Abstract
The research presented in this manuscript is focused on the pyrolysis of waste pharmaceutical blisters, which are a large and neglected group of waste, that could be possibly recovered. The studies were focused on the analysis of the chemical composition, as well as fuel properties of the char generated during the process and its possible applications. The process temperatures (400, 425, and 450 °C) were determined based on the thermogravimetric study that was performed prior to the pyrolysis tests. The selected materials included the pre- and post-consumer waste pharmaceutical blisters in order to determine the possible influence of impurities on the final products. The tests were performed on a laboratory scale fixed bed reactor. The obtained solid fractions (metal and char) were separated. Char was subjected to elemental analysis (C, H, N, Cl, S), as well as the heat of combustion and fuel-related properties (content of flammable, non-flammable, and volatile components) were determined. The results were used to compare the properties of char to the properties of active carbon. The market potential was analyzed.
1. Introduction
Generation of waste is an inevitable phenomenon which is inexorably linked to humanity’s actions. Waste which is not subjected to recycling or reusing processes may negatively impact the environment, leading to, among others, pollution of air, soil, and surface- or groundwater [1]. Waste also have a long-lasting harmful footprint on local flora and fauna. Besides its impacts on the environment, excessive amounts of stored waste may lead to various social disputes, ranging from waste localization issues, economy’s deceleration due to irrational management of resources, to the obligation to financially cover infringements upon environmental laws or the necessity to recultivate polluted areas. All those reasons indicate a need to manage the resources and materials responsibly, as well as to implement effective and balanced production processes, which generate minimal (or next to naught) amounts of waste [2,3].
A classic lineal economic model is based upon the assumption, that the access to natural resources is unlimited. This model includes the following: extraction of resources, processing to obtain relevant product, their utility time and waste phase. In practice, however, particularly due to economic reasons, a part of the waste is recycled or (in case of wastes that arise in production processes) reused in the same process (or in others). Such approach is insufficient from the environment’s point of view and from the point of view of depletion of natural resources.
The difference between a circular and a linear model is based on the maximization of the reuse and recovery of materials, and the use of renewable energy sources instead of fossil fuels. Another point is the limitation of emissions and the total use of energy, as well as the use of renewable resources and recycling to their fullest potential. Closing the loop is done through reuse of products or materials, their repair, refurbish, renovation or sharing, which can bring upon diminished costs and creation of new jobs [4,5,6].
Recycling processes of renewable resources are currently most efficient in case of single group of waste, while waste consisting of multiple materials still pose a challenge owing to their complicated composition. Although the development of recycling methods fits the multicomponent refuse (such as e-waste) is in progress, there are still various types of municipal solid waste (MSW) that do not get the attention and reuse it deserve. A group of such neglected waste is represented by waste pharmaceutical blisters (WBP). In common households this used-up packaging are usually found in plastic refuse containers or mixed-refuse containers. In pharmacies it is common practice to collect blister packs together with expired medication and related products, and subsequently burned as hazardous waste. In this work the tests for the recovery of the metallic part of clean (unused) blister packs were performed, along with used (emptied) WBPs (which were contaminated with the residue of medical products that were initially inside them).
Waste blister packs (WBPs) were developed in 1960s by Karl Klein. At first they were mostly used to store birth control pills, since this type of packaging allows easy and precise determination of the type of substance and its dose. Later, other advantages of WBPs were notices, such as protection of the pharmaceutical products from contact with oxygen, sunlight, moisture, or bacteria [7].
In 2014, only packaging of pharmaceutical products constituted about 5% of the total packaging market. Medication is most frequently administered orally in form of capsules and pills (around 51%) that are packed in multicomponent packaging of the blister-type or in bottles made out of plastics [8]. It is estimated that 85% of medication that exists in the form of pills and capsules is enclosed within blister packs [7].
Such a large amount of waste is not handled properly. In order to fulfil the requirements of the circular economy, a more precise approach is necessary. The authors of this manuscript decided to analyze the potential of pyrolysis as a method to separate and recover as many valuable materials from waste blister packs as possible, and recover energy at the same time. Pyrolysis is a process that is proven to be efficient for different types of materials that are, for some reason, hard to process, including multimaterial waste [9,10,11,12,13,14].
Pyrolysis is widely used to process various materials and waste, because it allows the production of high quality fuels and limitations in the volumes of waste directed to waste disposal or storage plants. Pyrolysis is a process of thermal decomposition of organic substances that takes place in a closed system in anoxic atmosphere. Depending on the temperature one distinguishes a low-temperature pyrolysis (slow and intermediate pyrolysis, temperature around 250 °C–700 °C) and high-temperature pyrolysis (also called “coking”, fast pyrolysis, temperature around 900 °C–1000 °C). Products of the process, that is gases, oils, and solids, can be further used in energetic, chemical, construction, and farming industries. Factors affecting the course and products of pyrolysis are, among others, humidity, size distribution of the grain, temperature, pressure, and heating time [15].
Products of pyrolysis can be divided into solid (char and inorganic constituents of the substrate), gaseous (mainly CO, CO2, CH4, CnHm, H2, H2S, and others), and liquid (usually oil or wax). Larger amounts of char arise in processes run at lower temperatures with longer residence time, whereas at higher temperatures and shorter residence times mostly oil and gas are generated [16].
Pyrolysis was used during the presented research as a method to separate the layer of aluminum from plastics and coating used to identify the products. Another advantage of this solution is that the lacquer coatings need to be removed anyway, in order to recover aluminum, and, usually, pyrolysis is a process used for this purpose [17,18,19,20].
2. Materials and Methods
The presented research is focused on the method of utilization of waste pharmaceutical blisters with the application of pyrolysis process. The tests were performed on two types of blister packs: pre- and post-consumer. The pre-consumer WBPs, which comes up while packing diet supplements, was not in direct contact with impurities, while the post-consumer WBPs had trace amounts of them. The post-consumer product is closer to the real, varied stream of pharmaceutical blister waste.
2.1. Materials
According to literature data [7,15] blister packs are comprised of three parts:
- (a)
- the base foil, which is made out of plastics, molded such that the pills fit inside. Very often a thermoplastic polymer-based foil is employed, such as polypropylene (PP) or polyvinyl chloride (PVC, PVDC or PVC/CTFE). They are characterized with different prices, as well as by varying transmission of humidity, which is an important parameter from the point of view of the duration of stored product (humidity may cause active substance degradation and cracking, discoloration or deterioration of the aesthetic aspects),
- (b)
- covering lid made out of an aluminum alloy. The layer admits neither moisture nor sunlight and allows for an easy detection for the state of packaging’s integrity,
- (c)
- coating from which various imprints are made, that allow for identification of the products and its expiration date.
The blister pack with its components is presented in Figure 1.
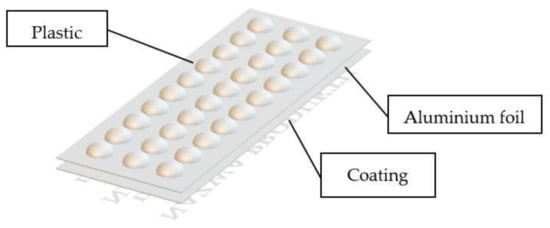
Figure 1.
Blister pack and its parts.
2.2. Methods
Pyrolysis tests were conducted on the laboratory scale fixed bed reactor. The volume of the reaction chamber is approximately 25 dm3. The tests were performed for both materials and at three maximum temperatures, determined based on the thermogravimetric study described in another paper [15] (400, 425, and 450 °C). Series 1 was performed on a pre-consumer waste material and Series 2 was performed on the post-consumer waste. The numbers of the tests (1, 2, and 3) correspond to the temperatures: 400, 425, and 450 °C, respectively. The process temperatures were carefully selected based on thermogravimetric study and including other parameters, such as melting temperature of aluminum.
The goal was to determine the optimal process temperature in order to balance the desired parameters and the energy consumption and analyze the potential of char to be used in further processes.
During the tests, samples of the gas fraction were taken and in due course were subjected to chemical analyses in order to obtain the contents of the flammable substances. Based on the analyses of the composition of gases, the heating value of gases created in the process was estimated. Solid products were subjected to mechanical separation. The elemental (C, H, N, Cl, S) composition of created char was determined, along with the heat of combustion and fuel-related properties (content of flammable, non-flammable, and volatile components).
Analysis was conducted using gas chromatography with the use of two separate chromatographs equipped with relevant separating columns and substance detection systems. Analog signals from detectors were processed into digital ones, which were analyzed with the use of a special integrating-calculating software “PeakSimple” provided by the analyzers’ vendor. The detailed information regarding the methods used to analyze the gas fraction were presented in another paper [21].
The received solid fraction underwent mechanical separation. Afterwards the char was subjected to chemical analysis in order to determine the content of following elements: carbon, hydrogen, nitrogen, chlorine, and sulphur.
Amount of nitrogen was measured using the Kjeldahl method. This method bases on the incineration of organic compounds carrying nitrogen in concentrated sulphuric acid with an addition of a catalyst and a catalyzing mixture. Nitrogen contained in the sample is transformed into ammonium sulphate, which is alkalized with sodium hydroxide and is marked as ammonia using distillation [20].
Sulphur content was measured with a high-temperature incineration method with alkalimetric titration, according to the PN-G-04584:2001 norm. The test is based on burning the weighed portion of the material in an oxygen stream at temperature of 1250 °C, absorption of created Sulphur oxides with hydrogen peroxide, alkalimetric marking of created sulphuric acid and finally calculating the total Sulphur content.
Carbon and hydrogen content was measured using the Sheffield method, which consists of the incineration of the weighed material within an oxygen stream, absorption of carbon dioxide and water (created during the process), and subsequently measuring their masses and calculating the carbon and hydrogen content. The analysis was performed according to the PN-G-04521:1973 norm.
Chlorine content was measured with use of the Eschka’s mixture, according to the PN-ISO 587 norm. This method is based on the incineration of the sample in direct contact with the Eschka’s mixture in oxidizing environment, in order to remove flammable substances and to transform chlorine into alkaline chlorides. Created chlorides were measured using the Mohr method, basing on heating of the solution until the boiling point, filtration and subsequent washing of the filter (five times) with hot demineralized water in an Erlenmeyer flask. Following that the sample is neutralized using nitric acid, 10 drops of potassium chromate are added and titration with a solution of silver nitrate (until first coloration) is employed.
Electron images of the morphology of the samples were taken using a JEOL JXA 8230 X-ray microprobe in two modes:
- under secondary electron illumination (SEI—the contrast depends mainly on the surface topography),
- in the light of backscattered electrons (COMPO—reveals differences in chemical composition).
3. Results and Discussion
The gaseous and solid (char) fractions, which were obtained during the pyrolysis of pharmaceutical blister packs underwent chemical analyses in order to determine the possible potential for fuel use. Exact composition of the gaseous fraction, as well as the yields of the products of pyrolysis of both pre- and post-consumer waste were presented in another publication [21].
3.1. Analysis of the Gaseous Fraction’s Heating Value
The heating value of the process gases as well as their composition was analyzed and described in detail in a previous publication [21,22]. The summary of the heating value for gases from series 1 and 2 were presented in Table 1 and Table 2.

Table 1.
Heating value of process gases at the start of the process, during and after achieving the maximum temperature, Serie 1 [21].

Table 2.
Heating value of process gases at the start of the process, during and after achieving the maximum temperature, Serie 2 [21].
Based on the calculations it is apparent that the heating value of the process gases and the quality of their potential as fuel increases with the maximum temperature and as the process continues [21,23]. The largest heating value of the gas was observed for the mixed post-consumer waste at the maximum temperature of 450 °C.
3.2. Analysis of the Char’s Heating Value
The solid fraction obtained during the pyrolysis, which contained char and metallic fraction, was subjected to a mechanical separation. Subsequently, the sieved char was subject to further analysis in order to determine its composition and fuel-related properties.
Char and metallic fraction (after the separation) are presented in Figure 2 and Figure 3, respectively. The results from chemical analyses of the char were presented in Table 3.

Figure 2.
Post-pyrolysis char obtained after the separation of the solid fraction.

Figure 3.
Post-pyrolysis metallic fraction obtained after the separation of the solid fraction.

Table 3.
Results of char’s chemical analyses. Serie 1—pre-consumer waste. Serie 2—post-consumer waste.
The metal fraction collected after separation contained 85–90% (average of 86.4% for pre-consumer waste and 88.5% for post-consumer waste) of aluminum, and the level of oxidation of Al was approximately 1%. All the paint and coatings were removed during the thermal process. During the tests performed on a laboratory scale electric furnace with the addition of MgCl2, NaCl and Na3(AlF6) at 800 °C, the aluminum recovery level was almost 90%. Depending on the composition of the input material, 150–200 g of Al can be recovered from 1 kg of waste pharmaceutical blisters.
Based on the analyses of the elemental composition of chars obtained from pyrolysis of pre- and post-consumer WBPs, the carbon content fluctuates within the 71 to 80% range. No significant dependencies were observed between final temperature and carbon content in the samples. Carbon is the basic component of chars obtained during the pyrolysis of diverse refuse. Its content in the final char depends, among others, on the type of material subjected to pyrolysis or the method of conducting the process.
Percentage of hydrogen in the char decreases along with the maximum temperature of the process. Larger quantities of hydrogen were observed from samples obtained as a result of the Series 2 (post-consumer product), which can be a result of a more complex composition of the waste. Hydrogen is a component that has a potent influence on the susceptibility for combustion and the heating value [24].
The amount of chlorine in the samples increases along with the increase in the temperature. It is a result of a larger deficiency in samples’ masses at higher temperatures. According to literature data [25], such chlorine content in the char can be a result of chlorine’s presence accumulated in the form of chloro-organic compounds. It is important to note that the high chlorine content above 10% might require additional treatment in order for it to be suitable for further application, especially combustion.
The nitrogen content for all samples is about 1%. Slightly raised values were observed for the pre-consumer material.
Sulphur’s content for both series fluctuates between 1.4 and 3.3%. It can be observed that a slight increase in Sulphur’s amount at higher temperatures. Sulphur’s presence negatively influences the quality of char, particularly when considering its further use as a fuel [25].
The content of flammable, non-flammable and volatile substances in the char is presented in Table 4.

Table 4.
Results of the analyses of the char’s fuel-related properties. Series 1—pre-consumer waste. Series 2—post-consumer waste.
The content of volatile substances is significantly larger for the materials obtained from Series 2 (post-consumer waste). The lowest amount of volatile substances was observed for the char created during the pyrolysis of pre-consumer waste at the temperature of 450 °C. Volatile substances are created in pyrolytic conditions from gases and tar-related products. During combustion, fuels with large content of volatile substances demand additional oxygen for complete combustion and their flame is elongated. Large content of volatile substances in the fuel is associated with easier combustion, however their lower content is a testament of possible difficulties arising with fuel’s combustion [24].
The largest amount of flammable substances was observed for the pre-consumer waste at the temperature of 400 °C (91.5%), however for the post-consumer waste (at the same temperature) the result is very similar (91.4%).
The post-consumer waste at the temperature of 450 °C exhibited the largest content of flammable substances (16.2%), and (at the temperature of 400 °C) the lowest for the pre- (8.5%) and post-consumer waste (8.6%).
In order to further evaluate the fuel properties of the char generated during the pyrolysis of waste pharmaceutical blisters (WBP), the heat of combustion of the samples and the activated carbon was measured. Based on the information provided by the manufacturer, the purity of activated carbon is not lower than 95% and it contains <2% ash and Fe < 0.3%.
The moisture content is increasing for the samples generated at higher process temperatures. It is important to note that this is caused by the act that before the sample was removed from the furnace it was cooled down to a room temperature. This moisture was collected by the samples during the cooling procedure that lasted approximately 24 h. It can be concluded, that the materials produced at higher temperatures have a higher porosity and therefore are more prone to absorb water.
The calorific value of the char was measured using the PRECYZJA-BIT K-12Mn2 calorimeter as per PN-81/G-04513 standard. The accuracy of the digital temperature measurement is 0.001 K. The heating values were calculated according to the formula:
where:
- Wd—heating value
- w—moisture content ,
- Q—measured heat of combustion ,
- r—heat of water vaporization ,
- h—hydrogen content .
The summary of heating values calculated for the analyzed samples is presented in Table 5.

Table 5.
Results of analyses of the measured calorific values (Q) and heating values (Wd) for activated carbon and char.
Based on the obtained results it can be observed that for both series, the highest heating values are achieved at lower temperatures. It results from the lower degree of decomposition of flammable substances. In an industrial process, it is crucial to determine the desired properties of final products. Char with higher heating value can potentially be used, e.g., as a reducing agent in pyrometallurgical processes [26].
Results from the analysis of the heating values of the char produced during the pyrolysis of WBPs were presented in Table 5, along with the same results for activated carbon. It is concluded that the average heating value for pyrolysis at 400 °C, of both pre- and post-consumer waste, is almost as high as the average heating value of activated carbon. The heating values decrease with the process temperature, and the lowest heating value was observed for the char produced from post-consumer waste at 450 °C. For the applications where the char could be treated as a substitute for activated carbon, that include thermal processing, the most favorable material is generated at 400 °C. Additionally, the measured chlorine content takes the lowest values at this temperature.
SEI microscopic pictures of the analyzed activated carbon and char obtained during the pyrolysis of pre-consumer waste at 400 °C and post-consumer waste at 400 °C are presented in Figure 4 and Figure 5, respectively.
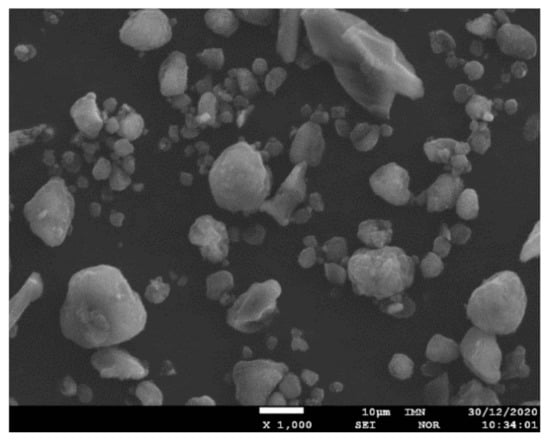
Figure 4.
SEI microscopic picture of the analyzed activated carbon; zoom 1000×.
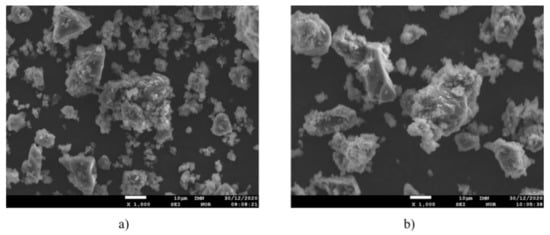
Figure 5.
SEI microscopic picture of the char obtained at the maximum temperature 400 °C; zoom 1000×. (a) pre-consumer waste, (b) post-consumer waste.
COMPO microscopic pictures of the analyzed activated carbon, char obtained during the pyrolysis of pre-consumer waste at 400 °C and postconsumer waste at 400 °C are presented in Figure 6 and Figure 7, respectively.

Figure 6.
COMPO microscopic picture of the analyzed activated carbon; zoom 500×.
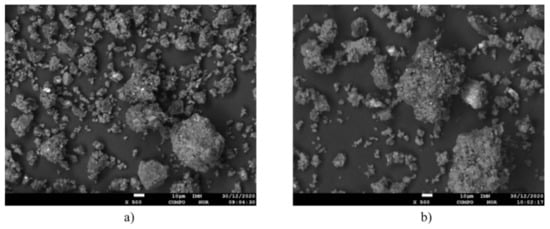
Figure 7.
COMPO microscopic picture of the char obtained at the maximum temperature 400 °C; zoom 500×. (a) pre-consumer waste, (b) post-consumer waste.
The sample of the activated carbon consists of two fractions: fine-grained, approximately 3–5 µm and larger, approximately 10–30 µm. The finer fraction contains grains of relatively uniform morphology and rounded shape. The larger fraction, on the other hand, is characterized by a more diverse morphology—the grains are partly oval in shape and partly elongated and irregular.
The sample of the char obtained from pre-consumer waste consists of two fractions: fine, about 5–10 µm and larger, about 30–50 µm. Agglomerates can be distinguished, consisting of very fine, rounded grains of about 1–2 µm, with varying degrees of agglomeration. Large grains are irregular in shape.
The sample of the char obtained during the pyrolysis of post-consumer waste has a morphology comparable to the sample obtained from pre-consumer waste, however the finer fraction is more frayed. Char obtained as a result of pyrolysis has many potential applications. Depending on the desired parameters, it can serve as a fuel (particularly the char obtained at lower temperatures, characterized by a heating value close to that of activated carbon, as well as lower chlorine content) used for co-combustion in cement kilns or in energetic processes as a soot substitute (e.g., for manufacturing of gum-related elements or as a filler used during manufacture of plastics). Another potential uses are the precursor of adsorbents (for manufacture of activated carbon), as a raw material in metallurgical processes (particularly as an alternative for coke as a reducing agent in rotary kilns), as a raw material in the fireproof materials’ industry, as a component of the coking coal mixture. Furthermore its use as one of the additives in asphalt mixtures and even ink toners (for printers) is predicted [27].
4. Conclusions
One of the primary goals of the described study, which was a part of a more extensive research, was to find a method that would allow the recovery of materials as well as energy from a group of waste that is currently neglected. As a multimaterial waste, waste blister packs are extremely hard to separate with application of mechanical methods, therefore a more complex approach is necessary.
Pyrolysis is a well-known process than is proven to be useful for different types of waste. Therefore, it might be a go-to process for the treatment of such troublesome material.
The presented tests were performed on two WBP materials—one recovered from the waste stream (called post-consumer waste) and one obtained from the manufacturer of the dietary supplements (pre-consumer waste) in order to analyze the possible differences between those two types of waste. Additionally, the tests were performed at three different temperatures, based on the initial thermogravimetry.
The percentages of components obtained in the solid fraction for the pre-consumer waste are:
- at Tmax = 400 °C: 59.5 % char and 40.5 % metal;
- at Tmax = 425 °C: 57.4 % char and 42.6 % metal; and
- at Tmax = 450 °C: 55.6 % char and 44.4 % metal.
The percentages of components obtained in the solid fraction for the postconsumer waste are:
- at Tmax = 400 °C: 63.6 % char and 36.4 % metal;
- at Tmax = 425 °C: 62.6 % char and 37.4 % metal; and
- at Tmax = 450 °C: 60.4 % char and 39.6 % metal.
The element with the dominant share in the elementary composition of char is carbon (its content in the samples ranges from 71 to 80%). The hydrogen content of the samples decreases with increasing process temperature and varies between 4.8 and 6.5%. The chlorine content of the char depends on the final process temperature and ranges from 4.5 to as much as 15.5%. The nitrogen content for all samples is approximately 1%. Slightly higher values were observed for pre-consumer material. The sulfur content for both series (for pre- and post-consumer waste) ranges from 1.4 to 3.3%.
The highest values of heat of combustion of char in each case were achieved at lower temperatures, which is related to a lower degree of decomposition of combustible substances. The highest heat of combustion value (32,534 kJ/kg) was recorded for the sample obtained by running the process on post-consumer material at 400 °C. Depending on the target process, char with a higher calorific value may, for example, be a replacement for coke in pyrometallurgical processes, while a lower calorific value may be desirable when used as a replacement for carbon black.
Those results show clearly that pyrolysis of WBPs has a huge potential to become a technology that would contribute to the implementation of circular economy in Europe. Particularly important is the development of technology that allows the processing of plastics such as PVC, which contain significant amounts of chlorine. The practical implementation of the objectives of a circular economy requires the development of recovery technologies tailored to specific groups of waste. Only this individual approach will maximize the use of secondary raw materials in a way that is both environmentally and economically sustainable.
Author Contributions
Conceptualization, K.P., W.Ś. and K.K.; methodology, W.Ś. and K.P.; software, K.K. and Ł.M.; validation, K.P., W.Ś. and K.K.; formal analysis, K.P.; investigation, W.Ś. and K.K.; resources, K.P.; data curation, Ł.M., A.J. and A.S.; writing—original draft preparation, K.K., A.S.; writing—review and editing, K.K., W.Ś.; visualization, A.J.; supervision, K.P. and W.Ś.; project administration, K.K.; funding acquisition, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
Publication was funded by research subsidy allocated for 2020 (08/030/BK_20/0079).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pikoń, K.; Kajda-Szczesniak, M.; Bogacka, M. Environmental effect of co-combustion of coal with wood waste in low-power boilers. Przem. Chem. 2015, 94, 1548–1550. [Google Scholar]
- Kozłowski, D.S.J.; Mikłasz, W.; Lewandowski, D.; Potempa, M.; Gawliczek, M. Recycling Odpadów Poużytkowych w Polsce Zawierających Metale Nieżelazne w Strategii na Rzecz Zrównoważonego Rozwoju; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2019. [Google Scholar]
- Pikoń, K.; Bogacka, M. Environmental and Socio-Economic Evaluation of Complex Process. In Proceedings of the International Multidisciplinary Scientific GeoConference-SGEM, Albena, Bulgaria, 18–24 June 2015; pp. 243–250. [Google Scholar]
- European Environment Agency. Circular economy in Europe: Developing the knowledge base. In European Environment Agency Report; European Environment Agency: København, Denmark, 2016. [Google Scholar] [CrossRef]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Czarnowska, L.; Stanek, W.; Pikoń, K. Environmental Quality Evaluation of Hard Coal Using LCA and Exergo-Ecological Cost Methodology. In Proceedings of the SDEWES: The 8th Conference on Sustainable Development of Energy, Water and Environment Systems Book Series: Chemical Engineering Transactions, Hong Kong, China, 16–20 September 2014; pp. 139–144. [Google Scholar]
- Nawrocki, T. Blistry—najpopularniejsze opakowania leków. Aptekarz Pol. 2014, 2. [Google Scholar]
- Dobrucka, R. Recent trends in packaging systems for pharmaceutical products. Sci. J. Logist. 2014, 10, 393–398. [Google Scholar]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Beyene, H.D.; Werkneh, A.A.; Ambaye, T.G. Current updates on waste to energy (WtE) technologies: A review. Renew. Energy Focus 2018, 24, 1–11. [Google Scholar] [CrossRef]
- Bean, T.G.; Bergstrom, E.; Thomas-Oates, J.; Wolff, A.; Bartl, P.; Eaton, B.; Boxall, A.B. Evaluation of a Novel Approach for Reducing Emissions of Pharmaceuticals to the Environment. Environ. Manage. 2016, 58, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Cepeliogullar, O.; Putun, A.E. Utilization of Two Different Types of Plastic Wastes from Daily and Industrial Life. J. Selcuk Univ. Nat. Appl. Sci. 2000, 2, 694–706. [Google Scholar]
- Ragaert, K.; Delva, L.; van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Zevenhoven, R.; Axelsen, E.P.; Hupa, M. Pyrolysis of waste-derived fuel mixtures containing PVC. Fuel 2002, 81, 507–510. [Google Scholar] [CrossRef]
- Klejnowska, K.; Pikoń, K.; Ścierski, W.L.; Zajączkowski, A. Termiczny rozkład wielomateriałowych odpadowych opakowań po produktach farmaceutycznych. Przem. Chem. 2019, 98, 1414–1416. [Google Scholar]
- Honus, S.; Kumagai, S.; Molnár, V.; Fedorko, G.; Yoshioka, T. Pyrolysis gases produced from individual and mixed PE, PP, PS, PVC, and PET—Part II: Fuel characteristics. Fuel 2018, 221, 361–373. [Google Scholar] [CrossRef]
- Nadziakiewicz, J.; Wacławiak, K.; Stelmach, S. Procesy Termiczne Utylizacji Odpadów; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2012. [Google Scholar]
- Orman, M.; Woźniak, Z. Metalurgia Aluminium; Wydawnictwo “Śląsk”: Katowice, Poland, 1972. [Google Scholar]
- Soo, V.K.; Peeters, J.; Paraskevas, D.; Compston, P.; Doolan, M.; Duflou, J.R. Sustainable aluminium recycling of end-of-life products: A joining techniques perspective. J. Clean. Prod. 2018, 178, 119–132. [Google Scholar] [CrossRef]
- Yin, S.; Rajarao, R.; Gong, B.; Wang, Y.; Kong, C.; Sahajwalla, V. Thermo-delamination of metallised composite plastic: An innovative approach to generate Aluminium from packaging plastic waste. J. Clean. Prod. 2019, 211, 321–329. [Google Scholar] [CrossRef]
- Bobrański, B. Analiza Ilościowa Związków Organicznych; PWN: Warszawa, Poland, 1979. [Google Scholar]
- Klejnowska, K.; Pikoń, K.; Ścierski, W.; Skutil, K.; Bogacka, M. Influence of temperature on the composition and calorific value of gases produced during the pyrolysis of waste pharmaceutical blisters. Appl. Sci. 2020, 10, 737. [Google Scholar] [CrossRef]
- Szargut, J. Termodynamika Techniczna; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 1997. [Google Scholar]
- Pikoń, K.; Krawczyk, P.; Badyda, K. Predictive Analysis of Waste Co-Combustion with Fossil Fuels Using the Life Cycle Assessment (LCA) Methodology. Energies 2019, 12, 3691. [Google Scholar] [CrossRef]
- Czop, M. Tests of basic fueling properties of polyolefin wastes. Arch. Waste Manag. Environ. Prot. 2013, 15, 71–80. [Google Scholar] [CrossRef][Green Version]
- Lu, P.; Huang, Q.; Bourtsalas, A.C.; Themelis, N.J.; Chi, Y.; Yan, J. Review on fate of chlorine during thermal processing of solid wastes. J. Environ. Sci. 2019, 78, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Badyda, K.; Krawczyk, P.; Pikoń, K. Relative environmental footprint of waste-based fuel burned in a power boiler in the context of end-of-waste criteria assigned to the fuel. Energy 2016, 100, 425–430. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).