Evolution of Internal Stress in Heterogeneous Electrode Composite during the Drying Process
Abstract
:1. Introduction
2. Methodology
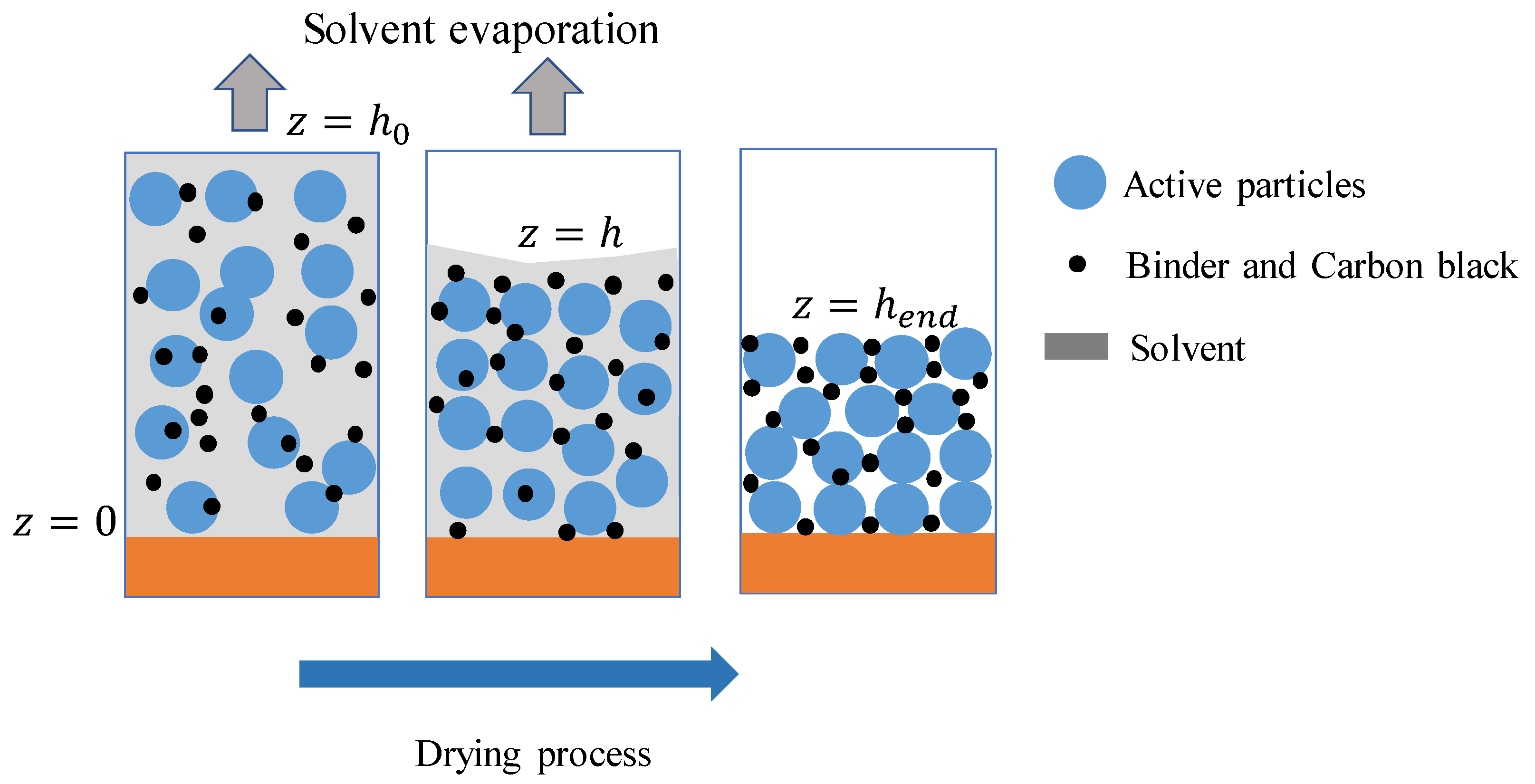
2.1. Drying Process Model
2.2. Drying Stress Model
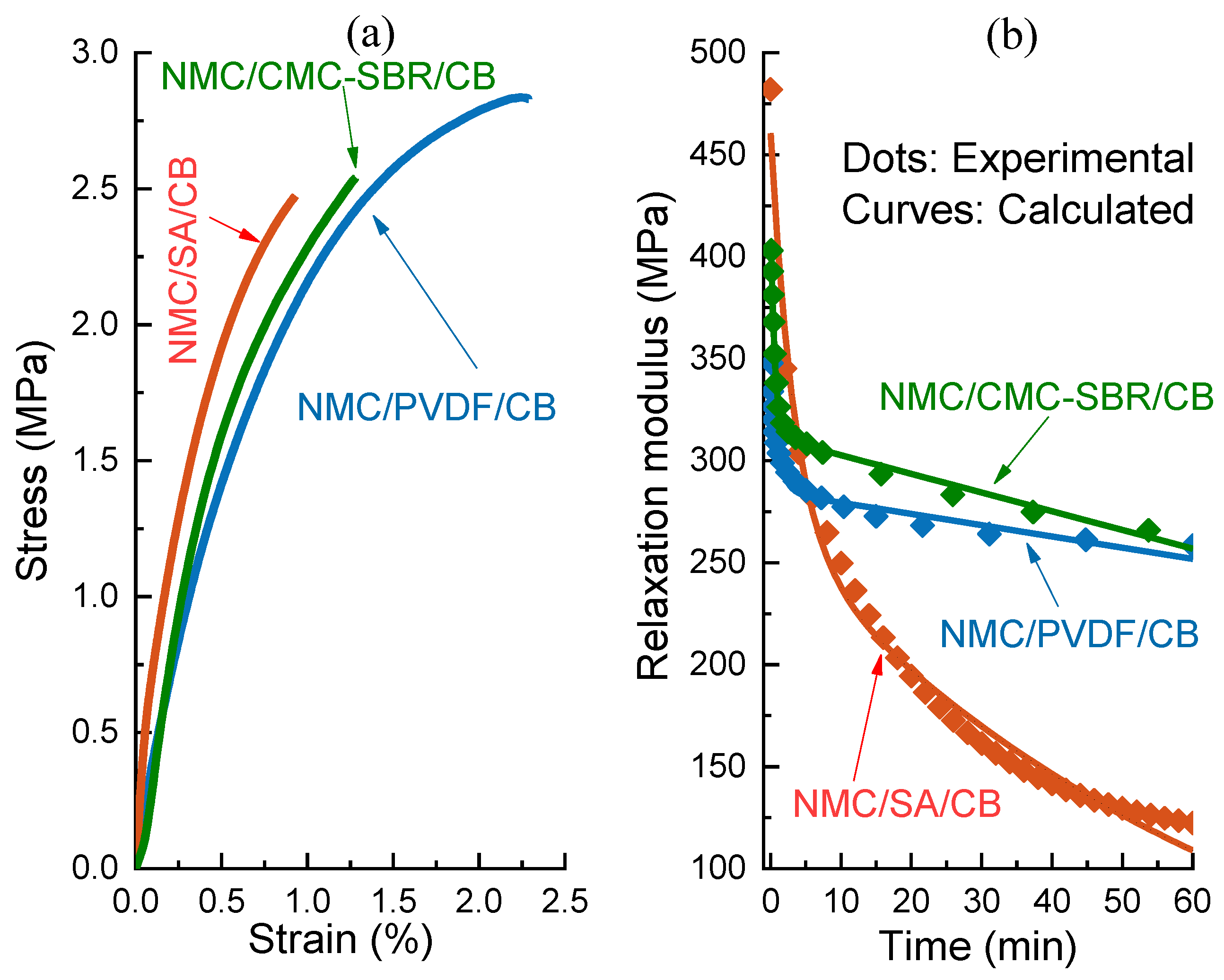
2.3. Mechanical Properties Measurement
3. Results and Discussion
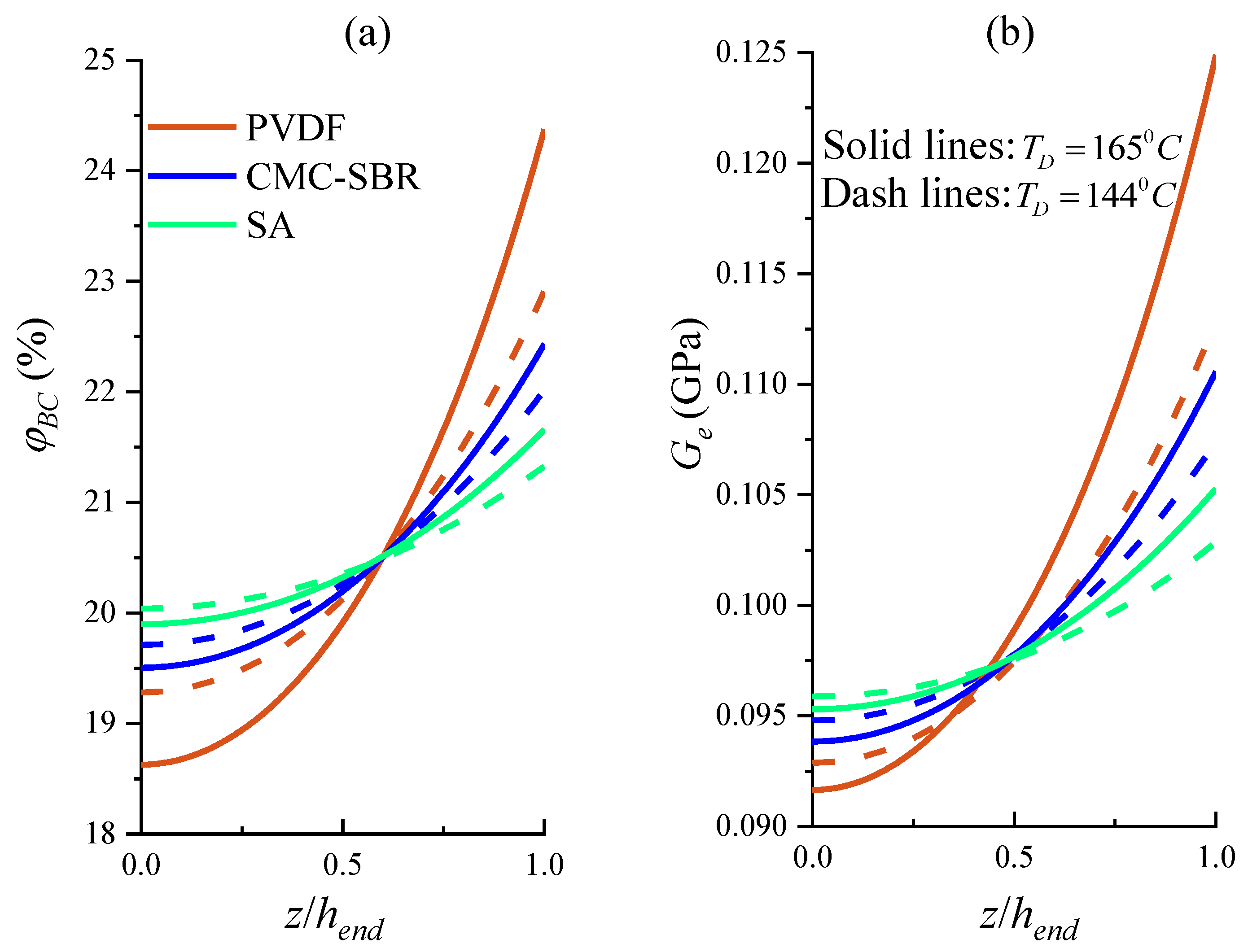
3.1. Gradient Distributed Physical Properties across Electrode Thickness
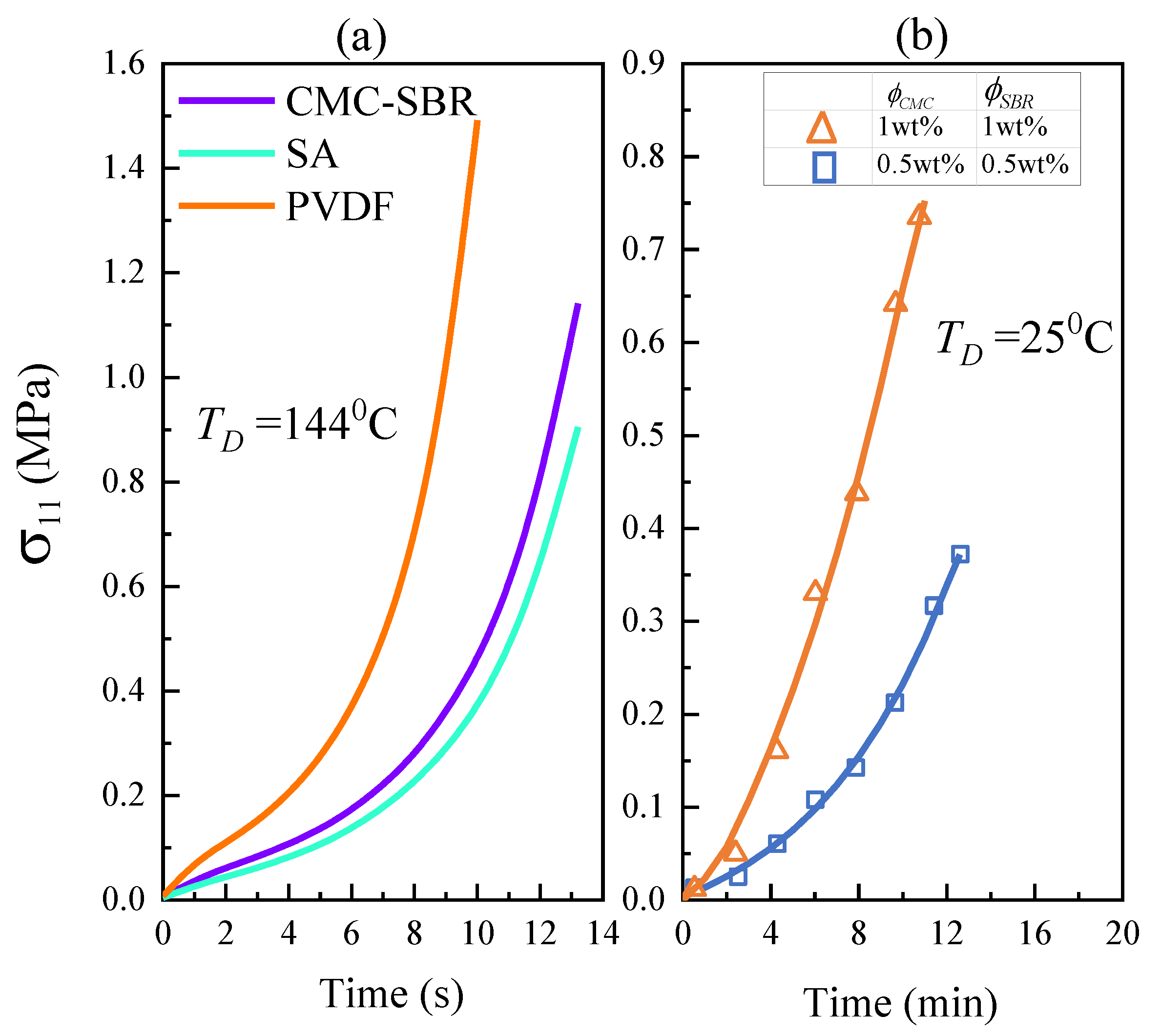
3.2. Evolution of Internal Stress Distribution during the Drying Process
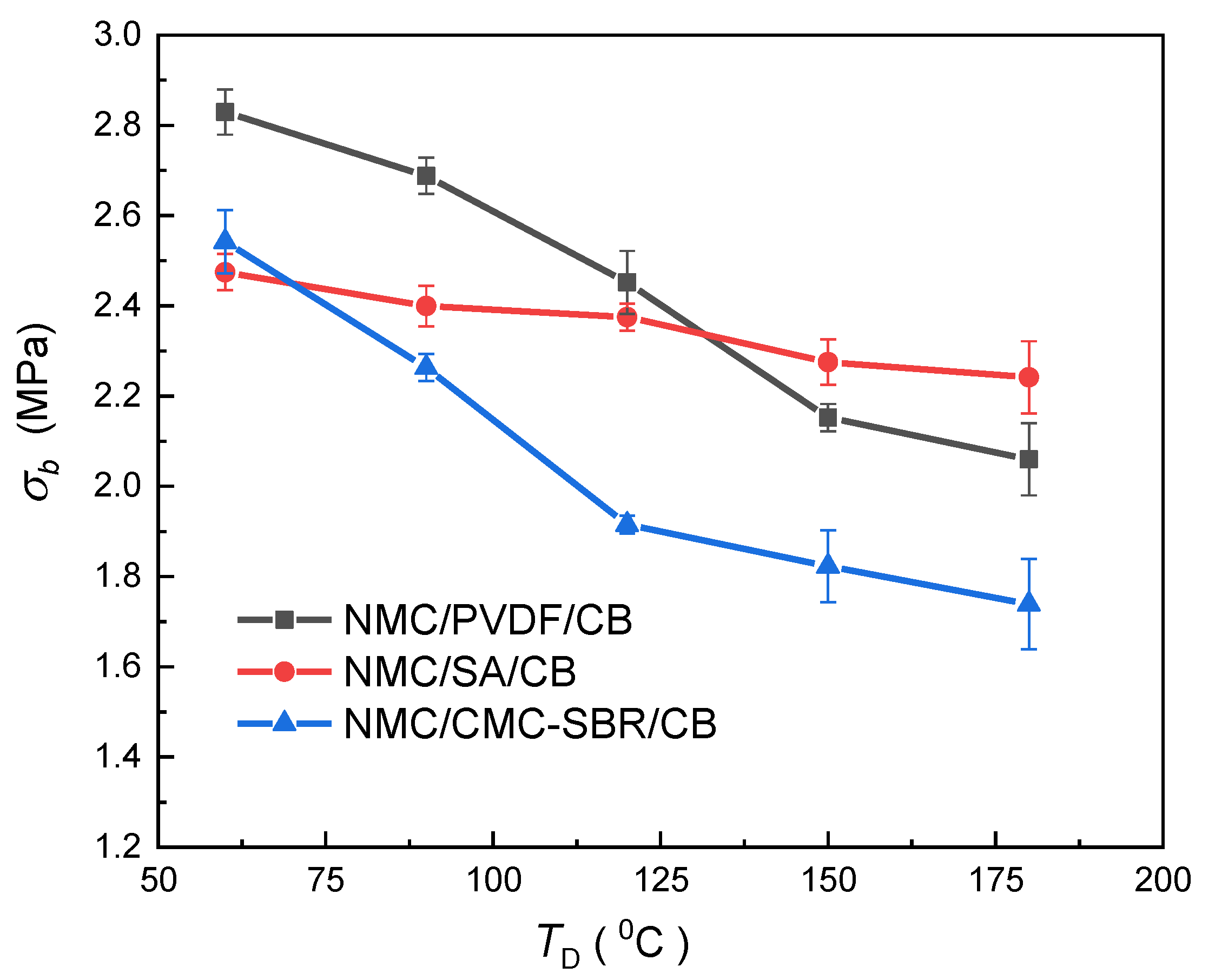
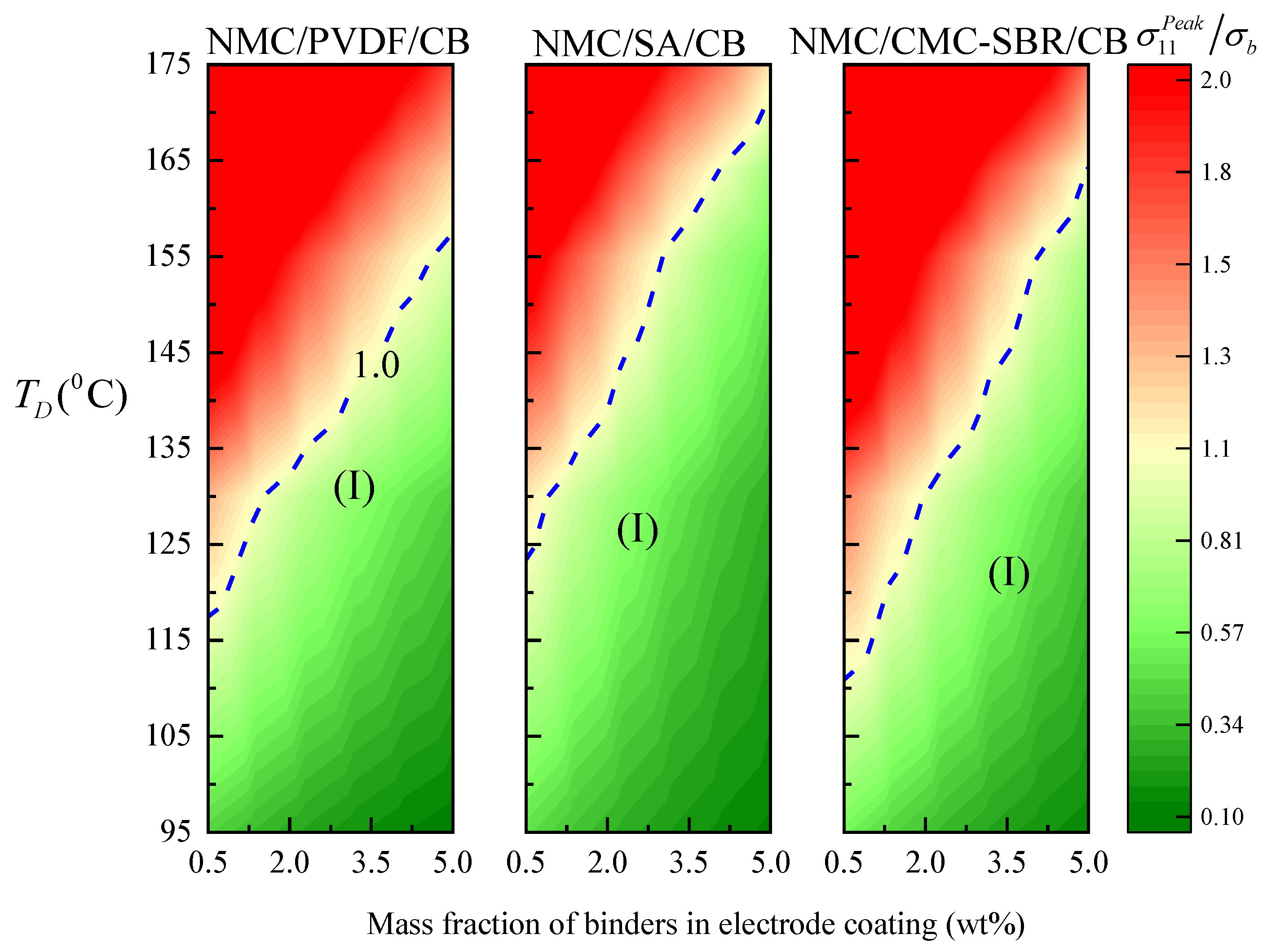
3.3. Dependence of Mechanical Integrity on Drying Temperature
4. Conclusions
- (1)
- The proposed model could reveal the gradient distributed inactive ingredients (binder and conductive agent, BC) across the electrode coating thickness. The calculated relaxation modulus of dried electrode composites is in good agreement with our experimental results. Noticeably, the predicted drying stress regarding the effect of interfacial tension in good accord with the reported test data.
- (2)
- Higher drying temperature accelerates the inhomogeneity of BC distribution in electrode composite under the drying process, leading to the significant increase in internal stress of the electrode and significantly impairing the tensile strength of electrode composite. It is also found that the PVDF system exhibits more marked drying temperature dependence.
- (3)
- Increasing the binder content can decrease the effective modulus of electrode composite, thus reducing the peak drying stress regardless of the processing temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Wang, Y.; Sahadeo, E.; Rubloff, G.; Lin, C.-F.; Lee, S.B. High-capacity lithium sulfur battery and beyond: A review of metal anode protection layers and perspective of solid-state electrolytes. J. Mater. Sci. 2019, 54, 3671–3693. [Google Scholar] [CrossRef]
- Rollag, K.; Juarez-Robles, D.; Du, Z.; Wood, D.L.; Mukherjee, P.P. Drying Temperature and Capillarity-Driven Crack Formation in Aqueous Processing of Li-Ion Battery Electrodes. ACS Appl. Energy Mater. 2019, 2, 4464–4476. [Google Scholar] [CrossRef]
- Jaiser, S.; Müller, M.; Baunach, M.; Bauer, W.; Scharfer, P.; Schabel, W. Investigation of film solidification and binder migration during drying of Li-Ion battery anodes. J. Power Sources 2016, 318, 210–219. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.; Li, J.; An, S.; Wood, M.; Sheng, Y.; Mukherjee, P.; Daniel, C.; Wood, D. Enabling aqueous processing for crack-free thick electrodes. J. Power Sources 2017, 354, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-C.; Wang, Y.-W. Binder Distributions in Water-Based and Organic-Based LiCoO2 Electrode Sheets and Their Effects on Cell Performance. J. Electrochem. Soc. 2011, 158, A1361–A1370. [Google Scholar] [CrossRef]
- Porcher, W.; Lestriez, B.; Jouanneau, S.; Guyomard, D. Design of Aqueous Processed Thick LiFePO[sub 4] Composite Electrodes for High-Energy Lithium Battery. J. Electrochem. Soc. 2009, 156, A133–A144. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-S.; Kim, Y.C.; Zang, D.S.; Paik, U. Dispersion properties of aqueous-based LiFePO4 pastes and their electrochemical performance for lithium batteries. Ultramicroscopy 2008, 108, 1256–1259. [Google Scholar] [CrossRef]
- Müller, M.; Pfaffmann, L.; Jaiser, S.; Baunach, M.; Trouillet, V.; Scheiba, F.; Scharfer, P.; Schabel, W.; Bauer, W. Investigation of binder distribution in graphite anodes for lithium-ion batteries. J. Power Sources 2017, 340, 1–5. [Google Scholar] [CrossRef]
- Morasch, R.; Landesfeind, J.; Suthar, B.; Gasteiger, H.A. Detection of Binder Gradients Using Impedance Spectroscopy and Their Influence on the Tortuosity of Li-Ion Battery Graphite Electrodes. J. Electrochem. Soc. 2018, 165, A3459–A3467. [Google Scholar] [CrossRef]
- Font, F.; Protas, B.; Richardson, G.; Foster, J. Binder migration during drying of lithium-ion battery electrodes: Modelling and comparison to experiment. J. Power Sources 2018, 393, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Baunach, M.; Jaiser, S.; Schmelzle, S.; Nirschl, H.; Scharfer, P.; Schabel, W. Delamination behavior of lithium-ion battery anodes: Influence of drying temperature during electrode processing. Dry. Technol. 2016, 34, 462–473. [Google Scholar] [CrossRef]
- Lim, S.; Kim, S.; Ahn, K.H.; Lee, S.J. Stress Development of Li-Ion Battery Anode Slurries during the Drying Process. Ind. Eng. Chem. Res. 2015, 54, 6146–6155. [Google Scholar] [CrossRef]
- Kumberg, J.; Müller, M.; Diehm, R.; Spiegel, S.; Wachsmann, C.; Bauer, W.; Scharfer, P.; Schabel, W. Drying of Lithium-Ion Battery Anodes for Use in High-Energy Cells: Influence of Electrode Thickness on Drying Time, Adhesion, and Crack Formation. Energy Technol. 2019, 7. [Google Scholar] [CrossRef]
- Li, J.; Rulison, C.; Kiggans, J.; Daniel, C.; Wood, D.L. Superior Performance of LiFePO4Aqueous Dispersions via Corona Treatment and Surface Energy Optimization. J. Electrochem. Soc. 2012, 159, A1152–A1157. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Li, J.; Liang, Y.; Wang, R.; Zhou, H.; Wood, D.L.; Singler, T.J.; Jin, C. Characterization of Surface Free Energy of Composite Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A2493–A2501. [Google Scholar] [CrossRef]
- Forouzan, M.M.; Chao, C.-W.; Bustamante, D.; Mazzeo, B.A.; Wheeler, D.R. Experiment and simulation of the fabrication process of lithium-ion battery cathodes for determining microstructure and mechanical properties. J. Power Sources 2016, 312, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Jaiser, S.; Kumberg, J.; Klaver, J.; Urai, J.L.; Schabel, W.; Schmatz, J.; Scharfer, P. Microstructure formation of lithium-ion battery electrodes during drying—An ex-situ study using cryogenic broad ion beam slope-cutting and scanning electron microscopy (Cryo-BIB-SEM). J. Power Sources 2017, 345, 97–107. [Google Scholar] [CrossRef]
- Malcolm Stein, I.V.; Aashutosh Mistry, P.P.M. Mechanistic Understanding of the Role of Evaporation in Electrode Processing. J. Electrochem. Soc. 2017, 164, A1616–A1627. [Google Scholar] [CrossRef] [Green Version]
- Westphal, B.G.; Kwade, A. Critical electrode properties and drying conditions causing component segregation in graphitic anodes for lithium-ion batteries. J. Energy Storage 2018, 18, 509–517. [Google Scholar] [CrossRef]
- Chen, Z.; Christensen, L.; Dahn, J.R. Mechanical and electrical properties of poly(vinylidene fluoride-tetrafluoroethylene-propylene)/Super-S carbon black swelled in liquid solvent as an electrode binder for lithium-ion batteries. J. Appl. Polym. Sci. 2004, 91, 2958–2965. [Google Scholar] [CrossRef]
- Christensen, R.; Lo, K. Solutions for effective shear properties in three phase sphere and cylinder models. J. Mech. Phys. Solids 1979, 27, 315–330. [Google Scholar] [CrossRef]
- Mori, T.; Tanaka, K. Average stress in matrix and average elastic energy of materials with misfitting inclusions. Acta Met. 1973, 21, 571–574. [Google Scholar] [CrossRef]
- McLaughlin, R. A study of the differential scheme for composite materials. Int. J. Eng. Sci. 1977, 15, 237–244. [Google Scholar] [CrossRef]
- Chen, J.-K.; Wang, W.-C.; Huang, Z.-P. Surface Energy Effect on Damage Evolution in a Viscoelastic Nanocomposite. Int. J. Damage Mech. 2010, 19, 949–970. [Google Scholar] [CrossRef]
- Li, D.; Hu, G.-K. Effective viscoelastic behavior of particulate polymer composites at finite concentration. Appl. Math. Mech. 2007, 28, 297–307. [Google Scholar] [CrossRef]
- Duan, H.; Wang, J.; Huang, Z.; Karihaloo, B. Size-dependent effective elastic constants of solids containing nano-inhomogeneities with interface stress. J. Mech. Phys. Solids 2005, 53, 1574–1596. [Google Scholar] [CrossRef]
- Madsen, B.; Thygesen, A.; Lilholt, H. Plant fibre composites—Porosity and stiffness. Compos. Sci. Technol. 2009, 69, 1057–1069. [Google Scholar] [CrossRef]
- Jones, E.M.C.; Silberstein, M.N.; White, S.R.; Sottos, N.R. In Situ Measurements of Strains in Composite Battery Electrodes during Electrochemical Cycling. Exp. Mech. 2014, 54, 971–985. [Google Scholar] [CrossRef]
- Huang, Z.P.; Sun, L. Size-dependent effective properties of a heterogeneous material with interface energy effect: From finite deformation theory to infinitesimal strain analysis. Acta Mech. 2006, 190, 151–163. [Google Scholar] [CrossRef]
- Tirumkudulu, M.S.; Russel, W.B. Role of Capillary Stresses in Film Formation. Langmuir 2004, 20, 2947–2961. [Google Scholar] [CrossRef]
- Tirumkudulu, M.S.; Russel, W.B. Cracking in Drying Latex Films. Langmuir 2005, 21, 4938–4948. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, H.; Hector, L.G., Jr.; Timmons, A. Threefold Increase in the Young’s Modulus of Graphite Negative Electrode during Lithium Intercalation. J. Electrochem. Soc. 2010, 157, A558–A566. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, K. Electronic Structure and Comparative Properties of LiNixMnyCozO2 Cathode Materials. J. Phys. Chem. C 2017, 121, 6002–6010. [Google Scholar] [CrossRef]
- Hu, H.; Tao, B.; He, Y.; Zhou, S. Effect of Conductive Carbon Black on Mechanical Properties of Aqueous Polymer Binders for Secondary Battery Electrode. Polymers 2019, 11, 1500. [Google Scholar] [CrossRef] [Green Version]
- Zaghib, K.; Shim, J.; Guerfi, A.; Charest, P.; Striebel, K. Effect of Carbon Source as Additives in LiFePO4 as Positive Electrode for Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2005, 8, A207–A210. [Google Scholar] [CrossRef]
- Susarla, N.; Ahmed, S.; Dees, D.W. Modeling and analysis of solvent removal during Li-ion battery electrode drying. J. Power Sources 2018, 378, 660–670. [Google Scholar] [CrossRef]
- Gordon, R.; Orias, R.; Willenbacher, N. Effect of carboxymethyl cellulose on the flow behavior of lithium-ion battery anode slurries and the electrical as well as mechanical properties of corresponding dry layers. J. Mater. Sci. 2020, 55, 15867–15881. [Google Scholar] [CrossRef]
- Ling, L.; Bai, Y.; Wang, Z.; Ni, Q.; Chen, G.; Zhou, Z.; Wu, C. Remarkable Effect of Sodium Alginate Aqueous Binder on Anatase TiO2 as High-Performance Anode in Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5560–5568. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Ahn, K.H.; Yamamura, M. Latex Migration in Battery Slurries during Drying. Langmuir 2013, 29, 8233–8244. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Sodium alginate solutions: Correlation between rheological properties and spinnability. J. Mater. Sci. 2019, 54, 8034–8046. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, S.; Park, J.H.; Park, J.D.; Ahn, K.H. Role of PVDF in Rheology and Microstructure of NCM Cathode Slurries for Lithium-Ion Battery. Materials 2020, 13, 4544. [Google Scholar] [CrossRef] [PubMed]
- Donev, A.; Cisse, I.; Sachs, D.; Variano, E.A.; Stillinger, F.H.; Connelly, R.; Torquato, S.; Chaikin, P.M. Improving the Density of Jammed Disordered Packings Using Ellipsoids. Science 2004, 303, 990–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routh, A.F.; Russel, W.B. A Process Model for Latex Film Formation: Limiting Regimes for Individual Driving Forces. Langmuir 1999, 15, 7762–7773. [Google Scholar] [CrossRef]
- Sahore, R.; Wood, D.L.; Kukay, A.; Grady, K.M.; Li, J.; Belharouak, I. Towards Understanding of Cracking during Drying of Thick Aqueous-Processed LiNi0.8Mn0.1Co0.1O2 Cathodes. ACS Sustain. Chem. Eng. 2020, 8, 3162–3169. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Stress generation and fracture in lithium insertion materials. J. Solid State Electrochem. 2006, 10, 293–319. [Google Scholar] [CrossRef]
- Mostafa, A.; Abouel-Kasem, A.; Bayoumi, M.; El-Sebaie, M. The influence of CB loading on thermal aging resistance of SBR and NBR rubber compounds under different aging temperature. Mater. Des. 2009, 30, 791–795. [Google Scholar] [CrossRef]
- Rezig, N.; Bellahcene, T.; Aberkane, M.; Abdelaziz, M.N. Thermo-oxidative ageing of a SBR rubber: Effects on mechanical and chemical properties. J. Polym. Res. 2020, 27, 1–13. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, D.; Li, D.; Hu, J.; Cheng, Y.-T. Influence of polymeric binders on mechanical properties and microstructure evolution of silicon composite electrodes during electrochemical cycling. J. Power Sources 2019, 425, 170–178. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; He, Y.; Hu, H.; Zhang, F. Evolution of Internal Stress in Heterogeneous Electrode Composite during the Drying Process. Energies 2021, 14, 1683. https://doi.org/10.3390/en14061683
Zhu Z, He Y, Hu H, Zhang F. Evolution of Internal Stress in Heterogeneous Electrode Composite during the Drying Process. Energies. 2021; 14(6):1683. https://doi.org/10.3390/en14061683
Chicago/Turabian StyleZhu, Zuoquan, Yaolong He, Hongjiu Hu, and Fangzhou Zhang. 2021. "Evolution of Internal Stress in Heterogeneous Electrode Composite during the Drying Process" Energies 14, no. 6: 1683. https://doi.org/10.3390/en14061683







