High Efficiency of the Removal Process of Pb(II) and Cu(II) Ions with the Use of Fly Ash from Incineration of Sunflower and Wood Waste Using the CFBC Technology
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.1.1. Sunflower Wood Fly Ash Preparation
2.1.2. Sunflower Wood Fly Ash Characterization
2.1.3. Pb(II) and Cu(II) Adsorption Process
3. Results and Discussion
3.1. Characterization of the Adsorbent
3.2. Adsorption Studies of Pb(II) and Cu(II) Ions
3.2.1. Analysis of pH Profile
3.2.2. Impact of Adsorbent Dosage
3.2.3. Impact of Initial Concentration of Pb(II) and Cu(II)
3.2.4. Reaction Kinetics
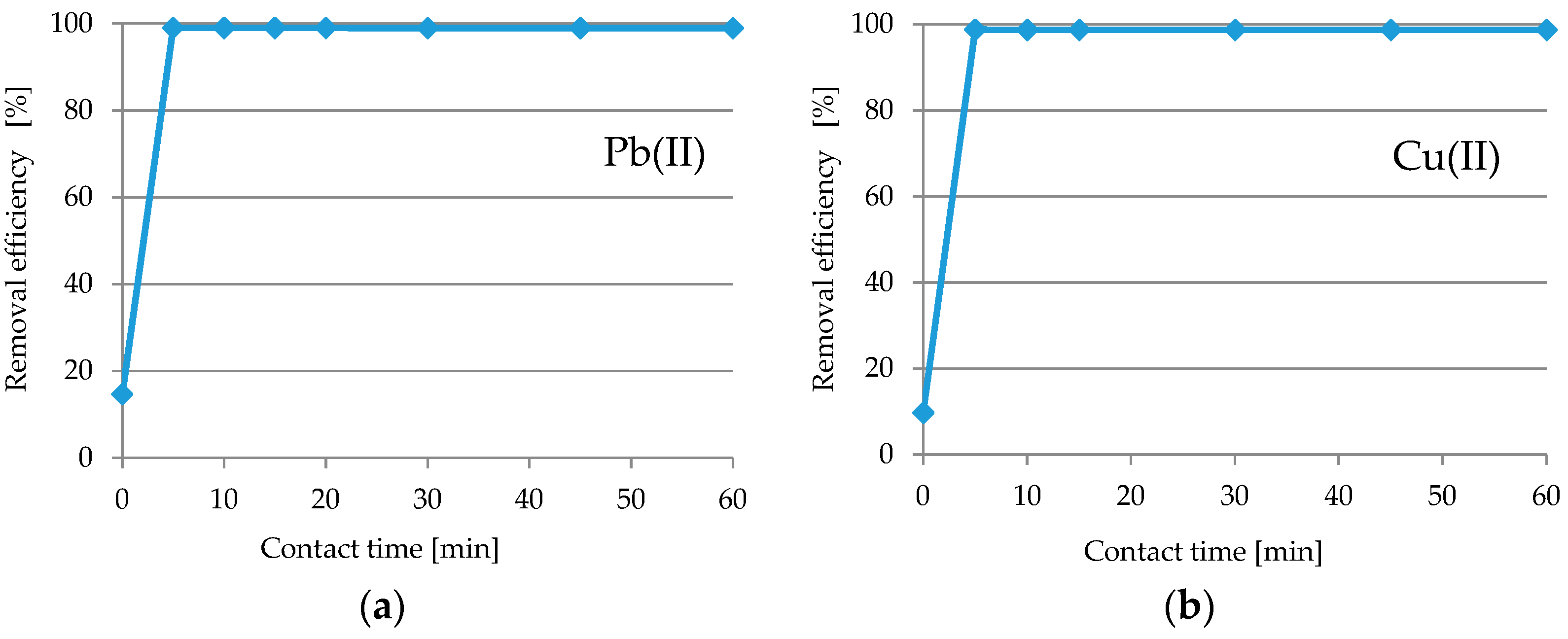
Contact Time Studies
Pseudo-First-Order and Pseudo-Second-Order Kinetic Models
3.2.5. Isothermal Studies
3.3. FT-IR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalak, T. Responsibility for Marine Litter Floating in the North Pacific Ocean; LAP Lambert Academic Publishing: Sunnyvale, CA, USA, 2018; ISBN 978-613-9-94366-1. [Google Scholar]
- Ramana, D.K.V.; Reddy, D.H.K.; Kumar, B.N.; Seshaiah, K.; Rao, G.P.C.; Lu, C. Adsorption of Pb(II) from Aqueous Solutions by Chemically Modified Zeolite supported Carbon Nanotubes: Equilibrium, Kinetic, and Thermodynamic Studies. Sep. Sci. Technol. 2013, 48, 403–412. [Google Scholar] [CrossRef]
- Assi, M.A.; Hezmee, M.N.M.; Haron, A.W.; Sabri, M.Y.M.; Rajion, M.A. The detrimental effects of lead on human and animal health. Veter World 2016, 9, 660–671. [Google Scholar] [CrossRef]
- Rousseau, M.-C.; Straif, K.; Siemiatycki, J. IARC Carcinogen Update. Environ. Health Perspect. 2005, 113, 580. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Larous, S.; Meniai, A.-H.; Lehocine, M.B. Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination 2005, 185, 483–490. [Google Scholar] [CrossRef]
- Araya, M.; Olivares, M.; Pizarro, F. Copper in human health. Int. J. Environ. Health 2007, 1, 608. [Google Scholar] [CrossRef]
- UNEP. Global Drinking Water Quality Index Development and Sensitivity Analysis Report; United Nations Environment Programme: Geneva, Switzerland, 2007. [Google Scholar]
- The, U.S. Environmental Protection Agency. Lead and Copper Rule for Drinking Water, Drinking Water Requirements for States and Public Water Systems; USEPA: Washington, DC, USA, 2016.
- Soliman, N.; Moustafa, A. Industrial solid waste for heavy metals adsorption features and challenges; A review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Filipowiak, K.; Wojciechowska, I.; Aksamitowski, P. Novel ionic liquid-modified polymers for highly effective adsorption of heavy metals ions. Sep. Purif. Technol. 2020, 236, 116313. [Google Scholar] [CrossRef]
- Sandoval-Flores, G.; Alvarado-Reyna, S.; Elvir-Padilla, L.G.; Mendoza-Castillo, D.I.; Reynel-Avila, H.E.; Bonilla-Petriciolet, A. Kinetics, Thermodynamics, and Competitive Adsorption of Heavy Metals from Water Using Orange Biomass. Water Environ. Res. 2018, 90, 2114–2125. [Google Scholar] [CrossRef]
- Ajmal, M.; Rao, R.A.K.; Ahmad, R.; Ahmad, J. Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni(II) from electroplating wastewater. J. Hazard. Mater. 2000, 79, 117–131. [Google Scholar] [CrossRef]
- Chiban, M.; Soudani, A.; Sinan, F.; Tahrouch, S.; Persin, M. Characterization and Application of Dried Plants to Remove Heavy Metals, Nitrate, and Phosphate Ions from Industrial Wastewaters. Clean Soil Air Water 2011, 39, 376–383. [Google Scholar] [CrossRef]
- Osman, A.I.; Ahmed, A.T.; Johnston, C.R.; Rooney, D.W. Physicochemical characterization of miscanthus and its application in heavy metals removal from wastewaters. Environ. Prog. Sustain. Energy 2018, 37, 1058–1067. [Google Scholar] [CrossRef]
- Aman, T.; Kazi, A.A.; Sabri, M.U.; Bano, Q. Potato peels as solid waste for the removal of heavy metal copper(II) from waste water/industrial effluent. Colloids Surf. B Biointerfaces 2008, 63, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Mata, Y.; Blázquez, M.; Ballester, A.; González, F.; Muñoz, J. Sugar-beet pulp pectin gels as biosorbent for heavy metals: Preparation and determination of biosorption and desorption characteristics. Chem. Eng. J. 2009, 150, 289–301. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Demir, M.; Gosu, V. Adsorptive removal of heavy metals from industrial effluents using cow dung as the biosorbent: Kinetic and isotherm modeling. Environ. Qual. Manag. 2020, 30, 51–60. [Google Scholar] [CrossRef]
- Joshi, N.C.; Singh, A.; Rajput, H. Utilization of Waste Leaves Biomass of Myrica Esculenta for the Removal of Pb (II), Cd (II) and Zn (II) Ions from Waste Waters. Orient. J. Chem. 2018, 34, 2548–2553. [Google Scholar] [CrossRef]
- Czikkely, M.; Neubauer, E.; Fekete, I.; Ymeri, P.; Fogarassy, C. Review of Heavy Metal Adsorption Processes by Several Organic Matters from Wastewaters. Water 2018, 10, 1377. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Eom, J.-H.; Lee, S.-L.; Hwang, S.-W.; Kim, S.-H.; Kang, S.-W.; Yun, J.-J.; Cho, J.-S.; Lee, Y.-H.; Seo, D.-C. Exploration of the potential capacity of fly ash and bottom ash derived from wood pellet-based thermal power plant for heavy metal removal. Sci. Total Environ. 2020, 740, 140205. [Google Scholar] [CrossRef]
- Qiu, R.; Cheng, F.; Huang, H. Removal of Cd2+ from aqueous solution using hydrothermally modified circulating fluidized bed fly ash resulting from coal gangue power plant. J. Clean. Prod. 2018, 172, 1918–1927. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible applications of coal fly ash in wastewater treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef]
- Buema, G.; Harja, M.; Lupu, N.; Chiriac, H.; Forminte, L.; Ciobanu, G.; Bucur, D.; Bucur, R.D. Adsorption Performance of Modified Fly Ash for Copper Ion Removal from Aqueous Solution. Water 2021, 13, 207. [Google Scholar] [CrossRef]
- Wu, P.; Tang, Y.; Cai, Z. Dual role of coal fly ash in copper ion adsorption followed by thermal stabilization in a spinel solid solution. RSC Adv. 2018, 8, 8805–8812. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Goswami, L.; Singh, A.K.; Sikandar, M. Valorization of coal fired-fly ash for potential heavy metal removal from the single and multi-contaminated system. Heliyon 2019, 5, e02562. [Google Scholar] [CrossRef] [Green Version]
- Xue, Q.; Li, J.-S.; Wang, P.; Liu, L.; Li, Z.-Z. Removal of Heavy Metals from Landfill Leachate Using Municipal Solid Waste Incineration Fly Ash as Adsorbent. Clean Soil Air Water 2014, 42, 1626–1631. [Google Scholar] [CrossRef]
- Yadla, S.V.; Sridevi, V.; Chandana Lakshmi, M.V.V. Adsorption Performance of Fly Ash for the Removal of Lead. Int. J. Eng. Res. Technol. 2012, 1, 1–7. [Google Scholar]
- Centrum Inżynierii Minerałów Antropogenicznych (Center for Engineering of Anthropogenic Minerals). Available online: http://www.cima.ibs.pw.edu.pl (accessed on 12 February 2021).
- Filipiak, J. Wykorzystanie ubocznych produktów spalania jako stabilizatora do wzmacniania gruntów organicznych (The use of combustion by-products as a stabilizer to strengthen organic soils). Rocz. Ochr. Sr. (Yearb. Environ. Prot.) 2013, 15, 1153–1163. [Google Scholar]
- Spalarnie Odpadów (Waste Incinerators). Available online: http://www.spalarnie-odpadow.pl (accessed on 11 February 2021).
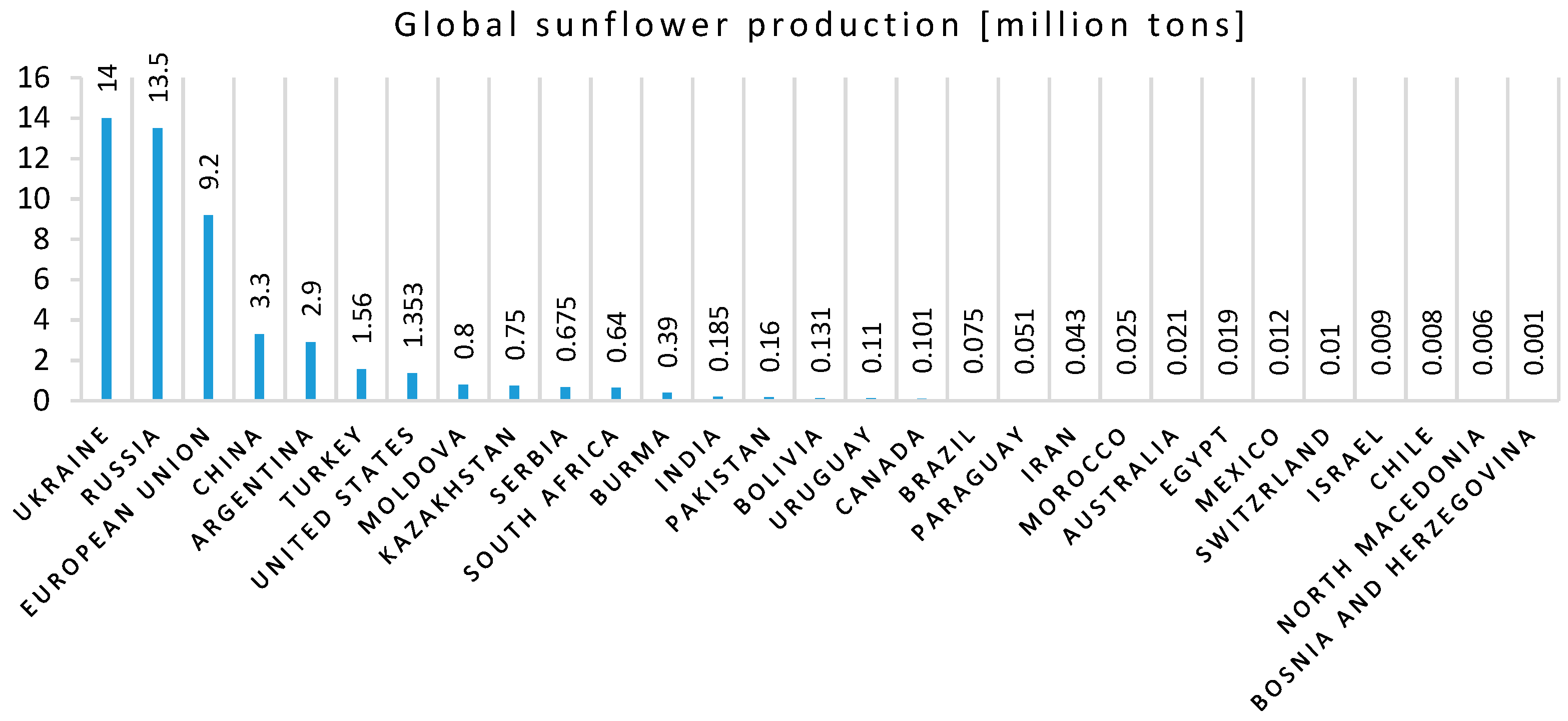
- World Agricultural Production. World Sunflower Production 2020/2021. Available online: http://www.worldagriculturalproduction.com/crops/sunflower.aspx (accessed on 11 January 2021).
- Pilorgé, E. Sunflower in the global vegetable oil system: Situation, specificities and perspectives. OCL 2020, 27, 34. [Google Scholar] [CrossRef]
- Shahbazpanahi, S.; Faraj, R.H. Feasibility study on the use of shell sunflower ash and shell pumpkin ash as supplementary cementitious materials in concrete. J. Build. Eng. 2020, 30, 101271. [Google Scholar] [CrossRef]
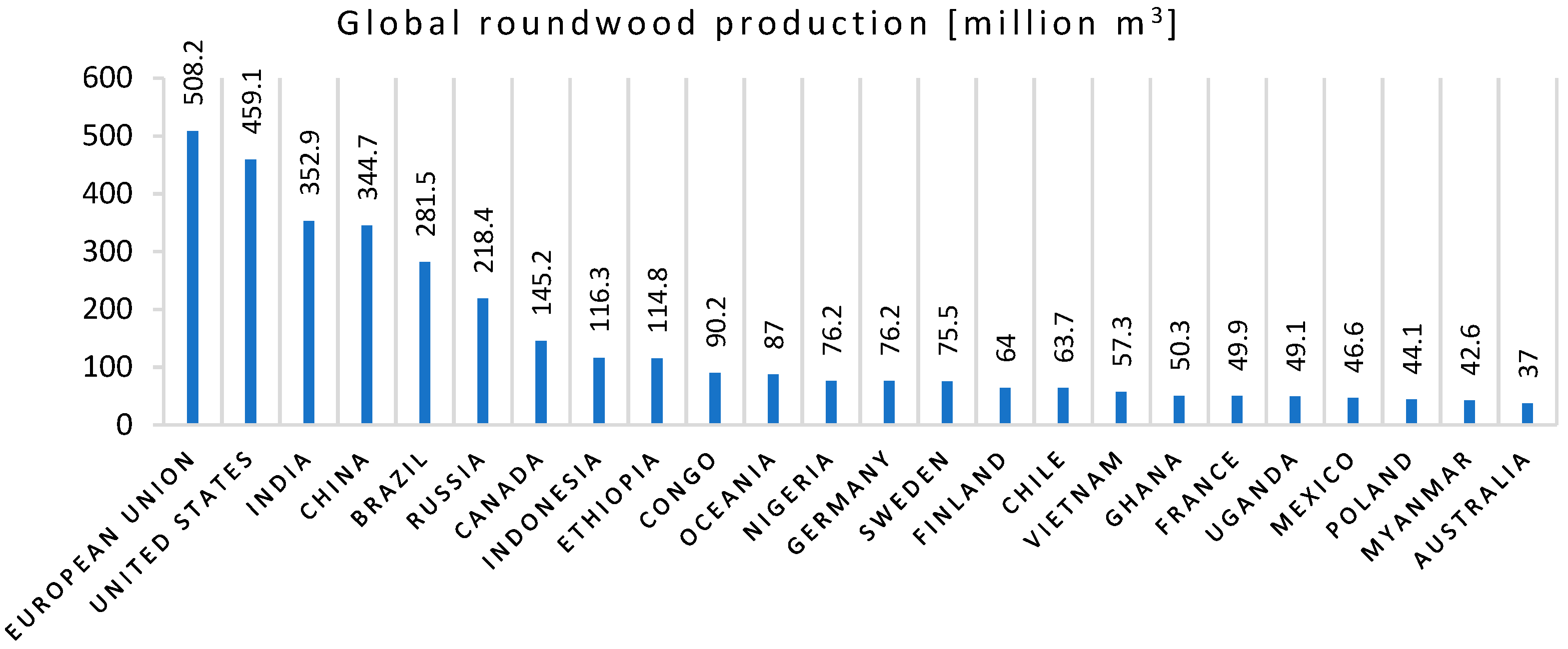
- UNdata, FOASTAT, Food and Agriculture Organization, Global Roundwood Production 2019. Available online: http://data.un.org/Data.aspx?d=FAO&f=itemCode%3a1861 (accessed on 10 February 2021).
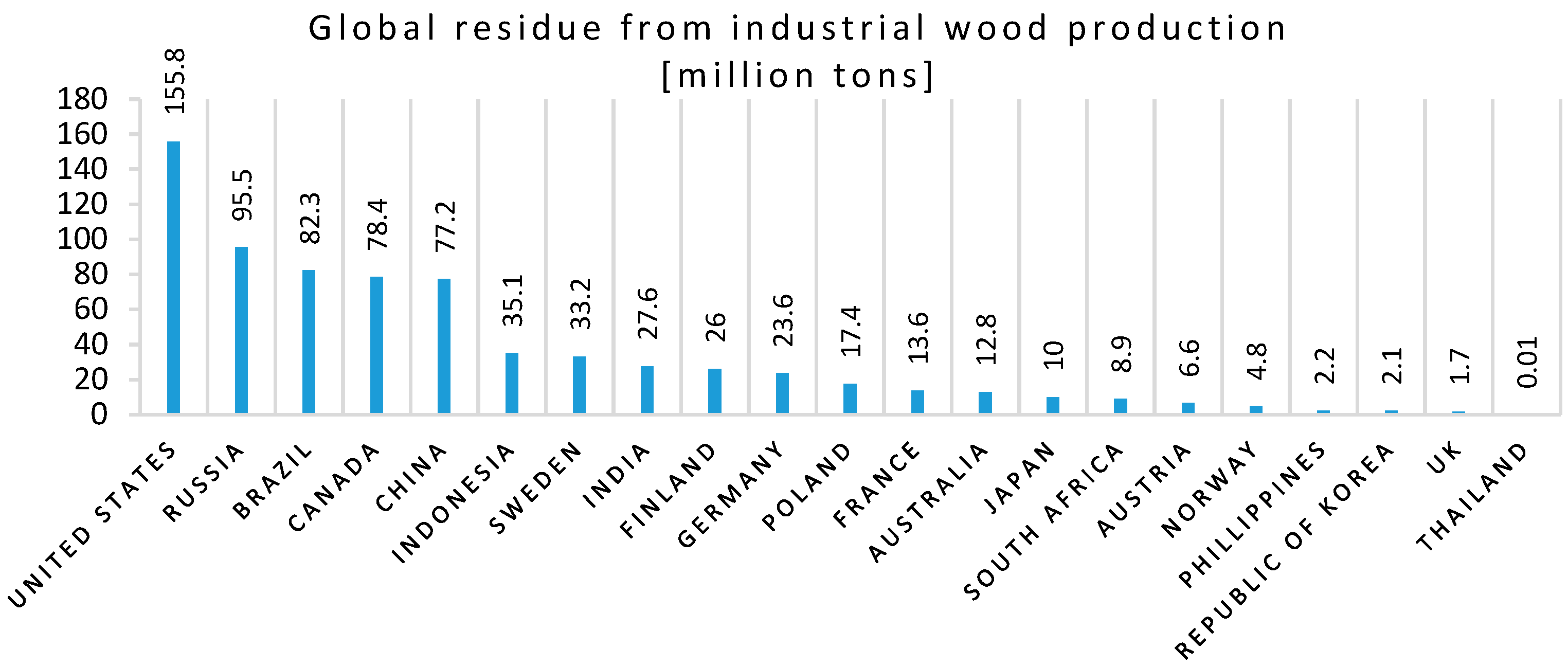
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.A.; Hora, G. State-of-the-art of waste wood supply chain in Germany and selected European countries. Waste Manag. 2017, 70, 189–197. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Shim, Y.-S.; Kim, Y.-K.; Kong, S.-H.; Rhee, S.-W.; Lee, W.-K. The adsorption characteristics of heavy metals by various particle sizes of MSWI bottom ash. Waste Manag. 2003, 23, 851–857. [Google Scholar] [CrossRef]
- Police, S.; Maity, S.; Chaudhary, D.K.; Dusane, C.K.; Sahu, S.K.; Kumar, A.V. Effect of coal fly ash’s particle size on U adsorption in water samples and thermodynamic study on adsorption. Environ. Chem. Ecotoxicol. 2020, 2, 32–38. [Google Scholar] [CrossRef]
- Itskos, G.; Koukouzas, N.; Vasilatos, C.; Megremi, I.; Moutsatsou, A. Comparative uptake study of toxic elements from aqueous media by the different particle-size-fractions of fly ash. J. Hazard. Mater. 2010, 183, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Lanzerstorfer, C. Fly ash from coal combustion: Dependence of the concentration of various elements on the particle size. Fuel 2018, 228, 263–271. [Google Scholar] [CrossRef]
- Kara, S.; Aydiner, C.; Demirbas, E.; Kobya, M.; Dizge, N. Modeling the effects of adsorbent dose and particle size on the ad-sorption of reactive textile dyes by fly ash. Desalination 2007, 212, 282–293. [Google Scholar] [CrossRef]
- Kalak, T.; Kłopotek, A.; Cierpiszewski, R. Effective adsorption of lead ions using fly ash obtained in the novel circulating flu-idized bed combustion technology. Microchem. J. 2019, 145, 1011–1025. [Google Scholar]
- Kalak, T.; Cierpiszewski, R. Comparative studies on the adsorption of Pb(II) ions by fly ash and slag obtained from CFBC technology. Pol. J. Chem. Technol. 2019, 21, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Señas, L.; Priano, C.; Marfil, S.; Maiza, P.; Valea, J. Final Disposal of Ashes from Sunflower Husk in Cementitious Mortars. Eur. J. Sci. Res. 2012, 74, 292–300. [Google Scholar]
- Brännvall, E.; Andreas, L.; Sjöblom, R.; Diener, S.; Lagerkvist, A. Factors Influencing Chemical and Mineralogical Changes in RDF Fly Ashes during Aging. J. Environ. Eng. 2014, 140, 04013014. [Google Scholar] [CrossRef]
- Sevim, O.; Demir, I. Particle Size Optimization of Fly Ash. Int. J. Adv. Mech. Civ. Eng. 2017, 4, 85–87. [Google Scholar]
- Nnaji, C.C.; Emefu, S.C. Effect of Particle Size on the Sorption of Lead from Water by Different Species of Sawdust: Equilibrium and Kinetic Study. Bioresources 2017, 12, 4123–4145. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, J.; Mohapatra, S.K. Influence of Particle Size on Leaching characteristic of fly ash. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 31 August–2 September 2017; pp. 1–7. [Google Scholar]
- Dos Santos, R.P.; Martins, J.; Gadelha, C.; Cavada, B.; Albertini, A.V.; Arruda, F.; Vasconcelos, M.; Teixeira, E.; Alves, F.; Filho, J.L.; et al. Coal Fly Ash Ceramics: Preparation, Characterization, and Use in the Hydrolysis of Sucrose. Sci. World J. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paya, J.; Monzo, J.; Borrachero, M.V.; Perris, E.; Amahjour, F. Thermogravimetric methods for determinig carbon content in fly ashes. Cem. Concr. Res. 1998, 28, 675–686. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Effects of Sample Preparation and Interpretation of Thermogravimetric Curves on Calcium Hydroxide in Hydrated Pastes and Mortars. Transp. Res. Rec. J. Transp. Res. Board 2012, 2290, 10–18. [Google Scholar] [CrossRef]
- Zhou, Z.; Sofi, M.; Lumantarna, E.; san Nicolas, R.; Hadi, K.G.; Mendis, P. Strength Development and Thermogravimetric Investigation of High-Volume Fly Ash Binders. Materials 2019, 12, 3344. [Google Scholar] [CrossRef] [Green Version]
- López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Strategic Design of Heavy Metals Removal Agents through Zeta Potential Measurements. In Heavy Metals; Saleh, H.E.-D.M., Aglan, R.F., Eds.; InTechOpen: London, UK, 2018. [Google Scholar]
- Cherian, C.; Siddiqua, S. Pulp and Paper Mill Fly Ash: A Review. Sustainability 2019, 11, 4394. [Google Scholar] [CrossRef] [Green Version]
- Topini, D.; Toraldo, E.; Andena, L.; Mariani, E. Use of recycled fillers in bituminous mixtures for road pavements. Constr. Build. Mater. 2018, 159, 189–197. [Google Scholar] [CrossRef]
- Silva, R.; de Brito, J.; Lynn, C.; Dhir, R. Environmental impacts of the use of bottom ashes from municipal solid waste incineration: A review. Resour. Conserv. Recycl. 2019, 140, 23–35. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press, Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2005. [Google Scholar]
- Li, X.; Gao, Z.; Fang, S.; Ren, C.; Yang, K.; Wang, F. Fractal Characterization of Nanopore Structure in Shale, Tight Sandstone and Mudstone from the Ordos Basin of China Using Nitrogen Adsorption. Energies 2019, 12, 583. [Google Scholar] [CrossRef] [Green Version]
- Horikawa, T.; Do, D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface Sci. 2011, 169, 40–58. [Google Scholar] [CrossRef]
- Weng, C.-H.; Huang, C. Adsorption characteristics of Zn(II) from dilute aqueous solution by fly ash. Colloids Surf. A Physicochem. Eng. Asp. 2004, 247, 137–143. [Google Scholar] [CrossRef]
- Mroczek, K.; Kalisz, S.; Pronobis, M.; Sołtys, J. The effect of halloysite additive on operation of boilers firing agricultural biomass. Fuel Process. Technol. 2011, 92, 845–855. [Google Scholar] [CrossRef]
- Gao, J.; Wang, T.; Zhao, J.; Hu, X.; Dong, C. An Experimental Study on the Melting Solidification of Municipal Solid Waste Incineration Fly Ash. Sustain. J. Rec. 2021, 13, 535. [Google Scholar] [CrossRef]
- Bada, S.O.; Potgieter-Vermaak, S. Evaluation and Treatment of Coal Fly Ash for Adsorption Application. Leonardo Electron. J. Pract. Technol. 2008, 12, 37–48. [Google Scholar]
- Farghali, A.; Bahgat, M.; Allah, A.E.; Khedr, M. Adsorption of Pb(II) ions from aqueous solutions using copper oxide nanostructures. Beni Suef Univ. J. Basic Appl. Sci. 2013, 2, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Franus, M.; Bandura, L. Sorption of heavy metal ions from aqueous solution by glauconite. Fresenius Environ. Bull. 2014, 23, 825–839. [Google Scholar]
- Cuppett, J.D.; Duncan, S.E.; Dietrich, A.M. Evaluation of Copper Speciation and Water Quality Factors That Affect Aqueous Copper Tasting Response. Chem. Senses 2006, 31, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudero, R.; Espinoza, E.; Tavera, F.J. Precipitation of Lead Species in a Pb—H2O System. Res. J. Recent Sci. 2013, 2, 1–8. [Google Scholar]
- Lee, J.-Y.; Chen, C.H.; Cheng, S.; Li, H.-Y. Adsorption of Pb(II) and Cu(II) metal ions on functionalized large-pore mesoporous silica. Int. J. Environ. Sci. Technol. 2016, 13, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Darmayanti, L.; Notodarmodjo, S.; Damanhuri, E. Removal of Copper (II) Ions in Aqueous Solutions by Sorption onto Fly Ash. J. Eng. Technol. Sci. 2017, 49, 546. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Ogata, F.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removal of Pb2+ from Aqueous Solutions Using K-Type Zeolite Synthesized from Coal Fly Ash. Water 2020, 12, 2375. [Google Scholar] [CrossRef]
- Vu, N.H.; Kristianová, E.; Dvořák, P.; Abramowski, T.; Dreiseitl, I.; Adrysheva, A. Modified Leach Residues from Processing Deep-Sea Nodules as Effective Heavy Metals Adsorbents. Metals 2019, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Fang, S.; Yao, Y.; Li, T.; Ni, Q.; Yang, X.; He, Z. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathi, B.D. Utilization of fly ash in adsorption of heavy metals from wastewater. Toxicol. Environ. Chem. 2008, 90, 1091–1097. [Google Scholar] [CrossRef]
- Sočo, E.; Kalembkiewicz, J. Comparison of adsorption of Cd(II) and Pb(II) ions on pure and chemically modified fly ashes. Chem. Process. Eng. 2016, 37, 215–234. [Google Scholar] [CrossRef]
- Harja, M.; Buema, G.; Lupu, N.; Chiriac, H.; Herea, D.D.; Ciobanu, G. Fly Ash Coated with Magnetic Materials: Improved Adsorbent for Cu (II) Removal from Wastewater. Materials 2020, 14, 63. [Google Scholar] [CrossRef]
- Militaru, B.A.; Pode, R.; Lupa, L.; Schmidt, W.; Tekle-Röttering, A.; Kazamer, N. Using Sewage Sludge Ash as an Efficient Adsorbent for Pb (II) and Cu (II) in Single and Binary Systems. Molecules 2020, 25, 2559. [Google Scholar] [CrossRef]
- Igberase, E.; Osifo, P.; Ofomaja, A. The Adsorption of Pb, Zn, Cu, Ni, and Cd by Modified Ligand in a Single Component Aqueous Solution: Equilibrium, Kinetic, Thermodynamic, and Desorption Studies. Int. J. Anal. Chem. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zaranyika, M.F.; Chirinda, T. Heavy metal speciation trends in mine slime dams: A case study of slime dams at a goldmine in Zimbabwe. J. Environ. Chem. Ecotoxicol. 2011, 3, 103–115. [Google Scholar]
- Igwe, J.; Abia, A. Adsorption isotherm studies of Cd (II), Pb (II) and Zn (II) ions bioremediation from aqueous solution using unmodified and EDTA-modified maize cob. Eclética Química J. 2007, 32, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Kalak, T.; Dudczak-Hałabuda, J.; Tachibana, Y.; Cierpiszewski, R. Effective use of elderberry (Sambucus nigra) pomace in biosorption processes of Fe(III) ions. Chemosphere 2020, 246, 125744. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Foroutan, R. Investigation into ion exchange and adsorption methods for removing heavy metals from aqueous solution. Int. J. Biol. Pharm. Allied Sci. 2015, 4, 620–629, ISSN: 2277–4998. [Google Scholar]
- Hałas, P.; Kołodyńska, D.; Płaza, A.; Gęca, M.; Hubicki, Z. Modified fly ash and zeolites as an effective adsorbent for metal ions from aqueous solution. Adsorpt. Sci. Technol. 2017, 35, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Alinnor, I. Adsorption of heavy metal ions from aqueous solution by fly ash. Fuel 2007, 86, 853–857. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Kinetic and Pseudo-Second-Order Modeling of Lead Biosorption onto Pine Cone Powder. Ind. Eng. Chem. Res. 2010, 49, 2562–2572. [Google Scholar] [CrossRef]
- Kalembkiewicz, J.; Galas, D.; Sitarz-Palczak, E. The Physicochemical Properties and Composition of Biomass Ash and Evalu-ating Directions of its Applications. Pol. J. Environ. Stud. 2018, 27, 2593–2603. [Google Scholar] [CrossRef]


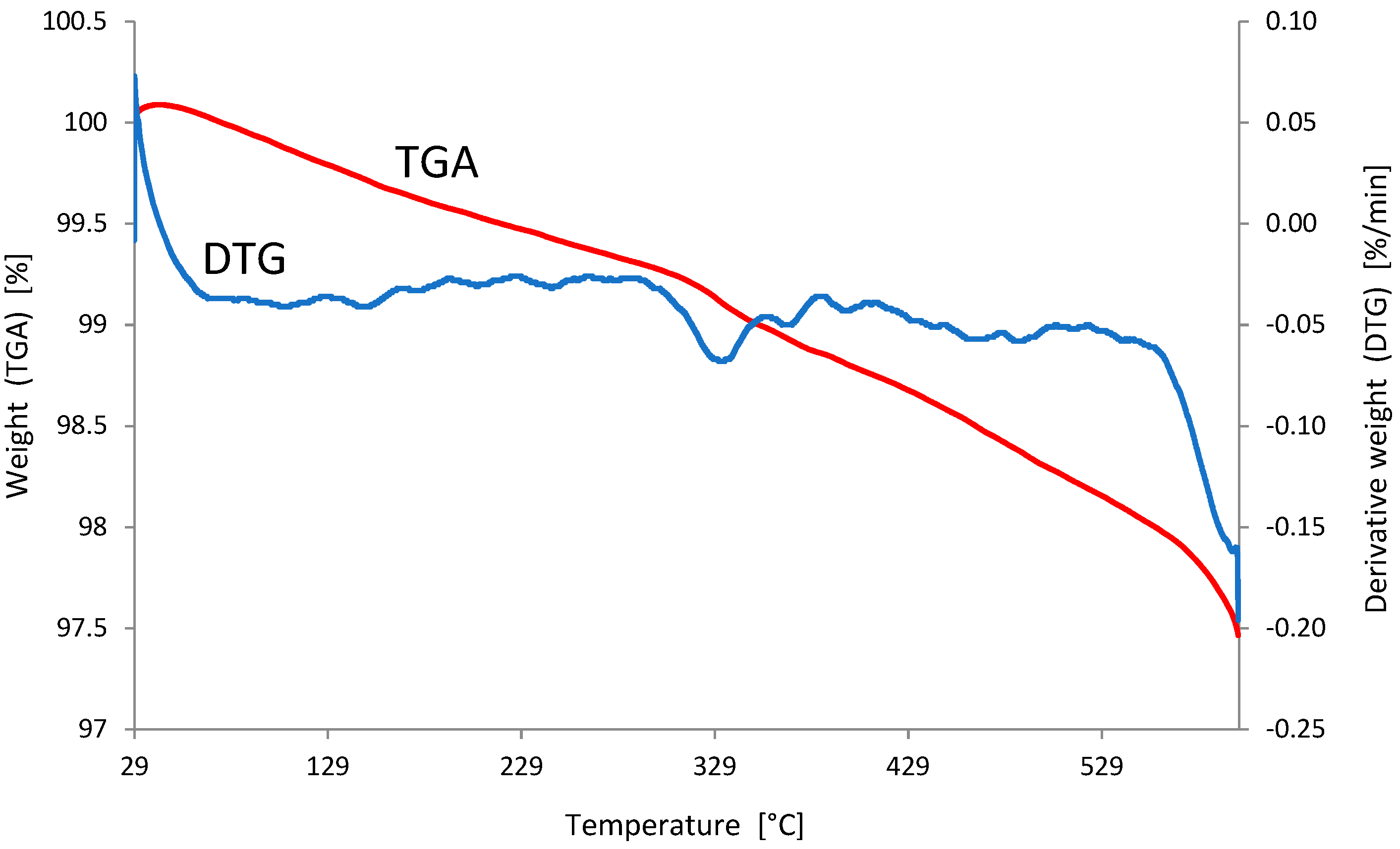
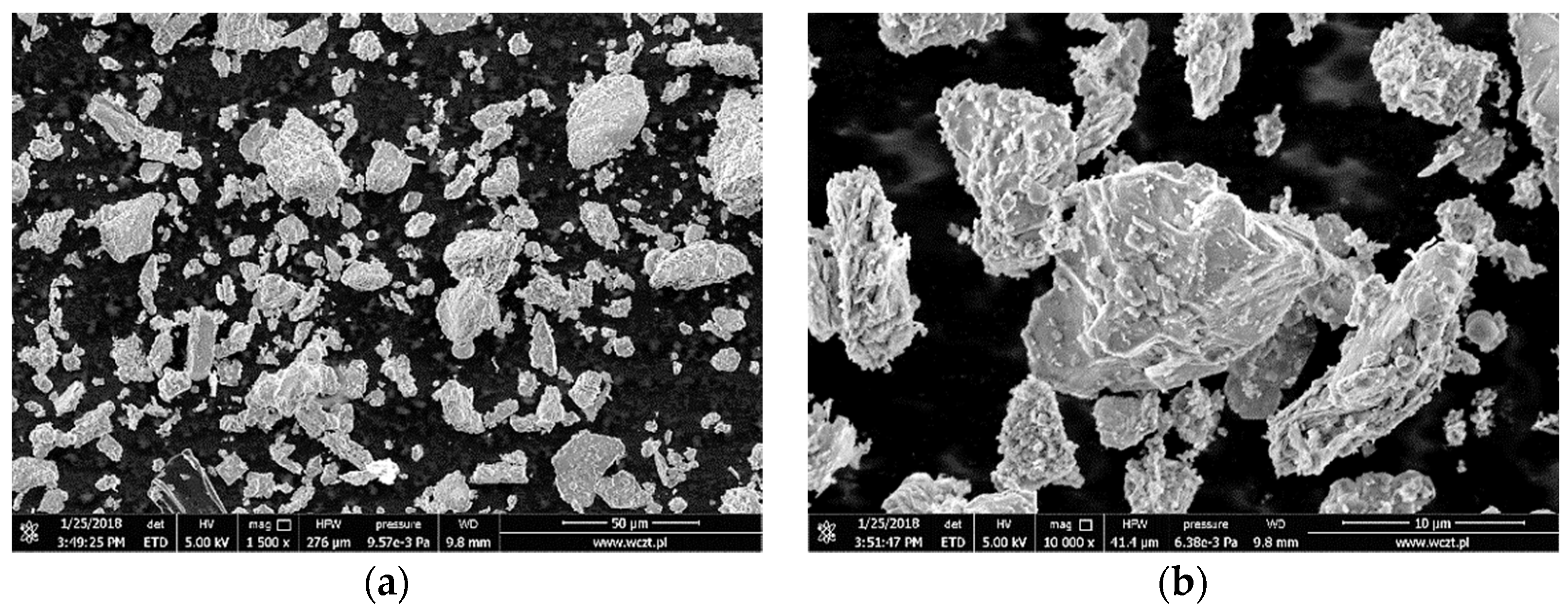
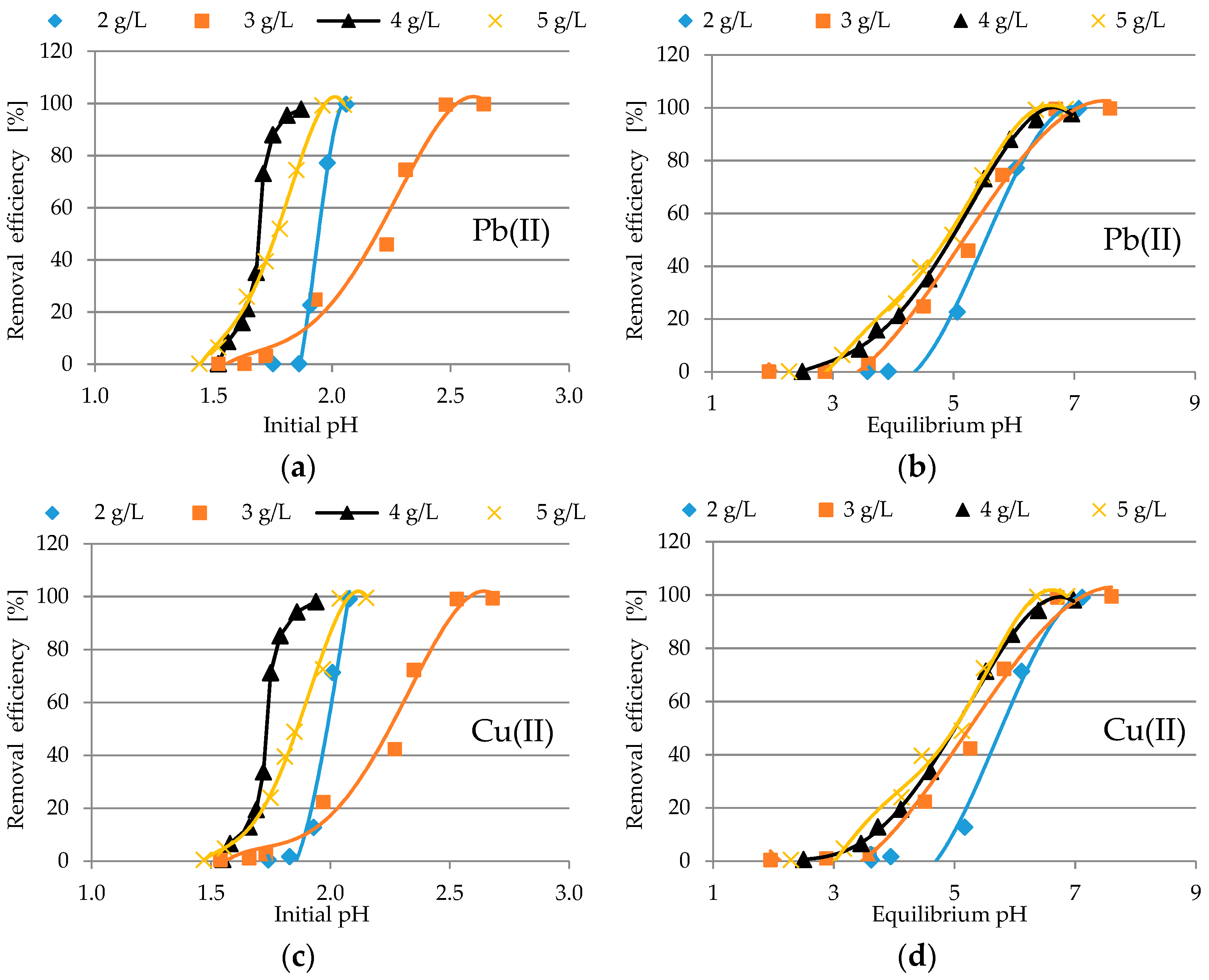

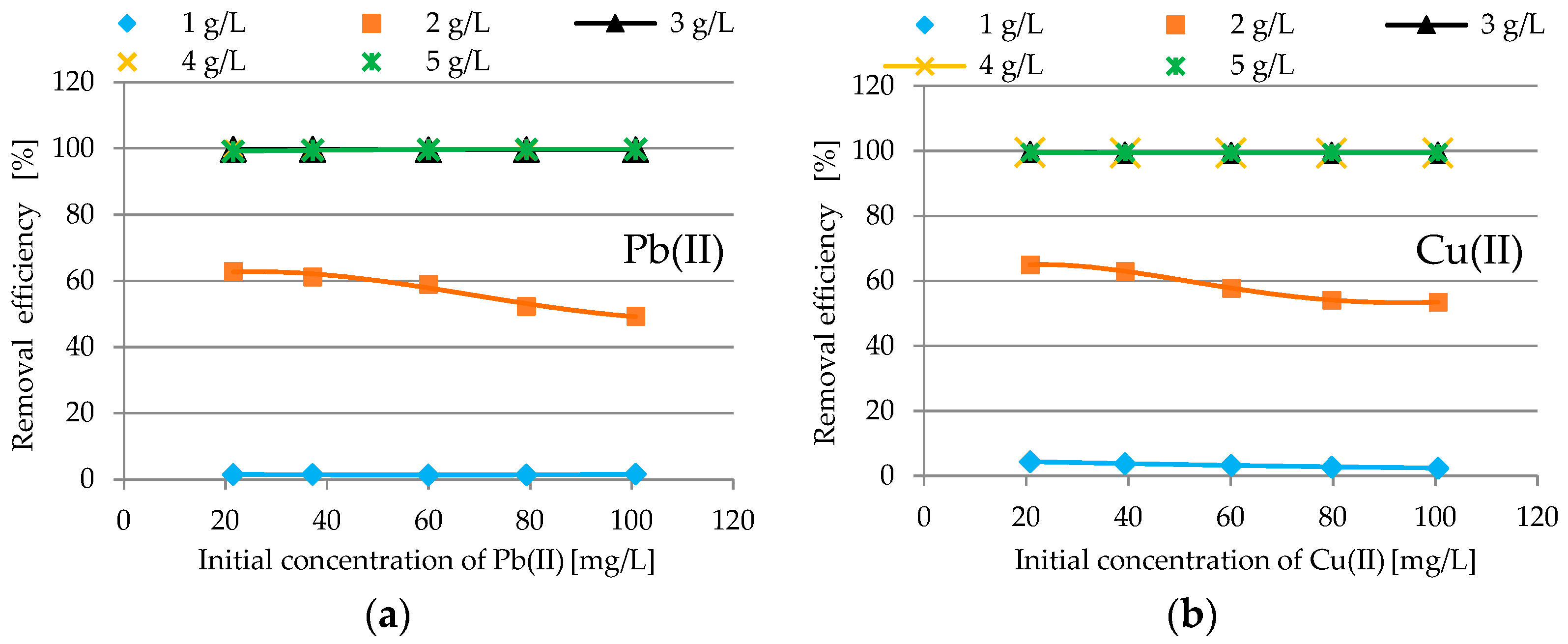

| Oxide | Content [%] | Oxide | Content [%] |
|---|---|---|---|
| SiO2 | 50.20 | BaO | 0.06 |
| Al2O3 | 12.29 | ZnO | 0.05 |
| CaO | 11.82 | SrO | 0.046 |
| K2O | 7.99 | ZrO2 | 0.025 |
| MgO | 3.34 | CuO | 0.019 |
| P2O5 | 2.04 | PbO | 0.016 |
| Fe2O3 | 1.46 | Rb2O | 0.014 |
| Na2O | 0.44 | Cr2O3 | 0.012 |
| TiO2 | 0.30 | NiO | 0.006 |
| MnO | 0.28 |
| Parameters | Values |
|---|---|
| BET adsorption cumulative surface area (SBET) [m2/g] | 3.264 |
| BET desorption cumulative surface area (SBET) [m2/g] | 3.660 |
| BJH adsorption cumulative volume of pores (Vpa) [cm3/g] | 0.014325 |
| BJH desorption cumulative volume of pores (Vpd) [cm3/g] | 0.014752 |
| BJH adsorption average pore diameter (Apda) [nm] | 17.553 |
| BJH desorption average pore diameter (Apdd) [nm] | 16.1218 |
| Metal Ion | Adsorbent Dosage [g/L] | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| kad [min−1] | qe [mg/g] | R2 | k [g/mg min] | qe [mg/g] | R2 | ||
| Pb(II) | 5 | 0.086 | 0.052 | 0.828 | 106.14 | 0.212 | 0.999 |
| Cu(II) | 5 | 0.124 | 0.046 | 0.878 | 72.66 | 0.257 | 0.999 |
| Metal Ion | Adsorbent Dosage [g/L] | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| Calculated qm [mg/g] | KL [L/mg] | R2 | Kf [mg/g] [L/mg](1/n) | n | R2 | ||
| Pb(II) | 1 | 16.18 | 0.0009 | 0.998 | 0.019 | 1.080 | 0.999 |
| 2 | 46.37 | 0.024 | 0.956 | 2.145 | 1.584 | 0.990 | |
| 3 | 68.76 | 2.228 | 0.966 | 57.344 | 1.430 | 0.989 | |
| 4 | 72.92 | 1.325 | 0.963 | 57.504 | 1.167 | 0.967 | |
| 5 | 138.37 | 0.537 | 0.992 | 61.209 | 1.045 | 0.996 | |
| Cu(II) | 1 | 7.57 | 0.007 | 0.953 | 0.148 | 1.608 | 0.984 |
| 2 | 48.53 | 0.02 | 0.981 | 1.686 | 1.390 | 0.993 | |
| 3 | 65.63 | 1.597 | 0.957 | 46.24 | 1.357 | 0.982 | |
| 4 | 72.63 | 0.818 | 0.979 | 35.48 | 1.223 | 0.989 | |
| 5 | 97.43 | 0.462 | 0.989 | 34.08 | 1.102 | 0.990 | |
| FT-IR Band [cm−1] | Assignment (Vibrations, Species) |
|---|---|
| 3258.6, 3261.8, 3314.28 | stretching vibrations O–H |
| 1410.2, 1412.4, 1414.53 | valence vibration of carbonate ions |
| 984.7, 984.27, 985.1 | asymmetric stretching vibrations of silica Si–O–Si and Al–O–Si |
| 873.3, 874.03, 874.28 | symmetric stretching of Al–O–M, vibration of carbonates (calcite) |
| 713 | symmetric stretching of Si–O–Si and Al–O–Si |
| 678.6, 693.5, 678.6 | stretching vibrations Al–O |
| 612.2 | stretching vibrations Al–O |
| 594.6 | vibrations Si-O-Pb |
| 396.3, 395.7, 387.3, 422.8, 430.9, 432.2 | bond bending vibrations Si-O-Si |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalak, T.; Cierpiszewski, R.; Ulewicz, M. High Efficiency of the Removal Process of Pb(II) and Cu(II) Ions with the Use of Fly Ash from Incineration of Sunflower and Wood Waste Using the CFBC Technology. Energies 2021, 14, 1771. https://doi.org/10.3390/en14061771
Kalak T, Cierpiszewski R, Ulewicz M. High Efficiency of the Removal Process of Pb(II) and Cu(II) Ions with the Use of Fly Ash from Incineration of Sunflower and Wood Waste Using the CFBC Technology. Energies. 2021; 14(6):1771. https://doi.org/10.3390/en14061771
Chicago/Turabian StyleKalak, Tomasz, Ryszard Cierpiszewski, and Małgorzata Ulewicz. 2021. "High Efficiency of the Removal Process of Pb(II) and Cu(II) Ions with the Use of Fly Ash from Incineration of Sunflower and Wood Waste Using the CFBC Technology" Energies 14, no. 6: 1771. https://doi.org/10.3390/en14061771
APA StyleKalak, T., Cierpiszewski, R., & Ulewicz, M. (2021). High Efficiency of the Removal Process of Pb(II) and Cu(II) Ions with the Use of Fly Ash from Incineration of Sunflower and Wood Waste Using the CFBC Technology. Energies, 14(6), 1771. https://doi.org/10.3390/en14061771