Conversion of Slaughterhouse Wastes to Solid Fuel Using Hydrothermal Carbonization
Abstract
:1. Introduction
2. Materials and Methods
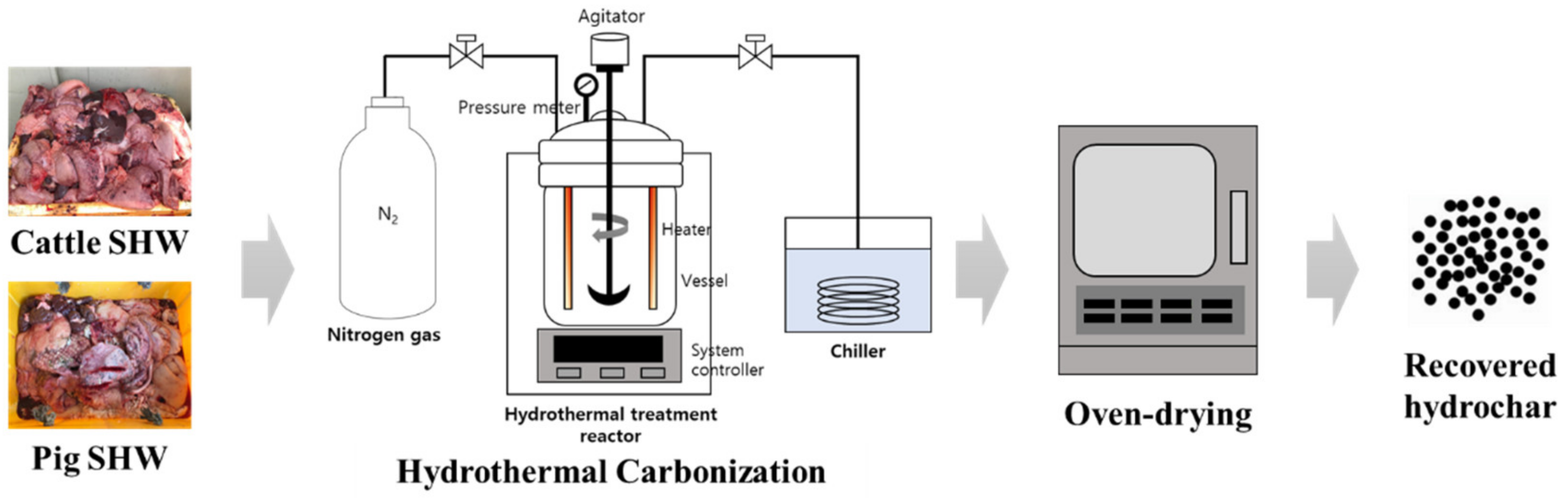
2.1. Feedstock
2.2. Hydrothermal Carbonization (HTC)
where HHV is the higher heating value
2.3. Analytical Methods
3. Results and Discussion
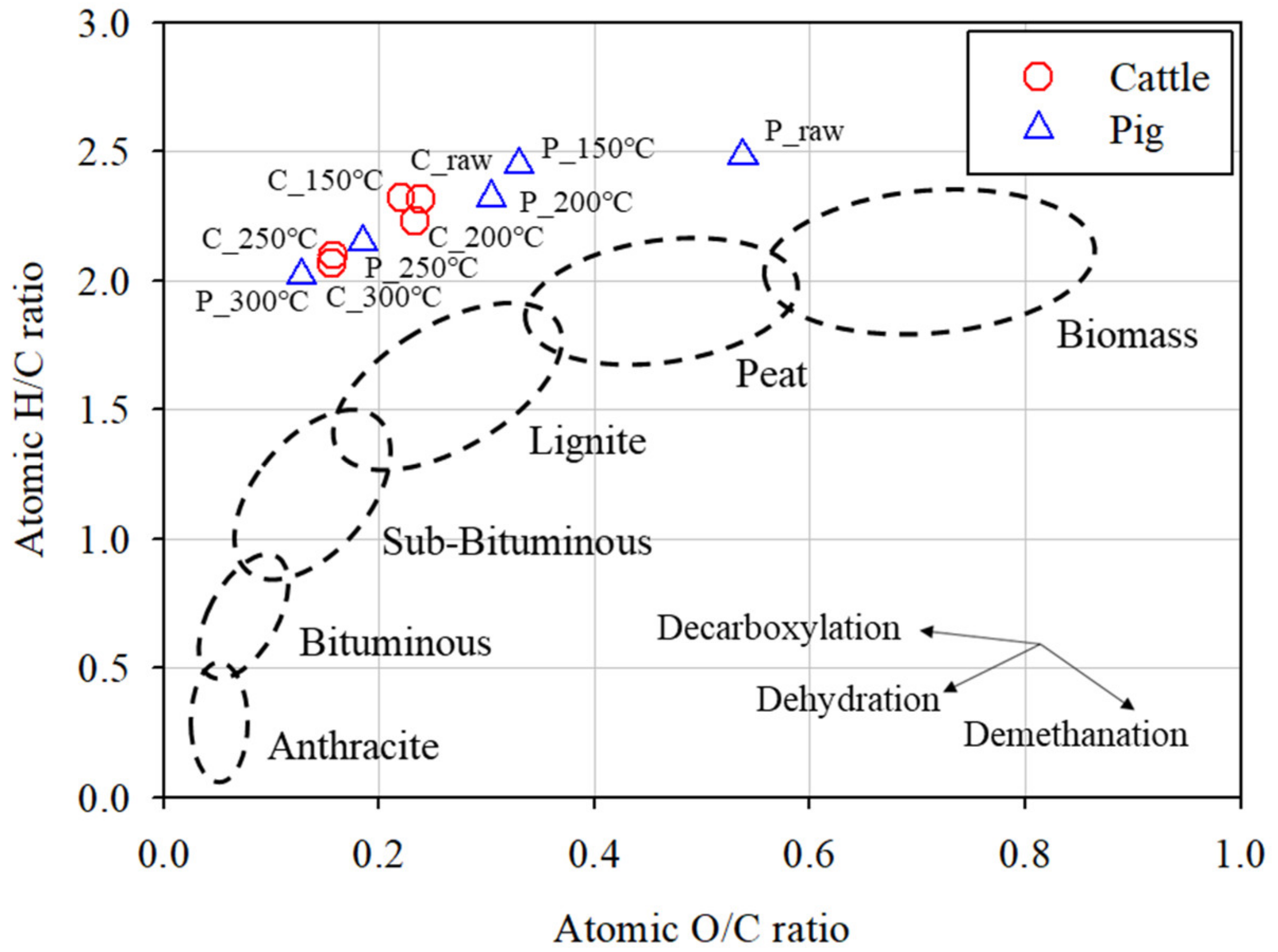
3.1. Properties of SHW and Hydrochar
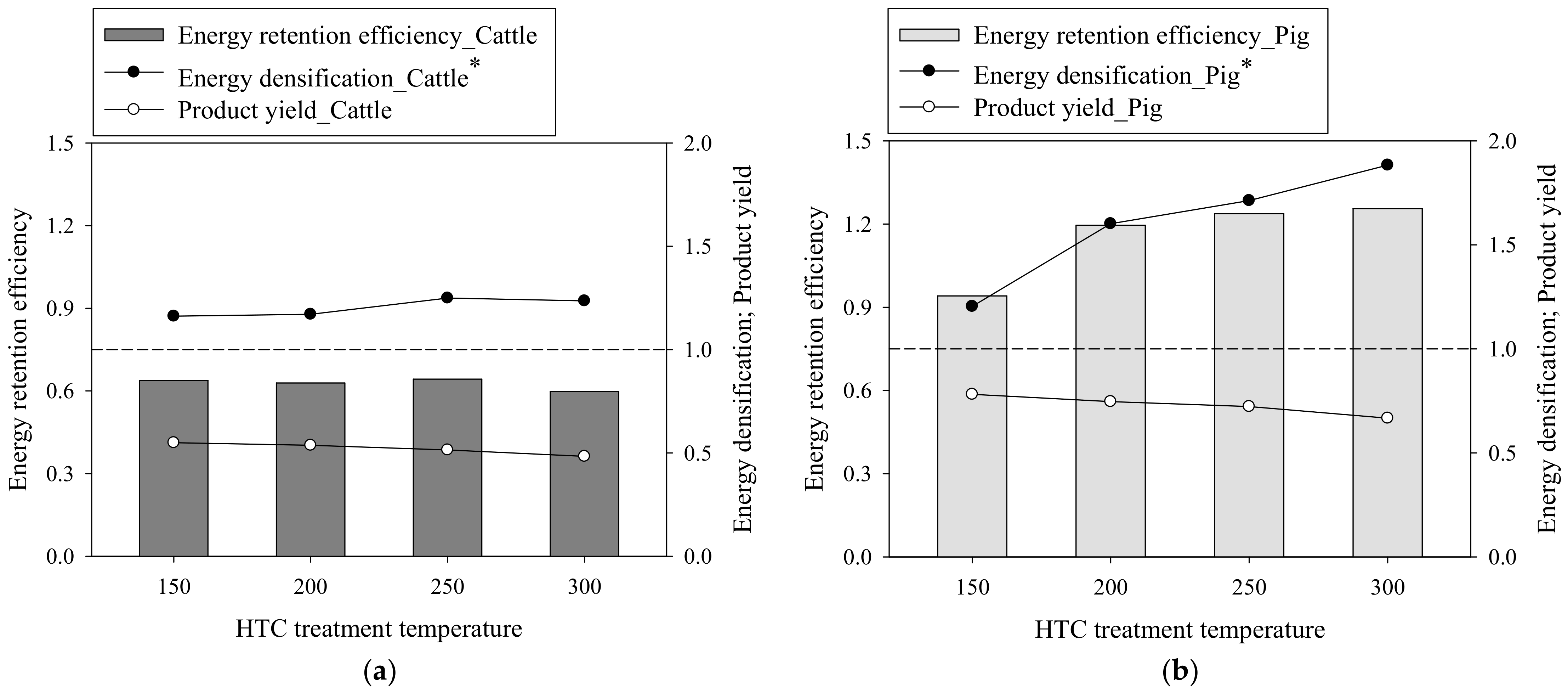
3.2. Improvements in the SHW Properties
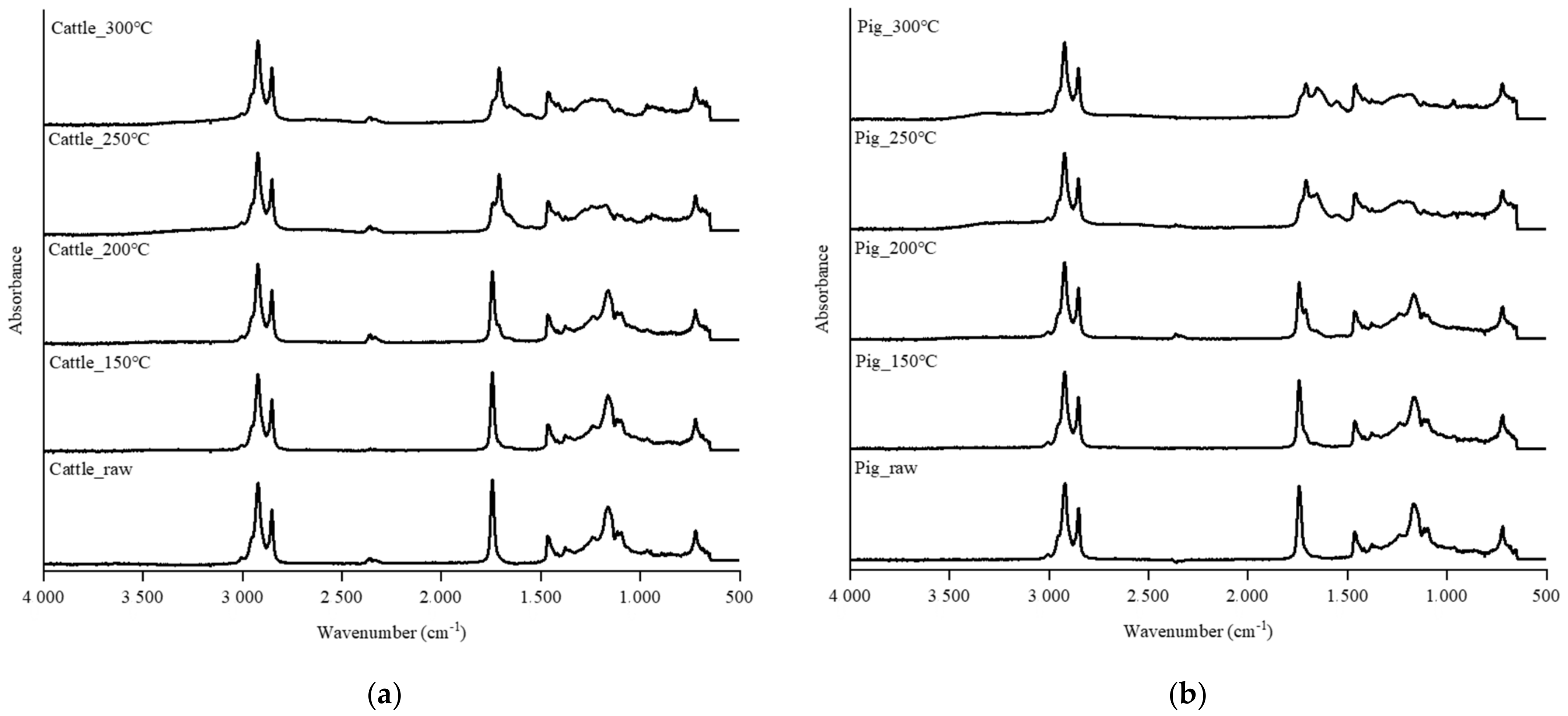
3.3. Changes of Functional Groups during the HTC Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of slaughterhouse waste in value-added applications: Recent advances in the development of wood adhesives. Polymers 2018, 10, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeker, D.L. North American Rendering: Processing high quality protein and fats for feed. Rev. Bras. Zootec. 2009, 38, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Gwyther, C.L.; Williams, A.P.; Golyshin, P.N.; Edwards-Jones, G.; Jones, D.L. The environmental and biosecurity characteristics of livestock carcass disposal methods: A review. Waste Manag. 2011, 31, 767–778. [Google Scholar] [CrossRef] [Green Version]
- Lasekan, A.; Bakar, F.A.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Bah, C.S.; Bekhit, A.E.D.A.; Carne, A.; McConnell, M.A. Slaughterhouse blood: An emerging source of bioactive compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Mekonnen, T.H.; Mussone, P.G.; Stashko, N.; Choi, P.Y.; Bressler, D.C. Recovery and characterization of proteinacious material recovered from thermal and alkaline hydrolyzed specified risk materials. Process Biochem. 2013, 48, 885–892. [Google Scholar] [CrossRef]
- Palatsi, J.; Viñas, M.; Guivernau, M.; Fernandez, B.; Flotats, X. Anaerobic digestion of slaughterhouse waste: Main process limitations and microbial community interactions. Bioresour. Technol. 2011, 102, 2219–2227. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Insam, H. Treatment alternatives of slaughterhouse wastes, and their effect on the inactivation of different pathogens: A review. Crit. Rev. Microbiol. 2013, 39, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Broch, A.; Jena, U.; Hoekman, S.K.; Langford, J. Analysis of solid and aqueous phase products from hydrothermal carbonization of whole and lipid-extracted algae. Energies 2013, 7, 62–79. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Choi, O.K.; Oh, D.; Lee, K.; Park, K.Y.; Kim, D. Stimulation of Lipid Extraction Efficiency from Sewage Sludge for Biodiesel Production through Hydrothermal Pretreatment. Energies 2020, 13, 6392. [Google Scholar] [CrossRef]
- Holubčík, M.; Klačková, I.; Ďurčanský, P. Pyrolysis conversion of polymer wastes to noble fuels in conditions of the Slovak Republic. Energies 2020, 13, 4849. [Google Scholar] [CrossRef]
- Li, Z.; Yi, W.; Li, Z.; Tian, C.; Fu, P.; Zhang, Y.; Zhou, L.; Teng, J. Preparation of Solid Fuel Hydrochar over Hydrothermal Carbonization of Red Jujube Branch. Energies 2020, 13, 480. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, D.; Lee, K.; Park, K.Y. Solid fuel production through hydrothermal carbonization of sewage sludge and microalgae Chlorella sp. from wastewater treatment plant. Chemosphere 2019, 230, 157–163. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.Y. Conversion of heavy metal-containing biowaste from phytoremediation site to value-added solid fuel through hydrothermal carbonization. Environ. Pollut. 2020, 269, 116127. [Google Scholar] [CrossRef]
- Lee, J.; Hong, J.; Jang, D.; Park, K.Y. Hydrothermal carbonization of waste from leather processing and feasibility of produced hydrochar as an alternative solid fuel. J. Environ. Manag. 2019, 247, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Dutta, A.; Gallant, J. Hydrothermal carbonization of peat moss and herbaceous biomass (Miscanthus): A potential route for bioenergy. Energies 2018, 11, 2794. [Google Scholar] [CrossRef] [Green Version]
- Lucian, M.; Fiori, L. Hydrothermal carbonization of waste biomass: Process design, modeling, energy efficiency and cost analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, Y.; Yang, H.; Gentili, F.G.; Söderlind, U.; Wang, X.; Zhang, W.; Chen, H. Hydrothermal carbonization of natural microalgae containing a high ash content. Fuel 2019, 249, 441–448. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.; Balasubramanian, R. Hydrothermal carbonization of sewage sludge for energy production with coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Sheng, C.; Azevedo, J. Estimating the higher heating value of biomass fuels from basic analysis data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Thipkhunthod, P.; Meeyoo, V.; Rangsunvigit, P.; Kitiyanan, B.; Siemanond, K.; Rirksomboon, T. Predicting the heating value of sewage sludges in Thailand from proximate and ultimate analyses. Fuel 2005, 84, 849–857. [Google Scholar] [CrossRef]
- Rubiera, F.; Arenillas, A.; Pevida, C.; Garcıa, R.; Pis, J.; Steel, K.; Patrick, J. Coal structure and reactivity changes induced by chemical demineralisation. Fuel Process Technol. 2002, 79, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Ge, S.; Chen, Z.; Li, X. Study on pore characteristics of flocs and sludge dewaterability based on fractal methods (pore characteristics of flocs and sludge dewatering). Appl. Therm. Eng. 2013, 58, 217–223. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Xiao, K.; Jin, M.; Xiao, H.; Yao, H. Combustion and Pyrolysis Characteristics of Hydrochar Prepared by Hydrothermal Carbonization of Typical Food Waste: Influence of Carbohydrates, Proteins, and Lipids. Energy Fuels 2019, 34, 430–439. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Xiao, K.; Liu, X.; Hu, H.; Li, X.; Yao, H. Correlations between the physicochemical properties of hydrochar and specific components of waste lettuce: Influence of moisture, carbohydrates, proteins and lipids. Bioresour. Technol. 2019, 272, 482–488. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Silva, J.; Rodrigues Filho, G.; da Silva Meireles, C.; Ribeiro, S.D.; Vieira, J.G.; da Silva, C.V.; Cerqueira, D.A. Thermal analysis and FTIR studies of sewage sludge produced in treatment plants. The case of sludge in the city of Uberlândia-MG, Brazil. Thermochim. Acta 2012, 528, 72–75. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub-and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Liu, S. Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 2011, 346, 999–1004. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.; Laginhas, C.; Ledesma, B.; González, J. Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef]
- Wei, X.; Ma, X.; Peng, X.; Yao, Z.; Yang, F.; Dai, M. Comparative investigation between co-pyrolysis characteristics of protein and carbohydrate by TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2018, 135, 209–218. [Google Scholar] [CrossRef]

| Cattle | Pig | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | 150 | 200 | 250 | 300 | Raw | 150 | 200 | 250 | 300 | ||
| Moisture 1 | 26.26 | - | - | - | - | 48.39 | - | - | - | - | |
| Proximate analysis (wt.%, dry) | VM | 98.95 | 98.61 | 98.27 | 98.09 | 98.08 | 98.69 | 98.02 | 97.78 | 97.57 | 97.11 |
| Ash | 0.95 | 1.23 | 1.34 | 1.47 | 1.37 | 1.04 | 1.36 | 1.49 | 1.68 | 2.13 | |
| FC | 0.10 | 0.16 | 0.39 | 0.44 | 0.55 | 0.27 | 0.62 | 0.73 | 0.75 | 0.76 | |
| Ultimate analysis (wt.%, dry) | Carbon | 65.52 | 66.95 | 66.29 | 71.80 | 71.94 | 50.91 | 57.87 | 60.46 | 67.39 | 71.24 |
| Hydrogen | 5.63 | 5.95 | 5.32 | 5.55 | 5.40 | 6.53 | 6.81 | 5.70 | 6.07 | 5.99 | |
| Oxygen | 27.81 | 26.70 | 27.61 | 22.02 | 22.01 | 40.50 | 33.48 | 32.46 | 24.58 | 21.14 | |
| Nitrogen | 11.05 | 0.40 | 0.78 | 0.63 | 0.64 | 1.97 | 1.84 | 1.37 | 1.96 | 1.63 | |
| Sulfur | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Composition analysis (wt.%, dry) | Carbohydrate | 1.45 | 1.34 | 0.20 | 0.03 | 0.00 | 13.86 | 2.81 | 2.25 | 0.79 | 0.03 |
| Protein | 1.00 | 1.19 | 1.00 | 0.63 | 0.40 | 13.13 | 11.25 | 8.83 | 9.63 | 7.29 | |
| Lipid | 74.75 | 75.66 | 82.33 | 85.05 | 85.32 | 33.25 | 50.72 | 69.32 | 85.06 | 89.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Cho, S.; Kim, D.; Ryu, J.; Lee, K.; Chung, H.; Park, K.Y. Conversion of Slaughterhouse Wastes to Solid Fuel Using Hydrothermal Carbonization. Energies 2021, 14, 1768. https://doi.org/10.3390/en14061768
Lee J, Cho S, Kim D, Ryu J, Lee K, Chung H, Park KY. Conversion of Slaughterhouse Wastes to Solid Fuel Using Hydrothermal Carbonization. Energies. 2021; 14(6):1768. https://doi.org/10.3390/en14061768
Chicago/Turabian StyleLee, Jongkeun, Sungwan Cho, Daegi Kim, JunHee Ryu, Kwanyong Lee, Haegeun Chung, and Ki Young Park. 2021. "Conversion of Slaughterhouse Wastes to Solid Fuel Using Hydrothermal Carbonization" Energies 14, no. 6: 1768. https://doi.org/10.3390/en14061768
APA StyleLee, J., Cho, S., Kim, D., Ryu, J., Lee, K., Chung, H., & Park, K. Y. (2021). Conversion of Slaughterhouse Wastes to Solid Fuel Using Hydrothermal Carbonization. Energies, 14(6), 1768. https://doi.org/10.3390/en14061768









