1. Introduction
The properties of microalgae make them a useful resource for environmental engineering technologies, including wastewater treatment, bio-sequestration of carbon dioxide, manufacture of biofuels, and sorption of contaminants [
1,
2]. Microalgal biomass is also a source of value-added products useful in medicine, pharmaceuticals, fertilizer industry, animal feed industry, and the food sector [
3,
4]. The potential of the widespread industrial use of microalgae is limited by the lack of technologies that are process-efficient, simple in terms of design/technology, cost-effective to build and operate, and environmentally friendly [
5]. One of the major drivers of operating cost for microalgal biomass production systems are the chemical components of the growth medium [
6]. Therefore, there is a real need to seek, develop, and optimize methods that could serve as a competitive alternative to the current solutions. One of the most promising and encouraging options is found in the development of technologies that use waste substrates as the main growth medium ingredient [
7,
8], a method consistent with the idea of the circular economy and the principles of an integrated biorefinery approach [
9]. With the use of biorefinery complexity index (BCI) as an indicator of technical and economic risk, algal- and waste-based bio-refinery platforms are considered to be one of the most promising approaches for producing fuel, food, animal feed, food supplements, fertilizers, and pharmaceuticals [
10].
The use of waste glycerol as a carbon source for the heterotrophic cultivation of microalgae seems to be one of the viable trajectories for exploring such integrated technologies. The supply of waste glycerol is steadily growing due to the increasing global production of biodiesel, spurred by requirements for blending specific percentages of biocomponents with conventional fuels [
11]. Global biodiesel production totals about 41.3 billion liters, a process that involves the co-production of waste glycerol at over 12% of total esters produced, regardless of the catalyst or technological process used [
12,
13]. Crude glycerol is the primary by-product in the biodiesel industry. The utilization of the glycerol becomes an urgent issue for the biodiesel business for two reasons; the environment and cost reduction. Large-scale biodiesel producers can choose to upgrade glycerol and move it towards the chemical products market. However, the refining of crude glycerol has a high cost. For small to medium scale biodiesel companies this is very problematic [
14,
15]. Therefore, there is a real need to seek new technologies for harnessing and neutralizing glycerol waste, as well as refining existing ones [
15].
Glycerol has been thermochemically converted to dipropylene glycol [
16] and hydroxyacetone [
17]. Other processes have also been explored, such as the reformation of glycerin to produce hydrogen and synthesis gas [
18], production of epichlorohydrin [
19], etherification [
20], and hydrogenation [
21]. Glycerin can serve as a source of carbon in biochemical processes that produce 1,3-propanediol colorants or omega-3 fatty acids [
22]. Waste glycerin is also commonly used in energy production as a substrate for fermentative methane production [
23]. By harnessing specific strains of bacteria and synthesizing new enzymes, new conversion pathways become available, including ethanol production (
Saccharomyces cerevisiae bacteria) or β-carotene production (
Blakeslea trispora) [
24,
25].
One little-explored approach to utilizing the waste glycerol fraction and converting it into value-added biocomponents is to use the biomass of
Schizochytrium sp. heterotrophic microalgae. These algae accumulate large quantities of docosahexaenoic acid (DHA) in their cells, making them a prime resource for use in the food, pharmaceutical, and animal feed industries [
26,
27]. DHA is an unsaturated fatty acid belonging to the Omega-3 group, which is an important structural component of cell membranes in some tissues of the human body, such as in the phospholipids that make up the neurons of the cortex of the brain and the retina of the eye [
28]. DHA suppresses inflammatory responses, plays an important neuroprotective role, and prevents neuronal damage and apoptosis [
29]. It has been proven that DHA protects against the development of arterial hypertension and plays a large role in the proper development of the brain in newborns [
30]. DHA also increases calcium absorption, helps maintain normal levels of “bad” and “good” cholesterol in the body, and supports the immune system [
31]. It is necessary to supply DHA with food [
32], and currently its main source are vegetable oils and fats from fish meat [
33]. Due to the growing awareness of consumers, the demand for acids from the Omega-3 group is still growing [
34]. Therefore, there is a justified need to search for alternative methods for their production, which will be environmentally friendly, and justified in terms of technology and economics.
Schizochytrium sp. biomass has been shown to grow on a broad range of carbon sources [
35], substantiating efforts to develop efficient methods of growing it on waste glycerol as a carbon source. Studies to date have examined the growth of
Thraustochytriacae microalgae on waste materials, such as breadcrumbs [
36], spent brewer’s yeast [
37], empty palm fruit bunches [
38], coconut water [
39], okara powder [
40], beer and potato processing residues [
41], and sweet sorghum juice [
42].
To ensure optimal
Schizochytrium sp. growth rate and enable culture scale-up, multiple variables must be considered and fine-tuned. They include physicochemical parameters, the nutritional value of the waste material, and the presence of growth inhibitors, as well as the availability and cost of the waste material [
43]. Considering the commercially and environmentally informed need to implement technologies for integrated waste neutralization, microalgal biomass production, energy recovery, and extraction of value-added substances, it is necessary to pursue research on optimizing technological parameters to achieve high performance [
44].
The aim of this research was to use a fractional Plackett–Burman design and the response surface methodology (RSM) to optimize docosahexaenoic acid (DHA) production by heterotrophic Schizochytrium sp., with waste glycerol used as a source of organic carbon.
2. Materials and Methods
2.1. General Design
The study aimed to identify and quantify the key process parameter values that affect the growth and DHA production of Schizochytrium sp. cultures, using glycerol as an external carbon source. The values were determined through a series of experiments structured using a Plackett–Burman design. After screening the most significant parameters having an effect on the performance, the parameter values resulting in highest dry cell weight (DCW) and DHA levels were determined using the response surface methodology. The modeled parameter values and predicted performance were then experimentally verified using a batch Schizochytrium sp. culture.
2.2. Materials
The study used
Schizochytrium sp., a strain of single-cell heterotrophic microalgae from the family
Thraustochytriaceae. The inoculum was obtained from the ATCC (American Type Culture Collection).
Schizochytrium sp. cells were maintained in sterile agar slants of ATCC790By+ medium containing 2% agar (
w/
w). The cells were spread into new agar slants on a monthly basis. To obtain enough inoculum for the experiment, the cells were transferred from the agar slants to 50 cm
3 Erlenmeyer flasks, containing 15 cm
3 of the agarless ATCC790By+ liquid medium with a pH of 6.5, with the composition given in
Table 1 and
Table 2.
The medium was autoclave-sterilized before use (Systec V-95 autoclave, parameters: 121 °C, 15 min, 2 bars, automatic demineralized water feed for steam generation was used). The flasks were agitated in a temperature-controlled orbital shaker (Excella E24R, New Brunswick/Eppendorf) set to 170 rpm at 25 °C. After 120 h, 10 cm3 of the culture (Schizochytrium sp. inoculum) was transferred into a 250 cm3 Erlenmeyer flask containing 90 cm3 of fresh ATCC790By+. The resultant 100 cm3 culture was inoculated into 500 cm3 bioreactors (with optimization performed using the Plackett–Burman design and response surface methodology) and a Biostat B Twin (Sartorius Stedim) bioreactor with a working capacity of 2.0 dm3 (for the verification step).
Crude glycerin sourced from the PKN Orlen Południe S.A. plant in Trzebinia (Poland) was used as the substrate and the sole carbon source in the cultures. During the production of biodiesel, pressed rapeseed oil is filtered and transesterified with the use of NaOH catalyst in the amount of 1.2% to the weight of the oil (process temperature is 25 °C, reaction time 45 min). The ester and glycerol phases are separated by gravity or by centrifugation and the methanol is recovered from both phases by distillation. The substrate contained at least 80% (w/w) glycerol, up to 5% (w/w) ash, up to 6% (w/w) MONG (matter organic non glycerol), and trace water, according to its safety data sheet. The liquid had pH = 5, a light-brown coloration, and a characteristic odor.
2.3. Experimental Equipment
Batch photobioreactors (Erlenmeyer flasks) with an active volume of 500 cm3 were used for the optimization step. The flasks were agitated in a temperature-controlled orbital shaker (Excella E24R, New Brunswick/Eppendorf) set to 170 rpm. During the validation step, the Schizochytrium sp. biomass was grown in a Biostat B Twin (Sartorius Stedim) bioreactor with a working capacity of 2 dm3. The bioreactor was fitted with acid, base, antifoaming agents, and organic substrate pumps. The system had a gas module for monitoring dissolved oxygen (DO), a system for stabilizing pH based on injecting acid or base via peristaltic pumps, and a temperature measurement/stabilization system. The vessels were stirred by a six-bladed Rushton turbine (53 mm diameter).
2.4. Analytical Methods
The crude glycerin concentration in the culture medium was determined by pre-centrifugation (8000× g, 4 min, 10 °C; UNIVERSAL 320 R centrifuge, Hettich, Westphalia, Germany). The supernatant was then filtered (pore size = 0.2 mm), and the filtrate was assayed for glycerol levels using a Glycerol GK Assay Kit (Megazyme). The test involved phosphorylating the glycerol with adenosine 5′-triphosphate (ATP), with the reaction product, adenosine 5′-diphosphate (ADP) then used to further phosphorylate d-glucose, which oxidizes producing nicotinamide adenine dinucleotide (NADH). The concentration of NADH was measured spectrophotometrically (Multiskan GO Microplate, Thermo Scientific, Waltham, MA, USA) at a wavelength of 340 nm.
The dry cell weight (DCW) of the microalgae was determined according to the method described by Chang et al., (2013) [
45]. It was done by transferring a 50 cm
3 sample of the culture to a pre-weighed centrifuge tube, which was then centrifuged (8000 g for 15 min, UNIVERSAL 320 R centrifuge, Hettich). The supernatant was discarded and the concentrated biomass was washed twice with distilled water, then dried at 60 °C for 12 h in a moisture balance (MAR, Radwag, Radom, Poland) to stabilize the biomass.
The lipid content of the biomass was determined by adding 7 cm
3 of a 20% hydrochloric acid solution to 1.0 g of freeze-dried biomass (ALPHA 1-4 LD plus freeze dryer, Christ), which was then placed in a water bath (GFL 1003) at 75 °C for 40 min. The sample was treated with 20 cm
3 of
n-hexane to extract the lipids and placed in a vacuum evaporator (Hei-VAP Advantage G3, Heidolph, Schwabach, Germany) to evaporate the solvent. The lipid content of the sample was measured gravimetrically. The determination of fatty acids in the microalgal biomass was done using a modified direct transmethylation process by Grayburn (1992) [
46]. The freeze-dried microalgal biomass (ALPHA 1-4 LD plus, Christ, Bethlehem, Palestine) of 20–100 mg was transferred to a reaction vial, enriched with 2 mg of triheptadecanoylglycerol as an internal standard, and spiked with 2 cm
3 of a 1% solution of H
2SO
4 in methanol. The vial was then thoroughly mixed and heated to a temperature of 80 °C for 2 h (MKR 13 blockthermostat–Ditabis, Pforzheim, Germany). After cooling, 2 cm
3 of chloroform and 1 cm
3 of distilled water were added. The vial was mixed at 1250 rpm (Vortex Reax top, Heidolph), and centrifuged at 1500×
g (UNIVERSAL 320 R, Hettich). The organic phase containing fatty acid methyl esters (FAME) was harvested and analyzed by chromatography. A Clarus 680 GC (Perkin Elmer, Waltham, MA, USA) gas chromatograph was used for FAME analysis, with helium used as the carrier gas. The column temperature was raised from 150 °C to 250 °C at 10 °C/min, then kept at 250 °C for 10 min. The injector was kept at 275 °C with an injection volume of 1 µL. Detection was made using a flame ionization detector (FID) at 280 °C. The peak areas were identified by comparing their retention times with those of standard mixtures.
2.5. Optimization Design
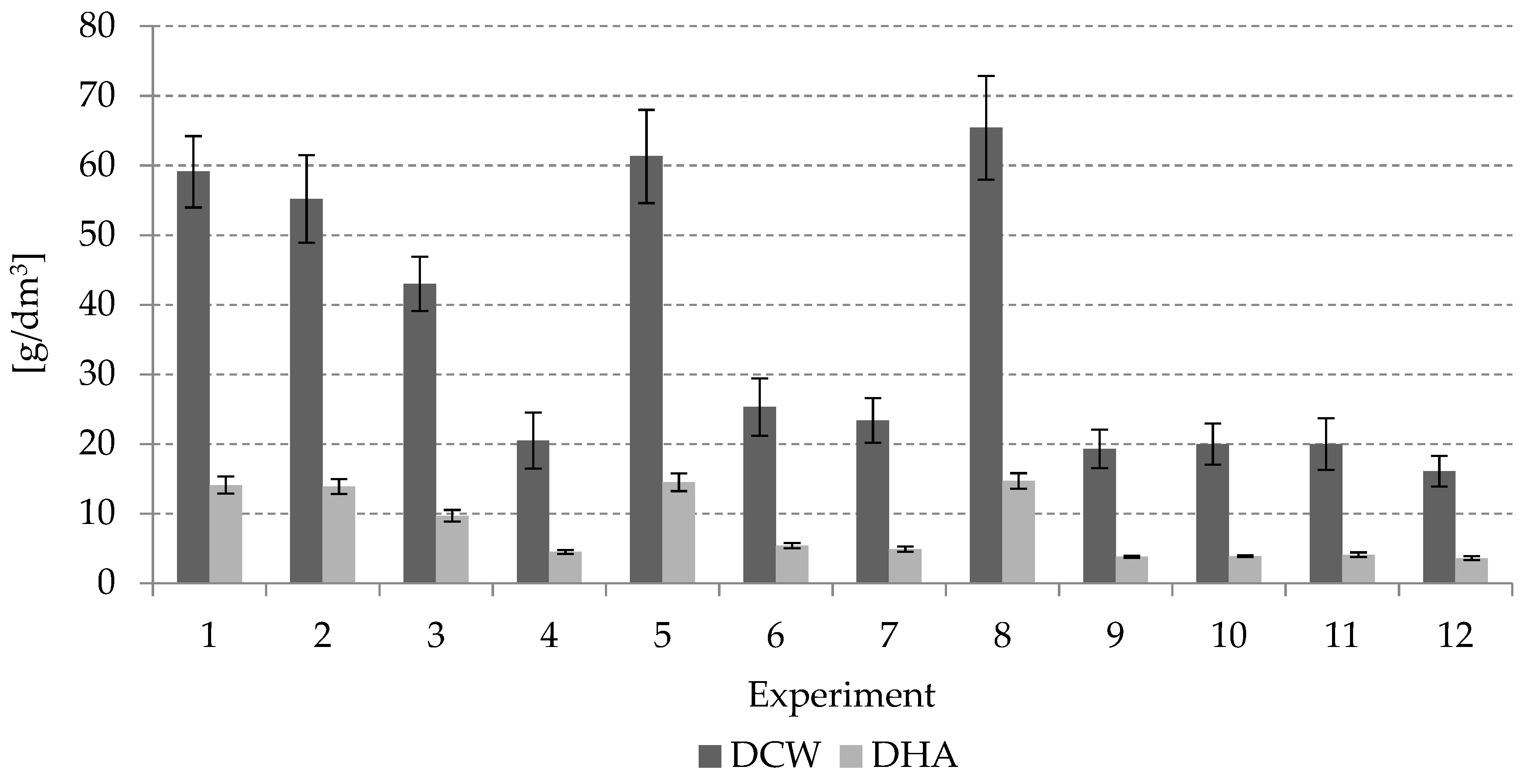
A Plackett–Burman based experimental design was used to screen the factors having significant effects on the DCW increase and DHA production (Design-Expert software by Stat-Ease Inc.). The evaluated parameters were as follows: temperature (°C), initial growth medium pH, volumetric air flow rate (Larea/min·Lcont.), oxygen in the growth medium (%), initial inoculum level (% v/v), concentration of crude glycerin (g/dm3), salinity (psu), concentration of yeast extract (g/dm3), turbine speed (rpm), and concentration of peptone (g/dm3). A design matrix of experiments with specific parameters was generated using the method. Parameters that significantly affected the values of: the rate of DHA production by microalgae—rDHA (g/dm3·h) and the growth rate of the microalgal biomass—rDCW (g/dm3·h), were identified in the course of the study.
Each independent variable was investigated at a low (−) and a high (+) level. The values of parameters for both levels were selected through the analysis of the available literature data. The low levels (−) of a variable always corresponded to the lowest parameter value at which Schizochytrium sp. growth and DHA production were possible. A high (+) level of a variable was taken as the lowest parameter value at which these parameters were not inhibited. Each experiment was conducted in triplicate.
Table 3 shows a list of the analyzed parameters and their values for each of the levels. Twelve experiments were established, as shown in
Table 4. To determine the significant level for each parameter, as prescribed by the Analytical Methods Committee method, an additional “dummy” variable, “d
1”, was added to the design matrix. The resultant effects of dummy variables reflected the standard error of the experiments, which was used to derive the significant level for each of the parameters. Parameters at
p < 0.10 were taken as significant factors, which were further optimized using response surface methodology. For the experiments designed using the response surface methodology matrix, the levels of non-significant parameters were maintained at −1. The effects of each variable (E
(xi)), the significant (P) levels, and the F-test results for the obtained data are presented herein.
The effect of each variable (E
(xi)) was derived using the equation:
where E
(xi) is the effect of the variable, M
i+ is theDCW or DHA concentration for the high (+) level of the variable, M
i− is the DCW or DHA concentration for the low (−) level of the variable, and N is the number of runs.
In order to determine how significant the effect of each parameter was on the technological process, ANOVA-derived calculations were used, i.e., the sum of squares of the effects (SS) of each parameter (with the exception of the dummy variable). The parameter was calculated according to the equation:
where SS is the sum of the squares of the effects of each variable, E
(xi) is the effect of the variable, and N is the number of runs.
The most significant parameters affecting the DCW and DHA concentration were screened, followed by the determination of the parameter values resulting in the highest DCW and DHA levels. This was achieved by using a statistical response surface methodology to formulate a list of experiments with Design-Expert software by Stat-Ease Inc. The method involved testing the parameters at five levels: −2, −1, 0, 1, and 2, and establishing an experimental design with central and axial points (
Table 5). Other parameters previously identified as non-significant were maintained at a constant level. The experimental design matrix consisted of a 2
4 full factor design combined with six central points, and eight axial points, where one variable was set at an extreme level while the others were set at the central point level (
Table 6).
Based on the experimental results, the DCW and DHA values were correlated by the second-order polynomial equation:
where Y is the predicted response of the design, β is the coefficient of the equation, and x
i and x
j are coded levels of parameters i and j, respectively.
The variable matrix equation was generated using Design-Expert software by Stat-Ease Inc. The significance level for the model was confirmed with an F-test. The selected cultural conditions resulting in highest DCW and DHA levels were verified through two experiments. The first experiment was to compare the experimental data with the predicted DCW levels, the other was to compare the experimental data with the predicted DHA concentrations.
2.6. Statistical Analysis
Each experimental variant was conducted in triplicate. The statistical analysis of experimental results was conducted using the STATISTICA 13.1 PL package. The hypothesis concerning the normality of distribution of each analyzed variable was verified using a W Shapiro–Wilk test. One-way analysis of variance (ANOVA) was conducted to determine differences between variables. Homogeneity of variance in groups was determined using a Levene test. The Tukey (HSD) test was applied to determine the significance of differences between the analyzed variables. In the tests, results were considered significant at α = 0.05.
4. Discussion
The present experiments are part of larger pioneer research efforts on the use of waste organic substrates to grow heterotrophic microalgae
Schizochytrium sp. for industrial purposes [
47,
48]. As these microalgae are able to grow on a variety of carbon sources, they may reasonably be grown on waste glycerol [
49]. The biomass of
Schizochytrium sp. heterotrophic microalgae contains high amounts of DHA and no heavy metals (e.g., mercury), making it highly suitable for use in food supplements, food products, and animal feed [
50,
51]. To ensure an optimum growth rate of
Schizochytrium sp. biomass and, above all, to enable scale-up of production for commercial purposes, a variety of factors need to be optimized and controlled for, including physicochemical parameters of the culture, properties of the waste substrate, presence of potential growth inhibitors, substrate availability and cost, culture productivity, investment costs, and the choice of the microalgae strain [
52]. Therefore, more advanced research is needed on this issue.
The present study showed that DCW and DHA levels in
Schizochytrium sp. microalgae are most significantly influenced by: temperature, crude glycerin concentration in the medium, oxygen concentration, and peptone concentration in the bioreactor. A temperature of 26 °C favored DHA production in the cells, whereas higher temperatures promoted biomass growth. These findings are corroborated by the authors of [
53,
54], who reported that temperature was the primary driver of cell concentration in the culture medium and the accumulation of bioactive compounds in the biomass. The optimal value for producing the highest biomass levels varies with the microalgae strain and its source environment. Many authors [
55,
56,
57,
58], have noted that fatty acid production in
Chlorella minutissima,
Pythium irregulare, and
Crypthecodinium cohnii cells consistently occurred at low temperatures, as opposed to temperature levels most favorable to biomass growth in process systems. According to [
59,
60], the increased PUFA content in the investigated microalgae cells at low culture temperatures was linked to increased cell membrane elasticity in the microorganisms, triggered as a defense mechanism. The increase might also be attributed to the higher availability of intracellular molecular oxygen at low temperatures, leading to the activation of oxygen-dependent enzymes that desaturate and elongate PUFAs in cells [
61,
62].
The present study demonstrated that a crude glycerin concentration of 150 g/dm
3 led to the highest DCW levels at 67.55 g/dm
3, as well as an increase of DHA concentration in
Schizochytrium sp. cells to 17.25 g/dm
3. Comparable results were obtained by Huang et al. (2012). Their study results indicated that at 100 g/dm
3 glycerol,
Aurantiochytrium limacinum SR21 achieved a biomass at 61.76 g/dm
3 and DHA concentration at 20.3 g/dm
3 [
63]. A lower production was observed by Chi et al. (2007), according to whom the optimal range of crude glycerol concentration is lower to support the growth of
Schizochytrium limacinum algae and DHA production, and amounts to 75–100 g/dm
3. A highest DHA yield of 4.91 g/dm
3 with 22.1 g/dm
3 DCW was obtained [
50]. Moreover, Scott et al. (2011) achieved less efficient results. Using 64.7 g/dm
3 of crude glycerol, they achieved 31.66 g/dm
3 biomass of
Thraustochytrium sp. ONC T18 and 4.41 g/dm
3 DHA [
64]. Other researchers have also noted how the type and level of the external carbon source affected microalgal cultures [
65,
66]. These authors reported that the carbon source affected biomass growth and might influence PUFA synthesis. Glycerol-based cultures produced higher yields than processes based on glucose, coconut oil, brewery wastes, or wastewater from soymilk production.
Thraustochytriacae microalgae biomass (
Y) grown on crude (waste) glycerol was found to produce higher DHA yields (166–550 mg/g) than a pure glycerol-based culture (110–223 mg/g) [
50,
67]. It is worth noting, however, that the final cell DHA levels are mainly determined by the cell growth phase at the time of DHA extraction.
Previous scientific reports have optimized the oxygen level in microalgae cultures for the production of PUFAs, including DHA. The study described in patent [
68] proposed the induction of higher DHA concentrations by lowering oxygen in the growth medium. Oxygen limitation caused a decrease in monounsaturated fatty acids and activated oxygen-dependent PUFA synthase, thus increasing cell DHA and DPA [
69,
70]. A study by Chi et al. (2009) [
71] showed that cultures grown at 10% oxygen exhibited low microalgal growth in favor of an increasing concentration of fatty acids in the biomass, whereas the reverse was true at 50% oxygen. These findings are corroborated by the present results, where the 30% oxygen concentration was found to favor DHA production in the cells, (DCW = 63.59 g/dm
3, C
DHA = 17.79 g/dm
3), and the highest biomass concentration was obtained when the system was oxygen-saturated to 50% (DCW = 67.55 g/dm
3, C
DHA = 15.53 g/dm
3). This can be explained by the fact that biomass growth necessitates the production of large quantities of primary metabolites, such as nucleic acids, enzymes, and proteins. The production of these compounds requires high oxygen in the process system [
71,
72].
The dry mass of
Schizochytrium sp. microalgae contains 14–20% nitrogen (
w/
w), mainly incorporated into the structure of proteins and nucleic acids [
53,
73]. In order to meet cellular nitrogen demand, growth mediums are spiked with various nitrogen sources, such as corn steep, peptone, ammonium sulfate, or mixtures thereof [
74,
75]. Other alternatives to cheap nitrogen sources include fish waste hydrolysate, silkworm larvae, and wheat bran extract [
76]. It is important to note that different nitrogen sources are preferred for different strains to ensure rapid cell growth and the accumulation of metabolites (such as DHA) in technological processes [
77,
78]. The present study confirms that a 9.99 g/dm
3 dose of peptone is required for an increased concentration of
Schizochytrium sp. cells in the growth medium, whereas only 2.21 g/dm
3 is needed at the lipid (DHA) accumulation stage. This observation supports the thesis that the generation of new cells and biomass requires a supply of primary metabolite components, such as enzymes, proteins, or nucleic acids. The nitrogen demand is less pronounced in cultures designed for secondary metabolite (Omega-3 acids) synthesis [
71,
79]. It is worth noting that peptone is a complex nitrogen source, meaning that it contains proteins, peptides, and free amino acids, while being low in carbohydrates, lipids, inorganic ions, vitamins, and growth factors. In addition to its primary function of supplying nitrogen to cell biomass, peptone also promotes overall cell development, granting an advantage over industrial nitrogen sources [
80,
81].
5. Conclusions
The experiments presented in this study showed the high potential of using the waste fraction of glycerol from biodiesel production to produce biomass and DHA by Schizochytrium sp. microalgae. In the course of designing and executing the study, as well as analyzing the results of optimization and experimental work, the theses made prior to the experiments were verified.
In the course of the study, parameters having a significant effect on Schizochytrium sp. cell growth and DHA accumulation were screened using the Plackett–Burman design and quantified using the response surface methodology.
The study has shown that the growth and DHA production of Schizochytrium sp. microalgae was most significantly influenced by the following physicochemical parameters: crude glycerin concentration in the growth medium (150 g/dm3), process temperature (27 °C), oxygen concentration in the bioreactor (49.99% v/v), and peptone concentration as a source of nitrogen (9.99 g/dm3). Other parameters, such as initial pH, volumetric air flow rate, inoculum level, salinity, turbine speed, and concentration of yeast extract, were not statistically significant and were therefore eliminated from further analyses.
The optimization methods used in the study identified the following physicochemical parameter values as optimal for producing high DHA concentrations in the biomass: temperature 26 °C, crude glycerin concentration 149.99 g/dm3, oxygen concentration 30% (v/v), and peptone concentration 2.21 g/dm3.
Laboratory verification produced DCW levels of 66.69 ± 0.66 g/dm3, i.e., 1.27% lower than the predicted value. When cell DHA levels are compared between the DHA-optimized culture (17.25 ± 0.33 g/dm3) and the value predicted using the response surface methodology (17.79 g/dm3), the former is found to be lower by 3.03%.
The results suggest that a two-step culture system should be employed, with the culture process divided into two phases. The first phase would target higher cell concentration, while the second, higher DHA yields from the biomass.