C.P.; Li, Z.; Deng, P.Y.; Pang, L.; Li, J.W.; Li, Y. Oxidation and thermal cycling behavior of c-AlPO4 and SiC whisker co-modified mullite deposited on SiC-C/SiC composites. Surf. Coat. Technol. 2020, 400, 126201. [Google Scholar] [CrossRef]
- Liu, D.Z.; Han, K.X.G.W.B.; Wang, G. Fabrication and properties of three-directional orthogonal aluminosilicate fiber fabric-reinforced mullite composite. Ceram. Int. 2020, 46, 23956–23963. [Google Scholar] [CrossRef]
- Ma, J.; He, B.D.C.; Zeng, S.H.; Hua, K.H.; Xi, X.A.; Luo, B.Y.; Shui, A.Z.; Tian, W. Corrosion Resistance Properties of Porous Alumina–Mullite Ceramic Membrane Supports. Adv. Eng. Mater. 2020, 22, 1901442. [Google Scholar] [CrossRef]
- Deshkar, A.; Youngman, O.G.R.E.; Mauroc, J.C.; Goel, A. Why does B2O3 suppress nepheline (NaAlSiO4) crystallization in sodium aluminosilicate glasses? Phys. Chem. Chem. Phys. 2020, 22, 8679. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Yadav, S.K.G.H.; Ranjane, P.; Kadam, R.M.; Achary, S.N.; Tyagi, A.K. A carnegieite type red emitting NaAlSiO4:Eu3+ phosphor: Concentration dependent time resolved photoluminescence and Judd-Ofelt analysis. J. Lumin. 2019, 209, 283–290. [Google Scholar] [CrossRef]
- Mahloujifar, M.M. Kaolinite fusion in carbonate media: KAlSiO4–NaAlSiO4 phase transformations and morphological study. Mater. Res. Express 2019, 6, 025040. [Google Scholar] [CrossRef]
- Zhao, M.; Molokeev, Z.G.X.M.S.; Ning, L.X.; Liu, Q.L. Temperature and Eu2+-Doping Induced Phase Selection in NaAlSiO4 Polymorphs and the Controlled Yellow/Blue Emission. Chem. Mater. 2017, 29, 6552–6559. [Google Scholar] [CrossRef]
- Deshkar, A.; Southern, J.M.S.A.; Kobera, L.; Bryce, D.L.; McCloy, J.S.; Goel, A. Understanding the structural origin of crystalline phase transformations in nepheline (NaAlSiO4)-based glass-ceramics. J. Am. Ceram. Soc. 2017, 100, 2859–2878. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ersoy, O. Fabrication and characterization of form-stable phase change material/xonotlite microcomposites. Sol. Energy Mater. Sol. Cells 2017, 168, 130–135. [Google Scholar] [CrossRef]
- Mu, S.Y.; Zhang, J.G.B.; Qi, S.W.; Yang, L.J.; Wang, D.; Zhang, S.; Yu, Y. On Preparation and Characterization of the Phase Change Nanofibers From the Copolymer of Poly(styrene-co-acrylonitrile) and Lauric Acid. J. Macromol. Sci. 2015, 52, 699–706. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, L.S.J.Y.; Cheng, J.; Wang, X.D. Fabrication of shape-stable composite phase change materials based on lauric acid and graphene/graphene oxide complex aerogels for enhancement of thermal energy storage and electrical conduction. Thermochim. Acta 2018, 664, 1–15. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wang, J.H.L.N.; Gao, Y.; Sun, H.; Li, W.L. A lauric acid-hybridized bentonite composite phase-changing material for thermal energy storage. RSC Adv. 2020, 10, 25864. [Google Scholar] [CrossRef]
- Wu, J.Q.; Xu, D.L.W.X.M.; Long, N.B.; Zhang, R.F. Efficient synthesis of DHA/EPA-rich phosphatidylcholine by inhibition of hydrolysis reaction using immobilized phospholipase A1 on macroporous SiO2/cationic polymer nano-composited support. Mol. Catal. 2021, 499, 111278. [Google Scholar] [CrossRef]
- Belgibayeva, A.; Taniguchi, I. Insights into the improved electrochemical performance of lithium–sulfur battery with free-standing SiO2/C composite nanofiber mat interlayer. J. Power Sources 2021, 484, 229308. [Google Scholar] [CrossRef]
- Belgibayeva, A.; Taniguchi, I. Synthesis and characterization of SiO2/C composite nanofibers as free-standing anode materials for Li-ion batteries. Electrochim. Acta 2019, 328, 135101. [Google Scholar] [CrossRef]
- Ma, J.H.; Zhou, Y.Y.L.X.R.; Yang, X.Y.; Alharthi, F.A.; Alghamdi, A.A.; Cheng, X.W.; Deng, Y.H. Au Nanoparticles Decorated Mesoporous SiO2–WO3 Hybrid Materials with Improved Pore Connectivity for Ultratrace Ethanol Detection at Low Operating Temperature. Small 2020, 16, 2004772. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Yubero, J.G.-R.F.; Espinos, J.P.; Gonzalez-Elipe, A.R. “In Operando” X-ray Absorption Spectroscopy Analysis of Structural Changes During Electrochemical Cycling of WO3 and WxSiyOz Amorphous Electrochromic Thin Film Cathodes. J. Phys. Chem. C 2015, 119, 644–652. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.P.Y.N.; Dong, Y.F.; Sun, Y.F.; Ji, W.H. Photo-to-thermal conversion and energy storage of lauric acid/expanded graphite composite phase change materials. Int. J. Energy Res. 2020, 44, 8555–8566. [Google Scholar] [CrossRef]
- Blohm, S.; Heinze, T. Mechanistic Considerations of Efficient Esterification of Starch with Propionic Anhydride/Lauric Acid in the Green Solvent Imidazole. Macromol. Chem. Phys. 2020, 221, 2000264. [Google Scholar] [CrossRef]
- Hirlekar, S.; Aswal, D.R.V.K.; Prabhune, A.A.; Nisal, A. Lauric Acid Sophorolipid: Accelerating the Gelation of Silk Fibroin. ACS Omega 2020, 5, 28571–28578. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Appukuttan, S. Preparation and thermal properties of lauric acid/myristyl alcohol as a novel binary eutectic phase change material for indoor thermal comfort. Energy Storage 2019, 1, e80. [Google Scholar] [CrossRef]


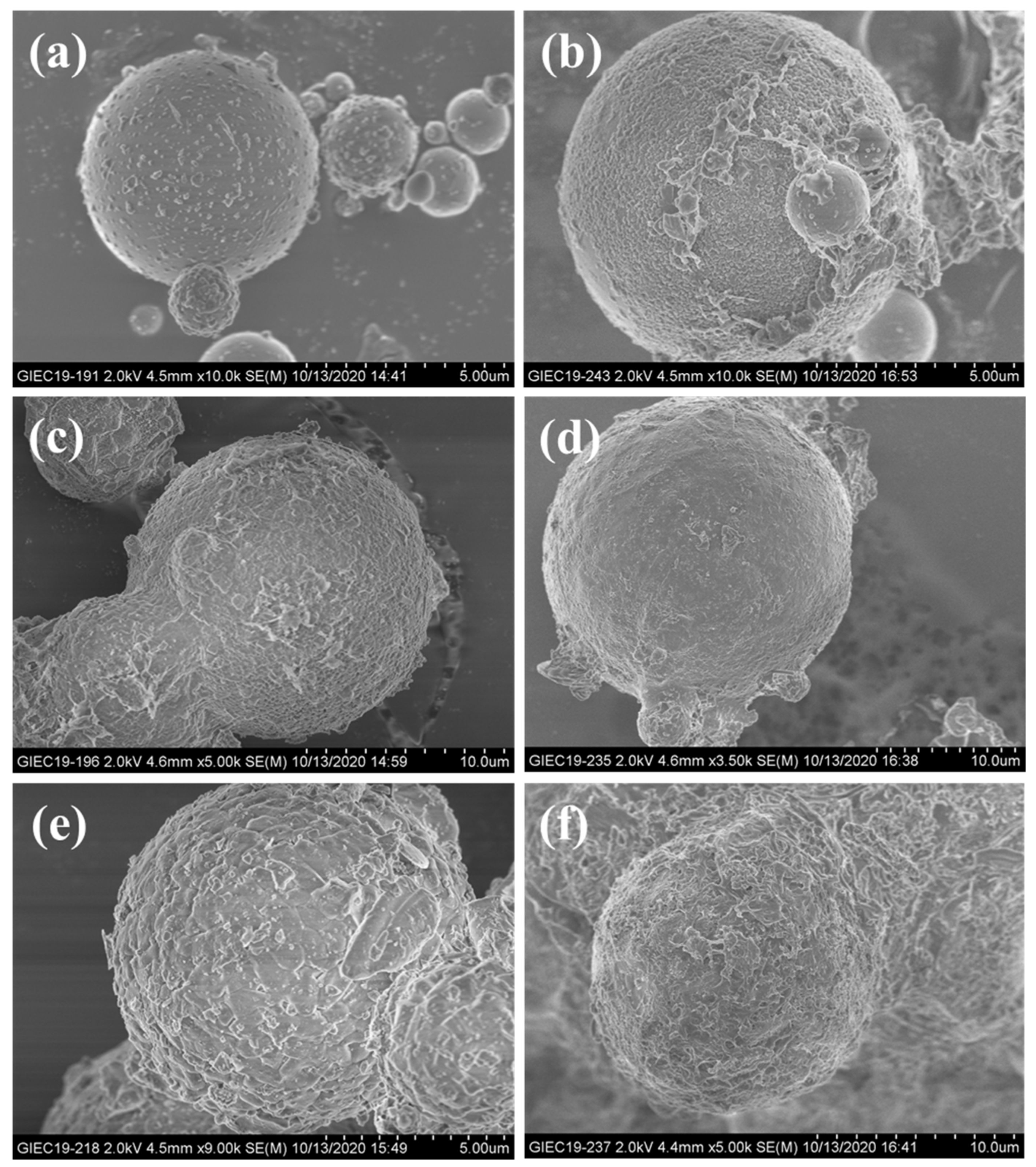
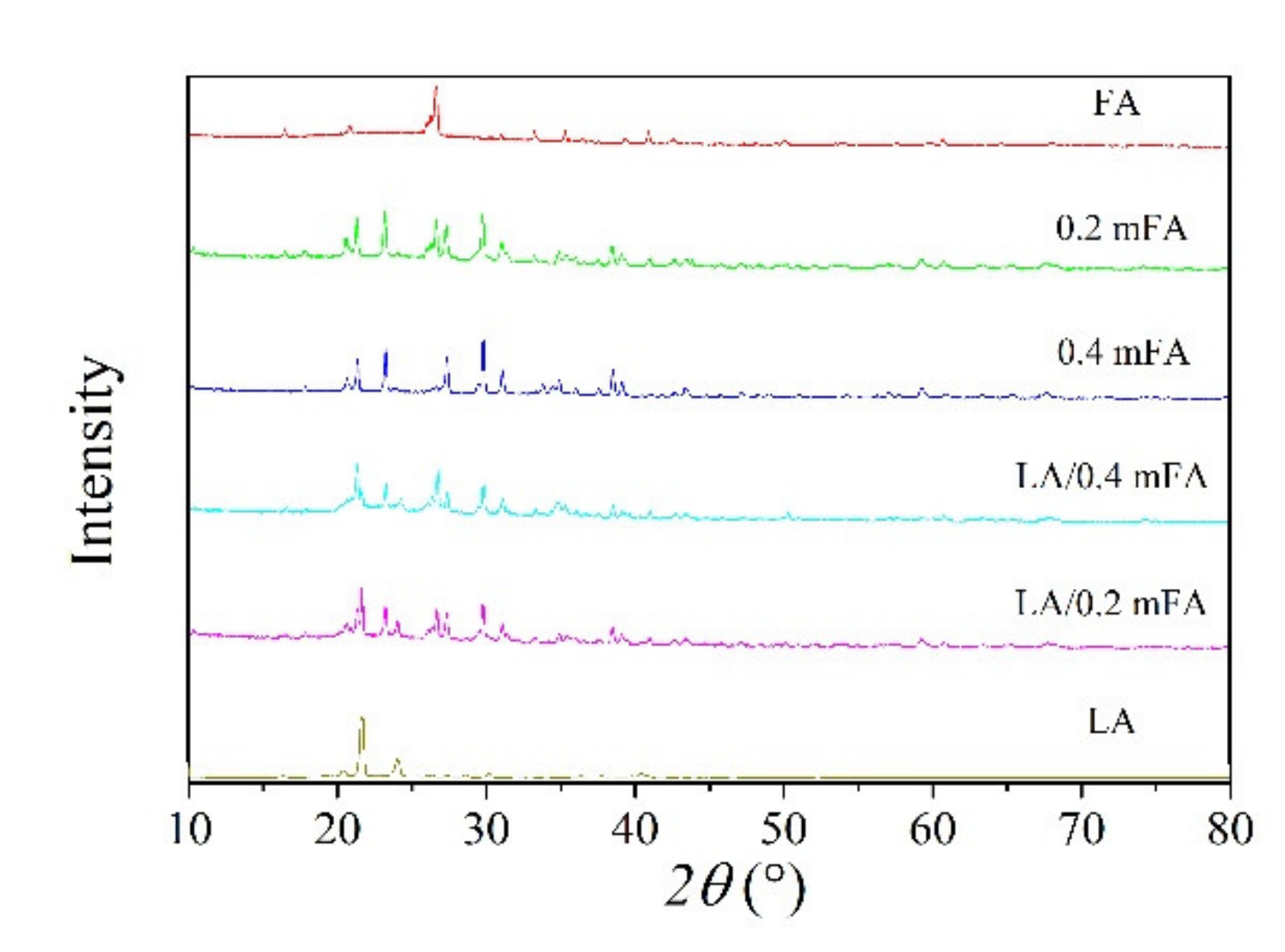
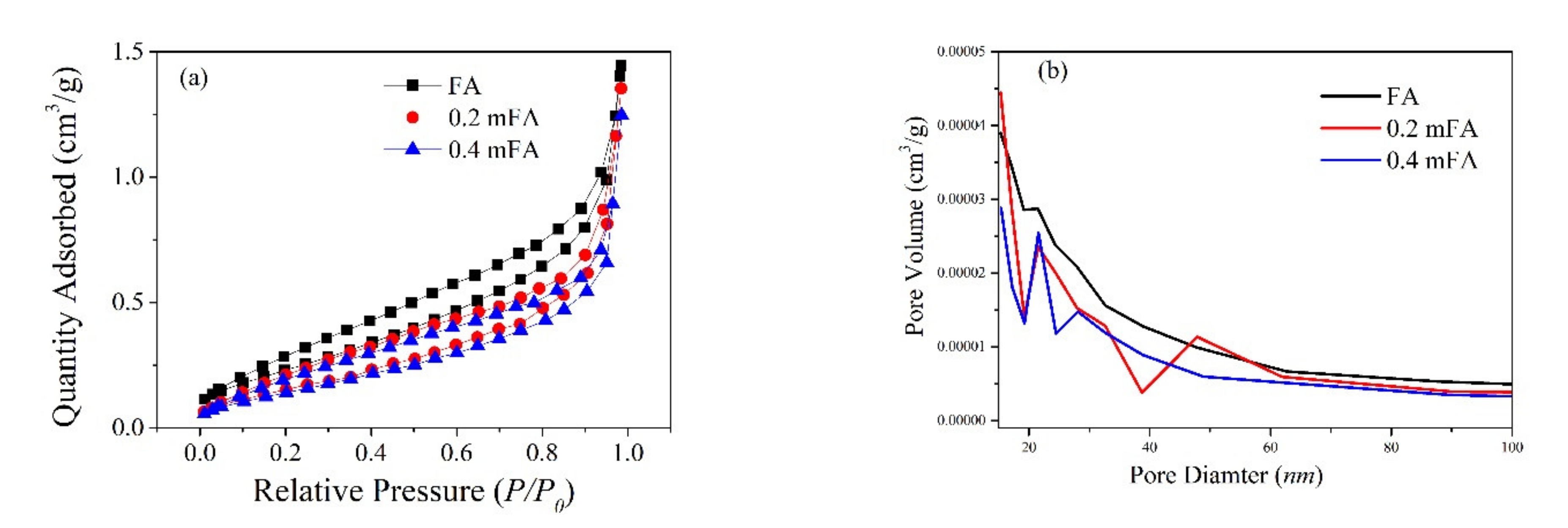

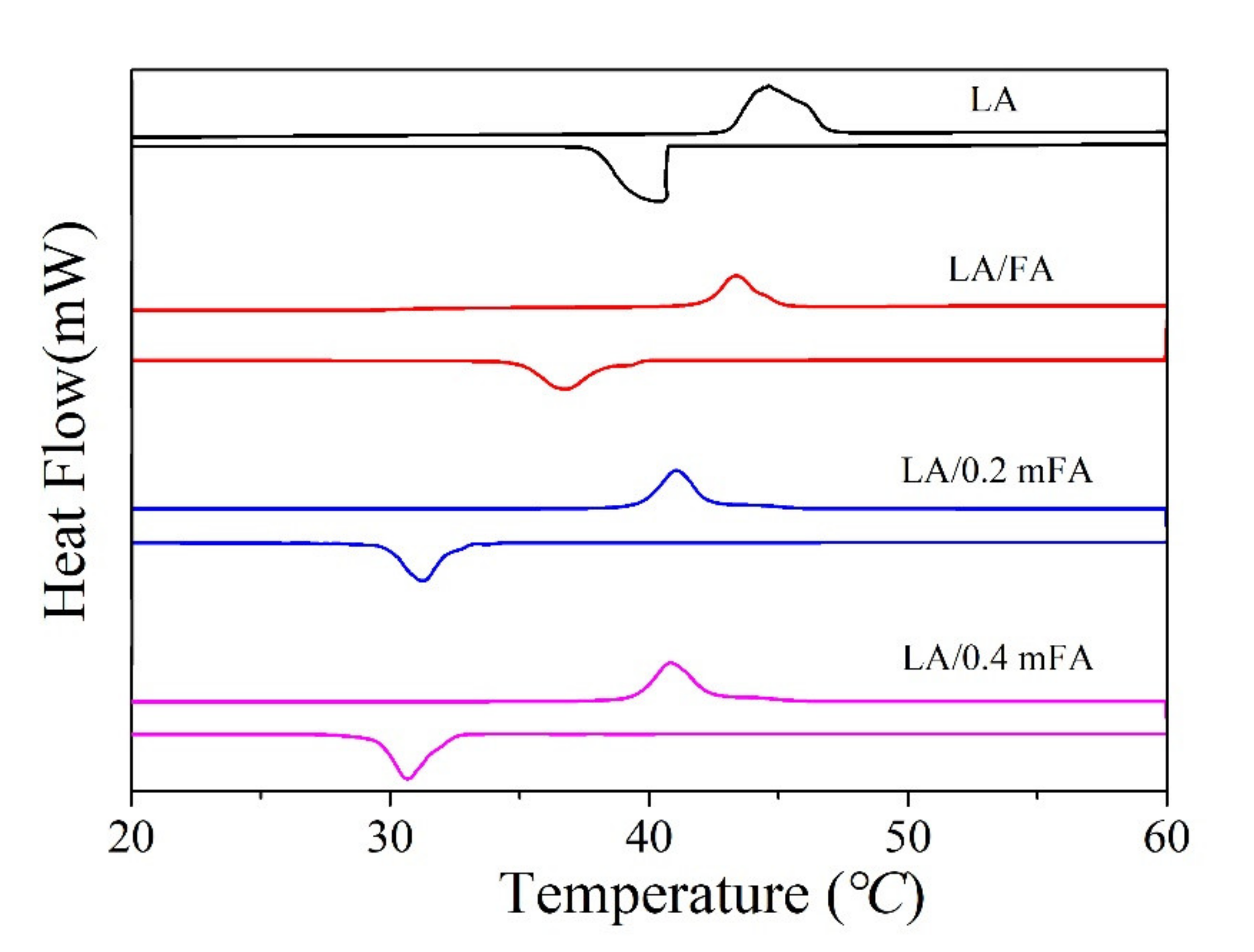
| Compound | SiO2 | Al2O3 | CaO | Fe2O3 | Na2O | Others |
|---|---|---|---|---|---|---|
| FA | 55.67 | 27.91 | 5.36 | 5.03 | 0.4 | 5.63 |
| 0.2 mFA | 47.20 | 25.61 | 6.02 | 5.68 | 10.22 | 15.49 |
| 0.4 mFA | 47.84 | 21.81 | 4.67 | 5.27 | 15.82 | 20.41 |
| PCM | Heating Process | Cooling Process | ||
|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | |
| LA | 43.1 | 186.6 | 40.3 | 183.5 |
| LA/FA | 42.3 | 16.7 | 38.5 | 15.8 |
| LA/0.2 mFA | 39.5 | 34.1 | 32.1 | 31.3 |
| LA/0.4 mFA | 39.7 | 31.5 | 32.4 | 27.6 |
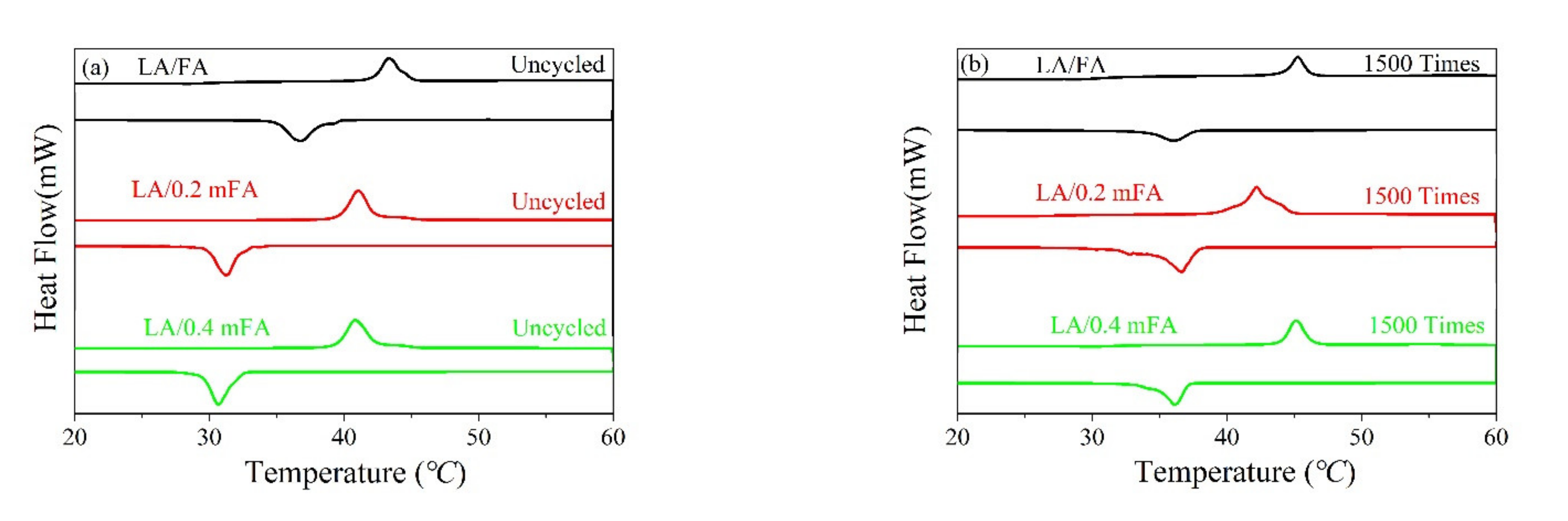
| PCM | Cycles | Heating Process | Cooling Process | ||
|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | ||
| LA/FA | 0 | 42.3 | 16.7 | 38.5 | 15.8 |
| 1500 | 41.9 | 7.5 | 38.9 | 6.5 | |
| LA/0.2 mFA | 0 | 39.5 | 34.1 | 32.1 | 31.3 |
| 1500 | 40.8 | 32.3 | 37.6 | 28.4 | |
| LA/0.4 mFA | 0 | 39.7 | 31.5 | 32.4 | 27.6 |
| 1500 | 42.2 | 30.9 | 38.9 | 26.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yang, J.; Chang, M.; Zhao, Y.; Yuan, H.; Chen, Y. Surface Roughness-Governed Shape Stability of the Coal Fly Ash-Based Phase Change Material: Molten Salt Processing and Thermal Properties. Energies 2021, 14, 1427. https://doi.org/10.3390/en14051427
Li D, Yang J, Chang M, Zhao Y, Yuan H, Chen Y. Surface Roughness-Governed Shape Stability of the Coal Fly Ash-Based Phase Change Material: Molten Salt Processing and Thermal Properties. Energies. 2021; 14(5):1427. https://doi.org/10.3390/en14051427
Chicago/Turabian StyleLi, Denian, Jizhang Yang, Menglei Chang, Yue Zhao, Haoran Yuan, and Yong Chen. 2021. "Surface Roughness-Governed Shape Stability of the Coal Fly Ash-Based Phase Change Material: Molten Salt Processing and Thermal Properties" Energies 14, no. 5: 1427. https://doi.org/10.3390/en14051427
APA StyleLi, D., Yang, J., Chang, M., Zhao, Y., Yuan, H., & Chen, Y. (2021). Surface Roughness-Governed Shape Stability of the Coal Fly Ash-Based Phase Change Material: Molten Salt Processing and Thermal Properties. Energies, 14(5), 1427. https://doi.org/10.3390/en14051427





