1. Introduction
During the last years, the number of research and development projects considering as main energy sources the so called Variable Renewable Energy Sources (VRES), such as photovoltaic (PV) and wind (WD), increased. These projects are largely driven by government regulations. Thus, the increase of RES challenged scientists and technicians to study and implement new strategies to improve the operation and stability of energy systems, and, in the meanwhile, to decrease the dependency by fossil fuels or, under a different point of view, lessening the emission of greenhouse gases [
1]. However, the intermittency typical of VRES introduces an additional risk which could compromise the successful satisfaction of the load [
2]. Therefore, the electrical system needs
flexibility, that can be provided in different ways. In [
3], the flexibility is guaranteed by coordinating several Peltier Effect regrigerator [
4]. In [
5] the flexibility is provided by a compression-based refrigerator, whereas in [
6] the exploitation of distributed multi-energy system is suggested as form of flexibility for the electrical grid. In this context, Power-to-X (P2X) technologies, such as Power-to-Gas (P2G), Power-to-Fuels (P2F), and Power-to-Heat (P2H), are gaining a leading role for providing flexibility to the electricity grid, because (i) they are able to exploit the existing infrastructure (such as the gas network and district heating) and (ii) allow decarbonising other sectors using the produced commodities (i.e., gas, liquids and heat) starting from an excess of VRES [
7]. Two of the above mentioned processes (i.e., P2G and P2F) are based on the production of
green hydrogen that can be successively converted in methane or liquid fuels by combining it with CO
2. However, this cannot determine the solution of the CO
2 emission. The only way is to use as energy carrier the green hydrogen and make use of it as basic element for the future energy system: this perspective, even though was indicated as possibile and desiderable in the past (see for example [
8]), is now is gaining a momentum. In particular, on the basis of the European Green Deal [
9], the European Commission presented the EU Hydrogen Strategy [
10], which includes two phases, having as objective to reach 6 GW of installed electrolysers up to the end of 2024 (with a production of up to 1 million tonnes of green hydrogen) and successively jump up to 40 GW at the end of 2030, with a production reaching 10 millions tonnes, to be compliant with the goal of covering around 14% of the European energy mix with green hydrogen [
11]. In China, the definition of the different types of hydrogen has been debated and a recent standard reports their characteristics, by defining low-carbon hydrogen, clean hydrogen and renewable hydrogen on the basis on their specific emission and the origin of the electricity [
12].
Several hydrogen-based energy projects around the world aimed or are aiming to study how to properly exploit the advantages from the coupling of RES-based power plants and hydrogen: taking as example the European Union, the activities in this topic are led by the
Fuel Cells and Hydrogen Joint Undertaking (FCH JU) and, since its foundation in 2008, more than 220 projects have been propitiated [
13]. This great interest is also due to the possibility to have a bi-directional conversion, i.e., from electricity to hydrogen (through electrolysers) and viceversa (by using fuel cells): this opens perspectives in which the hydrogen, properly stored, may be used as an
energy buffer that, combined with electrolysers and fuel cells, could improve the self sufficiency of the prosumers, by reducing the grid dependency. With this scope, the hydrogen-based system is used as an Energy Storage System (ESS) and the prosumer plant can be seen as a Hybrid Micro-Grid (H
μG).
A H
μG may combine RES power plants, gas-based devices and energy storage capability. It can supply remote customers with clean and cost-effective electricity [
14]: in fact, if properly designed, H
Gs can be operated also in islanded way [
15]. The structure to consider could be composed by a whole H
2 energy system, which employs mainly fuel cells, electrolysers and hydrogen tanks. Therefore, to present these new hybrid systems as a potential solution, two main aspects have to be considered: (i) novel algorithms to manage the surplus of energy associated with RES, and (ii) new strategies of energy management, in order to drive the power flow calculation of hydrogen systems integrated into the H
Gs [
1].
In literature, the contributions on this topic focus on specific aspects of the system. For example, in [
16], the author proposed the sizing of a hybrid plants (including photovoltaic and fuel cells) based on models included in the software HOMER. In [
17], the authors evaluated the sizing of batteries and hydrogen energy storage for a real domestic load, but with infinite storage capacity. In [
18] different meta-heuristic methods have been compared to optimally design an isolated H
G. All the above contributions consider simulation time steps of one hour, which are good enough for energy evaluation, but cannot properly represent the dynamics. In [
19], the time step is shorter (aroung 30 s), but the measurements of the electrical load were not based on real data and the response of electrolysers and fuel cells are based on simplified models. In [
20], the authors proposed a model implemented on a real-time simulation, by considering a DC grid layout. Also in this case, a simplified response of the electrolyser and fuel cell is considered. Even though considering dynamic behaviour of the components, other contributions investigates specific aspects with small size components. For example, the authors in [
21] presented a H
G including an electrolyser and a fuel cell of size around 1kW. The same layout has been employed in [
22] to investigate the use of Model Predictive Control in the energy management of the H
G.
In this paper, a H
G layout based on validated models of all the components is proposed.
The implemented control strategy aims to reduce as much as possible the dependence from the main grid, to improve the self-sufficiency of the prosumer. The main contributions of the paper are: (i) the hydrogen-based components, whose sizes are higher than the ones previously considered for fast dynamic studies (8 kW for the electrolyser and 12.5 kW for the fuel cells), (ii) the use of a high-speed sampled real electrical load, which has been measured at Politecnico di Torino (Italy) and (iii) the validation of all the components of the system, based on real data.
In particular, the validation data for the PEM fuel cell have been collected locally, whereas the validation data of the electrolyser operation have been collected remotely as in Reference [
23] with high sampling frequency
The paper is organized as follows:
Section 2 introduces the layout of the H
G and the dynamic models of the components i.e.,
PV and WD generation systems, electrolyser, fuel cell and hydrogen tank.
Section 3 shows the implemented control strategy, whereas
Section 4 presents the simulation results with different case studies. Finally, the last section lists the concluding remarks.
2. HμG Modelling
2.1. HμG Layout
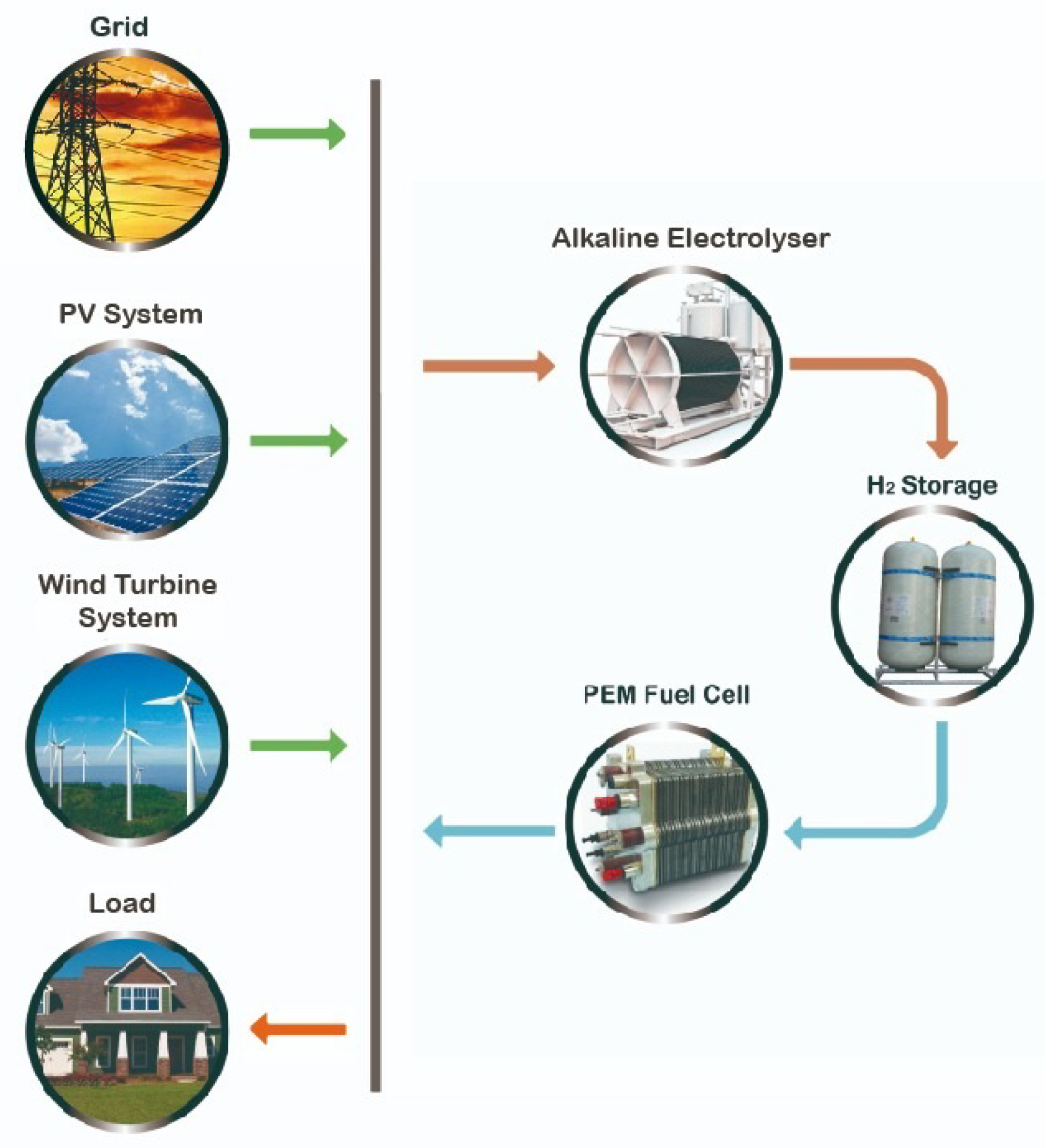
The HμG presented in this paper is composed of multiple generation sources, an EES, and a non-flexible load. The generation system is composed of a PV system, a WD generator and a Proton Exchange Membrane (PEM) fuel cell (FC). The ESS consists basically in a hydrogen storage tank (HST) coupled with an alkaline electrolyser (ELY). The ESS subsystem is connected to the non-flexible load and has enough capacity to supply the FC when needed.
A general model of hydrogen storage system is integrated to manage the production and consumption of energy from the electrolyser and the PEM FC respectively. The electrolyser is used to absorb rapidly the output power from RES and generates hydrogen as fuel for FCs [
24,
25].
The schematic diagram of the H
G is presented in
Figure 1. The sum of the demand and electrolyser power is equal to the sum of the grid contribution and the output power generated by WD and
PV generators and PEM fuel cells.
Equation (
1) represents the power balance in the H
G:
where
is the power demand,
is the power provided by the grid,
and
are the RES contributions, from the wind system and from the solar system respectively,
is the power produced by the PEM fuel cell and
the power consumed by the electrolyser.
In the following sections, a general description of the technical specifications for each component is presented. Furthermore, the models used to simulate each component are described, together with their testing and validation. All the components’ models and the overall HG system are simulated in Matlab Simulink®.
2.2. Load Demand
The load is considered to absorb only active power. The load profiles are loaded from a file. Two load profiles are considered in this work: (a) a synthetic load profile built with the aim to test the proper system operation and (b) a real load profile, obtained from real measurements.
The measurements were performed in a Medium Voltage (MV)/Low Voltage (LV) substation on a LV feeder supplying a data center at Politecnico di Torino on 18th and 19th June 2019. We used a high speed recorder Hioki 8880/20 MR with a direct connection to the feeder for voltage measurements and probes pico technology TA 167 for current measurements. The sampling time was set to 1 s.
The two load profiles are presented in
Figure 2.
2.3. Wind Turbine System
One of the renewable generators of the HG is the wind turbine system. The turbine considered in the HG has a rated power of 15 kW, a rated speed of 15 m/s, a cut-in speed of 5 m/s and a cut-out speed of 30 m/s. The average daily elecrical production is about 8 kWh.
2.4. PV System
The second renewable generator of the H
G is the
PV system; it delivers a maximum of 23 kW at 1000 W/m
sun irradiance. The technical specifications of the
PV system are summarized in
Table 1.
2.5. Alkaline Electrolyser
Electrolysers produce hydrogen from water dissociation. For the H
G described in this work, we modelled an alkaline electrolyser which, bytheway, it the most mature and available in the market [
26].
In an alkaline electrolyser, the alkaline solution (usually based on KOH at 30% in weight) is used as electrolyte. The modelled electrolyser has rate power 8 kW and rate hydrogen flow equal to 1.255 Nm
3/h, with pressure 12 barg and purity higher than 99.3%. The electrolyser comprises one stack of 8 kW, composed of 5 series-connected cells. The average efficiency is about 54.4% (low heating value). The electrolyser technical specifications are presented in
Table 2.
The alkaline electrolyser model presented is related to an Advanced Alkaline electrolyser. In the electro-chemical model temperature and pressure depends on Faraday efficiency, whereas the temperature depends on current-voltage relation [
27,
28].
The model may forecast the cell voltage, the hydrogen production, Faraday efficiency and the electrolyser operating temperature [
27]. In
Figure 3 the block diagram of the sub-models is presented. The sub-models are described in detail in the next paragraphs.
2.5.1. Thermodynamic Sub-Model
The reversible voltage is defined as the minimum voltage that, applied to the water molecule, allows its separation. The reversible voltage
is expressed in terms of the Gibbs Energy variation, as reported in Equation (
2).
The Gibbs energy can be associated with reversible voltage and themoneutral cell voltage as shown in Equation (
3).
where the reversible voltage
is measured in V and is sensible to reaction temperature and pressure,
z the hydrogen molecule electrons, and
F is the Faraday constant, equal to
C/mol. The total energy demand
refers to thermo-neutral cell voltage and it is expressed by Equation (
4).
2.5.2. Electrical Sub-Model
The electrical sub-model allows the estimation of the voltage-current relationship. The model inputs are the electrical power and the stack temperature, whereas the output is the voltage-current couple for every stack cell at different temperatures [
29].
The relationship of the electrical power on the electrolyser is given Equation (
5):
where
is the consumed power in W,
is the of stack cell number,
is the stack current of the electrolyser in A,
and is the electrolyser cell voltage in V.
In Equation (
5), the electrolyser cell voltage
represents an empirical
model for electrolysers. The kinetics of the cell electrode are modeled with the
curve, which includes the ohmic and stack temperature effects. The
curve can be expressed by the sum of three terms, as reported in Equation (
6): the reversible voltage
, the activation voltage
, and the ohmic overvoltage
. All the terms are expressed in V [
30].
The first term of the right-side of Equation (
6) represents the minimum voltage required to activate the ideal electrolyser cell, as expressed above in Equation (
3). The second and third terms refer to the activation and ohmic overvoltages that can be defined as in Equations (
7) and (
8), respectively.
where
and
are empirical coefficients referring to the electrodes’ overvoltages,
r represents the electrolyte ohmic effect
m
and
is the electrolyser stack area in m
.
2.5.3. Hydrogen Production Sub-Model
The hydrogen production is directly proportional to the current in the external circuit (i.e., transfer rate of electrons to electrodes):
where
is the hydrogen flow rate in mol/s,
is the Faraday efficiency, and
is the stack current in A.
2.5.4. Faraday Efficiency Sub-Model
The Faraday efficiency represents the losses caused by parasitic currents: their value is inversely proportional to the current density, i.e., they increase if the current density decreases. Moreover, the value of the parasitic currents increases with a temperature rise, which lowers the Faraday efficiency, as shown in Equation (
10):
where
and
are empirical constants taken from [
31], and
is the current density expressed by Equation (
11):
2.5.5. Thermal Sub-Model
The thermal behavior of the electrolyser cannot be neglected in case of connection to RES. In fact, the temperature variation impacts the hydrogen production over the time. The temperature of the electrolyte can be determined by solving the thermal energy balance in Equation (
12):
where
is the electrolyser stack thermal capacity in W/K,
is the electrolyser temperature in K,
is the generated heat during the electrolysis (in W),
is the cooling thermal power expressed in W, and
represents the heat losses (in W).
2.6. Simulation and Validation of the Alkaline Electrolyser
The dynamic model of the electrolyser has been implemented in Simulink. The unique input of the dynamic model is the power requested in kW, while the outputs are the temperature in °C, the stack voltage and current (in V and A, respectively), the hydrogen produced in lpm and the performance in %. The main parameters of the computation model are shown in
Table 3.
The model is validated using laboratory measurements on a 8 kW alkaline electrolyser. The input power has been raised up up to about 8 kW within a step. Then, the electorlyzer is operated at the nominal power for 4 min (
Figure 4).
In
Figure 4, the active power corresponds to the overall power of the system. i.e., power requested by the electrolyser and losses which are estimated as 400 W. The stack voltage and stack current of the validating model steadies at 44.02 V and 126 A with errors of 4.58% and 6.45% respectively. The temperature reaches its steady state to 30.65 °C after 150 s with an error of 2.13% and hydrogen production steadies at 19.91 lpm with an error of 1.89%. Furthermore, the characteristic curve of the electrolyser at 35 °C is presented. While the open circuit voltage of the ELY cell is 1.229 V, the output voltage when the electrolyser provides 50 A is 4.5 V. The results show that the model is quite accurate and may be used to predict the output variables of the electrolyser.
2.7. PEM Fuel Cell
The PEM FCproduces electricity by combining hydrogen (i.e., the fuel) and the oxygen (i.e., the oxidant). Reaction byproducts are heat and water [
26,
29]. The FC stack is composed of a number of cells connected in series, to have high output voltage and power. The modules obtained are able to offer as outputs powers lying in the range between 100 W to thousands kW, reaching average efficiencies between 40% and 60%. The FC modelled in the H
G is composed by one stack of 110 cells and provides a rated power of 12.5 kW. The PEM fuel cell is equipped to guarantee the self-humidification and get from the air the required oxygen. The fuel cell technical specifications are summarized
Table 4.
The architecture of the model is equivalent to the structure of the alkaline electrolyser (presented before, in
Figure 3), shown for the PEM fuel cell in
Figure 5. In the model, the following assumptions are considered [
29]:
There is a prevalent dimension, i.e., the spatial distribution can be considered one-dimensional
The gases follow the ideal gas law and are distributed in uniform way
It is considered a negligible pressure variation in the FC gas flow channels
Both the fuel (hydrogen) and the oxidant (air) are humidified
Thermodynamic properties refer to the mean temperature.
It is considered a negligible stack temperature variation
It is supposed a constant stack heat capacity
The presented model is based on three sub-models, which can be run separately, but are linked to each-other. The sub-models are the electrical sub-model, the thermal sub-model and the hydrogen consumption sub-model.
2.7.1. Electrical Sub-Model
As for the alkaline electrolyser, the PEM fuel cell model is based on a number of relationships, covering thermodynamics and heat transfer aspects [
27,
32]. The operating cell voltage is expressed by Equation (
13):
where
is the open circuit voltage,
indicates the activation overvoltage,
refers to the ohmic overvoltage, and
is the overvoltage term referring to the reactant concentration. All terms are measured in V.
2.7.2. Thermal Sub-Model
The fuel cell thermal sub-model is determined on the basis of a lumped thermal model. The heat generated during the fuel cell use is expressed through Equation (
14):
where
indicated the enthalpy related to the water formation,
is the energy incorporated produced as electricity, and
is heat dissipated through convection and the one removed through the cooling system.
2.7.3. Fuel Consumption Sub-Model
The required fuel (i.e., hydrogen) is obtained through Equation (
15).
where
indicated the hydrogen flow expressed in mol/s,
is FC cell number,
indicated the stack current (in A),
z number of molecule’s electrons, and
F is the Faraday constant in C/mol.
2.8. Simulation and Validation of the PEM FC
The dynamic model of the PEM FC has been implemented in Matlab Simulink®. The inputs of the model are the hydrogen requested in lpm and the nominal FC current in A, while the outputs are the produced power in kW, the FC voltage in V, the actual FC current in A and the hydrogen consumption in lpm.
The model is validated using laboratory measurements on a 12.5 kW PEM fuel cell stack composed by 110 cells (see
Figure 6). Measured data correspond to the following conditions: 40% of oxidant composition
, air pressure at 1 bar and variable load. During the validation tests the
consumption increases from 100 to 200 lpm, whereas the air flow rate is almost constant at 200 lpm. The maximum error obtained on the current is 0.1%, on the voltage 8.7% and on the power 7.5%.
2.9. Hydrogen Storage Tank
The storage of the hydrogen can be done under different forms, i.e., as gas, liquid or with an intermediate means. In the modelled H
G the produced hydrogen stored in a high pressure tank [
33]. The tank capacity is imposed equal to 9 m
. The tank specifications are summarized in
Table 5.
2.10. Hydrogen Tank Modelling
The hydrogen tank modelling approach is shown in Equation (
16).
where
is the hydrogen storage rate of the tank,
is the rate of hydrogen production,
represents the hydrogen flow withdrawn by the PEM FC, and
refers to the hydrogen leakages, all terms in
. Despising the leakage, Equation (
16) can be written again as Equation (
17).
where
and
are the stored hydrogen and the initial condition of the tank, respectively (both in in
). Assuming ideal gas conditions, the pressure inside the tank can be obtained as Equation (
18):
where
is the pressure in the tank,
indicates the stored moles of hydrogen in the tank,
R is the ideal gas constant,
refers to the stored hydrogen temperature, and
indicates the tank volume.
2.11. Simulating and Validating Hydrogen Storage Tank
The inputs of the model are the hydrogen production by the electrolyser in lpm and the hydrogen consumption by the PEM fuel cell in lpm. The output is the tank level expressed in % or m
. The hydrogen tank has a capacity of 9 m
. The model is validated with a constant flow rate of 20 lpm. It is required supplying 7.5 h with a constant inlet flow of 20 lpm of hydrogen to charge the tank from its minimum capacity (0 m
) until its maximum capacity (9 m
), see
Figure 7.
3. Control Strategy
This section shows the implemented control strategy for the H
G, receivingthe state variables as inputs and providing as outputsthe control signals. In the following, the conditions to enable/disable the single models and the control signals are described. The initialization of the state variables into the Central Control System (CCS) is required to avoid algebraic loops.The supervisory control of the CCS is presented in
Figure 8.
In the CCS all individual models are combined, and the connection among them is made by sending and receiving the system state variable.
Table 6 summarizes the inputs and outputs of the model.
The models related to RES are connected to the grid at the point of common coupling (PCC) and activated according to environmental conditions, i.e., wind speed or presence of clouds. Furthermore, an activation hysteresis has been considered to avoid an intermittent operation of the systems due to sudden changes of the environmental conditions. These models might be disconnected through an enabling signal which switches to 0 the output, i.e., the active power. On the one hand, when the wind speed is lying between 5 m/s and 30 m/s for at least 500 s, the WD turbine may inject active power into the grid or supply the alkaline electrolyser. On the other hand, when the solar irradiance is higher than 100 W/m for a 500s-period, the PV system may inject active power either to the local demand or to the electrolyser.
With reference to the electrolyser, it is switched
on when the following conditions are satisfied: (i) the sum of the power produced by the wind turbine
and photovoltaic system
is higher, for a period of 500 s, than the sum of the load power
and the minimum operating power of the electrolyser
; (ii) the level of hydrogen tank lies between its minimum and maximum capacity. These two conditions are shown in Equations (
19) and (
20).
Once the electrolyser is on, the PEM fuel cell is automatically blocked (i.e., switched off).
When instead the condition shown in Equation (
21) (which should be valid for a 500s-period) Equations (
22) and (
23) are satisfied, the electrolyser is turn
off.
being
and
the 20% and 15% of the electrolyser rated power, respectively.
By considering the operation of the PEM fuel cell, it is switched
on when the following conditions exist: (i) the tank level respects the condition (
20), (ii) the sum of
and
is lower than
for a period of 500 s (as shown in Equation (
24)), (iii) the load demand is higher than the minimum operating power of the PEM fuel cell (i.e.,
), as reported in Equation (
25).
when the
PEM fuel cell is switched
on the electrolyser is automatically turned
off.
The PEM fuel cell is switched
off when the following conditions are met: (i) the tank level is lower than its minimum level (as Equation (
23), (ii) the sum of
and
is higher than
, for a period of 500s (as reported in Equation (
26)), (ii) the load demand is lower than the PEM minimum operating power
, as shown in Equation (
27).
The values of and are chosen to be 20% and 15% of the PEM fuel cell rated power, respectively.
Finally, the last component of the HG is the hydrogen tank. It may operate between 10% and 95% of its capacity. As mentioned before, its level allows controlling the operation of electrolyser and PEM fuel cell: when the tank level reaches its maximum value, the electrolyser (if in operation) is turned off. Conversely, when the tank level reaches its minimum value, the PEM, if in operation, is turned off. The tank model has two enabling signals, i.e., the first one allows to activate the charging of the tank, while the second one activates the discharging.
5. Conclusions
This work presented a methodology to simulate the behavior of a Hybrid Microgrid, i.e., aWind-Solar-Hydrogen Energy System. The system can produce hydrogen from the generation surplus and can use the produced hydrogen, stored in a tank, as energy carrier to be used later by a fuel cell for electricity generation. Mathematical models to simulate the dynamic behaviour of every system component (i.e., photovoltaic system, wind generator, alkaline electrolyser, PEM fuel cell and hydrogen tank) have been developed. The models encompass both dynamics and low computational requirements. The validation of electrolyser and PEM fuel cell models are presented as well. The outputs confirmed that both models may successfully depict the validation data. Results in validation process indicated an average error less than 2% for hydrogen production by electolyzer and 6% for the power produced by PEM fuel cell. Furthermore, a supervisory control strategy for the Wind-Solar-Hydrogen system has been introduced as well.
The dynamic behavior of the Wind-Solar-Hydrogen system model has been shown by performing simulations in different wind velocity and solar irradiance conditions by considering a real load profile. The simulation results have shown a satisfactory operation, in which wind turbine, photovoltaic system and hydrogen-system are compensating each other, even if the complete self-sufficiency cannot be reached.
A sensitivity analysis has been also reported: it highlighted how the decoupling between the energy and power is positive to exploit the potential of the hydrogen system. In this way, the importance of the capability of the storage tank has been demonstrated for hydrogen systems integrated with RES.
In summary, the models of the different components developed in this paper were able to jointly operate under the supervision of the control logic, that was appositely designed to improve the self-sufficiency of the Hybrid Microgrid. The overall system has also proven to be a reliable tool for the performance evaluation of Wind-Solar-Hydrogen plants. Even though the developed model does not refer to any existing system, it allows to size the components of the hybrid microgrid at the design stage, by considering the real dynamics of both loads (electrolyser and passive load) and generation (fuel cell and renewable energy sources). Furthermore, it is possible to evaluate the overall system efficiency when the tank size is varied, as well as the self-sufficiency and the self-consumption of the system. This demonstration project can prepare the way for a future hydrogen marketplace and it will help researches to run scenario analysis, verify theoretical findings and optimize system operations. It is therefore expected that this work will help improving cost competitiveness of renewable energy and reduce market barriers for new energy and technology solutions in general, and hydrogen technology in particular. We trust that it is possible to supply remote areas with wind and solar power using hydrogen as storage medium, even if there are several aspects to improve in order to make the system competitive with respect to alternative systems (like wind-diesel).