Improved Formation Kinetics of Carbon Dioxide Hydrate in Brine Induced by Sodium Dodecyl Sulfate
Abstract
1. Introduction
2. Experimental
2.1. Experimental Materials and Apparatus
2.2. Experimental Section
2.2.1. Macroscopic Measurements
2.2.2. Data Processing
2.2.3. Microscopic Measurements
3. Results and Discussion
3.1. Macroscopic Experiments
3.2. Microscopic Experiments
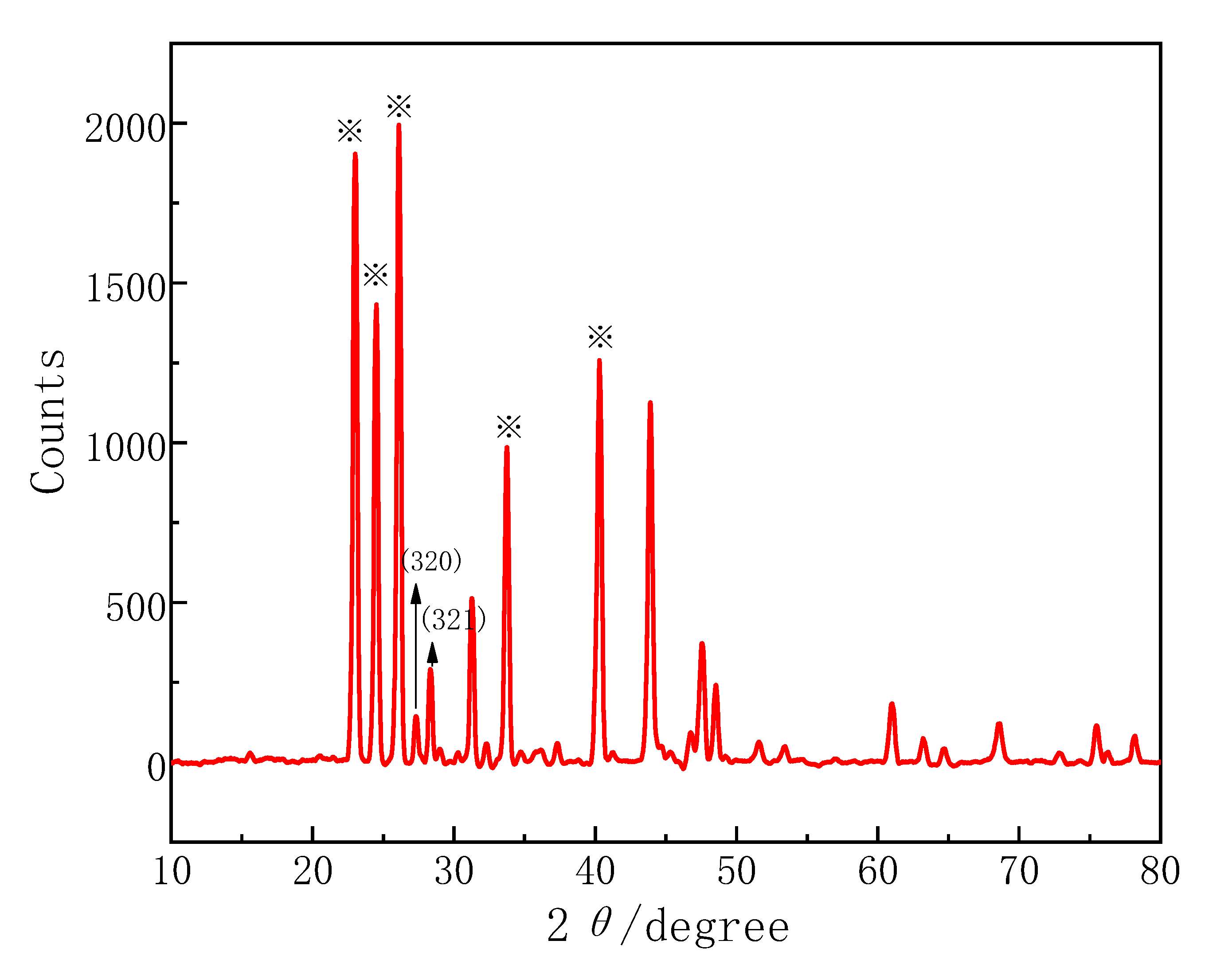
3.2.1. XRD
3.2.2. Cryo-SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.M. Advances in seawater desalination technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Park, K.N.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.G.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+). Desalination 2011, 274, 91–96. [Google Scholar] [CrossRef]
- Kalogirou, S.A. Seawater desalination using renewable energy sources. Prog. Energy Comb. Sci. 2005, 31, 242–281. [Google Scholar] [CrossRef]
- Englezos, P. Clathrate hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, W.; Lee, Y.; Jun, B.M.; Kwon, Y.N. Investigation of Hydrate-induced Ice Desalination (HIID) and its application to a pretreatment of reverse osmosis (RO) process. Desalination 2016, 395, 8–16. [Google Scholar] [CrossRef]
- Hong, S.; Moon, S.; Lee, Y.; Park, Y. Investigation of thermodynamic and kinetic effects of cyclopentane derivatives on CO2 hydrates for potential application to seawater desalination. Chem. Eng. J. 2019, 363, 99–106. [Google Scholar] [CrossRef]
- Han, S.; Rhee, Y.W.; Kang, S.P. Investigation of salt removal using cyclopentane hydrate formation and washing treatment for seawater desalination. Desalination 2017, 404, 132–137. [Google Scholar] [CrossRef]
- Cha, J.H.; Seol, Y. Increasing Gas Hydrate Formation Temperature for Desalination of High Salinity Produced Water with Secondary Guests. ACS Sustain. Chem. Eng. 2013, 1, 1218–1224. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zhang, Y.; Zang, X.Y.; Liang, D.Q. Formation Kinetics of the Mixed Cyclopentane-Carbon Dioxide Hydrates in Aqueous Sodium Chloride Solutions. Energies 2020, 13, 4388. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.B.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A review of clathrate hydrate based desalination to strengthen energy-water nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; Chong, Z.R.; Daraboina, N.; Albeirutty, M.; Bamaga, O.A.; Linga, P. Hydrate-based desalination (HyDesal) process employing a novel prototype design. Chem. Eng. Sci. 2020, 218, 115563. [Google Scholar] [CrossRef]
- He, T.B.; Nair, S.K.; Babu, P.; Linga, P.; Karimi, I.A. A novel conceptual design of hydrate based desalination (HyDesal) process by utilizing LNG cold energy. Appl. Energy 2018, 222, 13–24. [Google Scholar] [CrossRef]
- Kang, K.C.; Linga, P.; Park, K.N.; Choi, S.J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Unusual behavior of propane as a co-guest during hydrate formation in silica sand: Potential application to seawater desalination and carbon dioxide capture. Chem. Eng. Sci. 2014, 117, 342–351. [Google Scholar] [CrossRef]
- Linga, P.; Clarke, M.A. A review of reactor designs and materials employed for increasing the rate of gas hydrate formation. Energy Fuels 2017, 31, 1–13. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Chen, Z.Y.; Wu, H.J. Tetra-n-butyl ammonium bromide semi-clathrate hydrate process for post-combustion capture of carbon dioxide in the presence of dodecyl trimethyl ammonium chloride. Energy 2010, 35, 3902–3908. [Google Scholar] [CrossRef]
- Torre, J.P.; Ricaurte, M.; Dicharry, C.; Broseta, D. CO2 enclathration in the presence of water-soluble hydrate promoters: Hydrate phase equilibria and kinetic studies in quiescent conditions. Chem. Eng. Sci. 2012, 82, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.S.; Lee, J.W. Enhanced Kinetics of CO2 Hydrate Formation under Static Conditions. Ind. Eng. Chem. Res. 2009, 48, 5934–5942. [Google Scholar] [CrossRef]
- Arora, A.; Cameotra, S.S.; Kumar, R.; Balomajumder, C.; Singh, A.K.; Santhakumari, B.; Kumar, P.; Laik, S. Biosurfactant as a Promoter of Methane Hydrate Formation: Thermodynamic and Kinetic Studies. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Zhou, X.B.; Wan, L.H.; Long, Z.; Liang, D.Q. Kinetic measurements on CO2 adsorption and release using TBAB∙38H2O hydrates as adsorbents. Energy Fuels 2019, 33, 6727–6733. [Google Scholar] [CrossRef]
- Han, X.H.; Wang, S.J. Surfactant Accelerates Gas Hydrate Formation. In Proceedings of the 4th International Conference on Gas Hydrates, Yokohama, Japan, 19–23 May 2002; Volume 2, pp. 1036–1039. [Google Scholar]
- Jiang, L.L.; Li, A.R.; Xu, J.F.; Liu, Y.J. Effects of SDS and SDBS on CO(2)Hydrate formation, induction time, storage capacity and stability at 274.15 K and 5.0 MPa. Chemistryselect 2016, 1, 6111–6114. [Google Scholar] [CrossRef]
- Molokitina, N.S.; Nesterov, A.N.; Podenko, L.S.; Reshetnikov, A.M. Carbon dioxide hydrate formation with SDS: Further insights into mechanism of gas hydrate growth in the presence of surfactant. Fuel 2019, 235, 1400–1411. [Google Scholar] [CrossRef]
- Dicharry, C.; Duchateau, C.; Asbai, H.; Broseta, D.; Torre, J.P. Carbon dioxide gas hydrate crystallization in porous silica gel particles partially saturated with a surfactant solution. Chem. Eng. Sci. 2013, 98, 88–97. [Google Scholar] [CrossRef]
- Gayet, P.; Dicharry, C.; Marion, G.; Graciaa, A.; Lachaise, J.; Nesterov, A. Experimental determination of methane hydrate dissociation curve up to 55 MPa by using a small amount of surfactant as hydrate promoter. Chem. Eng. Sci. 2005, 60, 5751–5758. [Google Scholar] [CrossRef]
- Link, D.D.; Ladner, E.P.; Elsen, H.A.; Taylor, C.E. Formation and dissociation studies for optimizing the uptake of methane by methane hydrates. Fluid Phase Equilibria 2003, 211, 1–10. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, J.W. Kinetic behaviors of CO2 hydrates in porous media and effect of kinetic promoter on the formation kinetics. Chem. Eng. Sci. 2010, 65, 1840–1845. [Google Scholar] [CrossRef]
- Zhang, J.S.; Lo, C.; Somasundaran, P.; Lee, J.W. Competitive adsorption between SDS and carbonate on tetrahydrofuran hydrates. J. Colloid Interface Sci. 2010, 341, 286–288. [Google Scholar] [CrossRef]
- Torre, J.P.; Dicharry, C.; Ricaurte, M.; Daniel-David, D.; Broseta, D. CO2 capture by hydrate formation in quiescent conditions: In search of efficient kinetic additives. In 10th International Conference on Greenhouse Gas Control Technologies; Gale, J., Hendriks, C., Turkenberg, W., Eds.; Elsevier Science BV: Amsterdam, The Netherlands, 2011; Volume 4, pp. 621–628. [Google Scholar]
- Sun, J.Y.; Li, C.F.; Hao, X.L.; Liu, C.L.; Chen, Q.; Wang, D.G. Study of the surface Morphology of Gas Hydrate. J. Ocean Univ. China 2020, 19, 331–338. [Google Scholar] [CrossRef]
- Yi, L.Z.; Zhao, L.L.; Tao, S.H. Methane hydrate formation in an oil-water system in the presence of lauroylamide propylbetaine. RSC Adv. 2020, 10, 12255–12261. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zang, Q.; Long, Z.; Liang, D.Q. In situ PXRD analysis on the kinetic effect of PVP-K90 and PVCap on methane hydrate dissociation below ice point. Fuel 2021, 286, 119491. [Google Scholar] [CrossRef]
- Schicks, J.M.; Ripmeester, J.A. The Coexistence of Two Different Methane Hydrate Phases under Moderate Pressure and Temperature Conditions: Kinetic versus Thermodynamic Products. Angew. Chem. Int. Ed. 2004, 43, 3310–3313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.B.; Liang, D.Q. Enhanced performance on CO2 adsorption and release induced by structural transition that occurred in TBAB∙26H2O hydrates. Chem. Eng. J. 2019, 378, 122128. [Google Scholar] [CrossRef]
- Zhong, Y.; Rorges, R.E. Surfactant effects on gas hydrate formation. Chem. Eng. Sci. 2000, 55, 4175–4187. [Google Scholar] [CrossRef]
- Rorges, R.E. Chemical Engineering. U.S. Patent 6389820, 21 May 2002. [Google Scholar]
- Pahlavanzadeh, H.; Khanlarkhani, M.; Rezaei, S.; Mohammadi, A.H. Experimental and modelling studies on the effects of nanofluids (SiO2, Al2O3, and CuO) and surfactants (SDS and CTAB) on CH4 and CO2 clathrate hydrates formation. Fuel 2019, 253, 1392–1405. [Google Scholar] [CrossRef]
- Partoon, B.; Malik, S.N.A.; Azemi, M.H.; Sabil, K.M. Experimental investigations on the potential of SDS as low-dosage promoter for carbon dioxide hydrate formation. Asia-Pac. J. Chem. Eng. 2013, 8, 916–921. [Google Scholar] [CrossRef]
- Henning, R.W.; Schultz, A.J.; Thieu, V. Neutron diffraction studies of CO2 clathrate hydrate: Formation from deuterated ice. J. Phys. Chem. A 2000, 104, 5066–5071. [Google Scholar] [CrossRef]





| Reagent | Molecular Formula | Purity | Supplier |
|---|---|---|---|
| Sodium dodecyl sulfate (SDS) | C12H25O4NaS | 99.0 wt.% | Macklin Company, (Shanghai, China) |
| Sodium chloride | NaCl | 99.5 wt.% | |
| Carbon dioxide | CO2 | 99.99 mol% | Guangzhou Yuejia Gas Co (Guangdong, China) |
| Deionized water | H2O | Electrical resistivity 18.2 mΩ·cm−1 | Made in laboratory |
| System | n/mol | wt./% |
|---|---|---|
| 3.5 wt.% NaCl | 0.03130 | 3.997 |
| 3.5 wt.% NaCl+0.01 wt.% SDS | 0.04102 | 4.182 |
| 3.5 wt.% NaCl+0.02 wt.% SDS | 0.04102 | 4.178 |
| 3.5 wt.% NaCl+0.05 wt.% SDS | 0.05655 | 4.515 |
| 3.5 wt.% NaCl+0.07 wt.% SDS | 0.03041 | 4.101 |
| 3.5 wt.% NaCl+0.10 wt.% SDS | 0.03775 | 4.118 |
| System | Cw1/% | Cw2/% |
|---|---|---|
| 3.5 wt.% NaCl | 11.77 | 6.70 |
| 3.5 wt.% NaCl + 0.01 wt.% SDS | 15.34 | 34.7 |
| 3.5 wt.% NaCl + 0.02 wt.% SDS | 15.42 | 29.5 |
| 3.5 wt.% NaCl + 0.05 wt.% SDS | 21.26 | 30.0 |
| 3.5 wt.% NaCl + 0.07 wt.% SDS | 15.02 | 31.3 |
| 3.5 wt.% NaCl + 0.10 wt.% SDS | 14.19 | 36.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Yao, Y.; Zhou, X.; Zhang, Y.; Liang, D. Improved Formation Kinetics of Carbon Dioxide Hydrate in Brine Induced by Sodium Dodecyl Sulfate. Energies 2021, 14, 2094. https://doi.org/10.3390/en14082094
Liu L, Yao Y, Zhou X, Zhang Y, Liang D. Improved Formation Kinetics of Carbon Dioxide Hydrate in Brine Induced by Sodium Dodecyl Sulfate. Energies. 2021; 14(8):2094. https://doi.org/10.3390/en14082094
Chicago/Turabian StyleLiu, Lu, Yuanxin Yao, Xuebing Zhou, Yanan Zhang, and Deqing Liang. 2021. "Improved Formation Kinetics of Carbon Dioxide Hydrate in Brine Induced by Sodium Dodecyl Sulfate" Energies 14, no. 8: 2094. https://doi.org/10.3390/en14082094
APA StyleLiu, L., Yao, Y., Zhou, X., Zhang, Y., & Liang, D. (2021). Improved Formation Kinetics of Carbon Dioxide Hydrate in Brine Induced by Sodium Dodecyl Sulfate. Energies, 14(8), 2094. https://doi.org/10.3390/en14082094






