Numerical Analysis of the Influence of Different Flow Patterns on Power and Reactant Transmission in Tubular-Shaped PEMFC
Abstract
:1. Introduction
2. Mathematical Model
2.1. Assumptions
- The gas inlet conditions of the cathode and anode are considered to be completely humid.
- There are stationary and non-isothermal conditions.
- The laminar flow is established inside the channel.
- The system works under steady-state conditions.
- The fluid is an ideal incompressible fluid.
- The liquid water transfer mechanism in the membrane is controlled by permeation resistance and diffusion.
- Membrane, CL and GDL are considered to have a uniform porous area and to be isotropic.
2.2. Governing Equations
2.3. Boundary Conditions
3. Results and Discussion
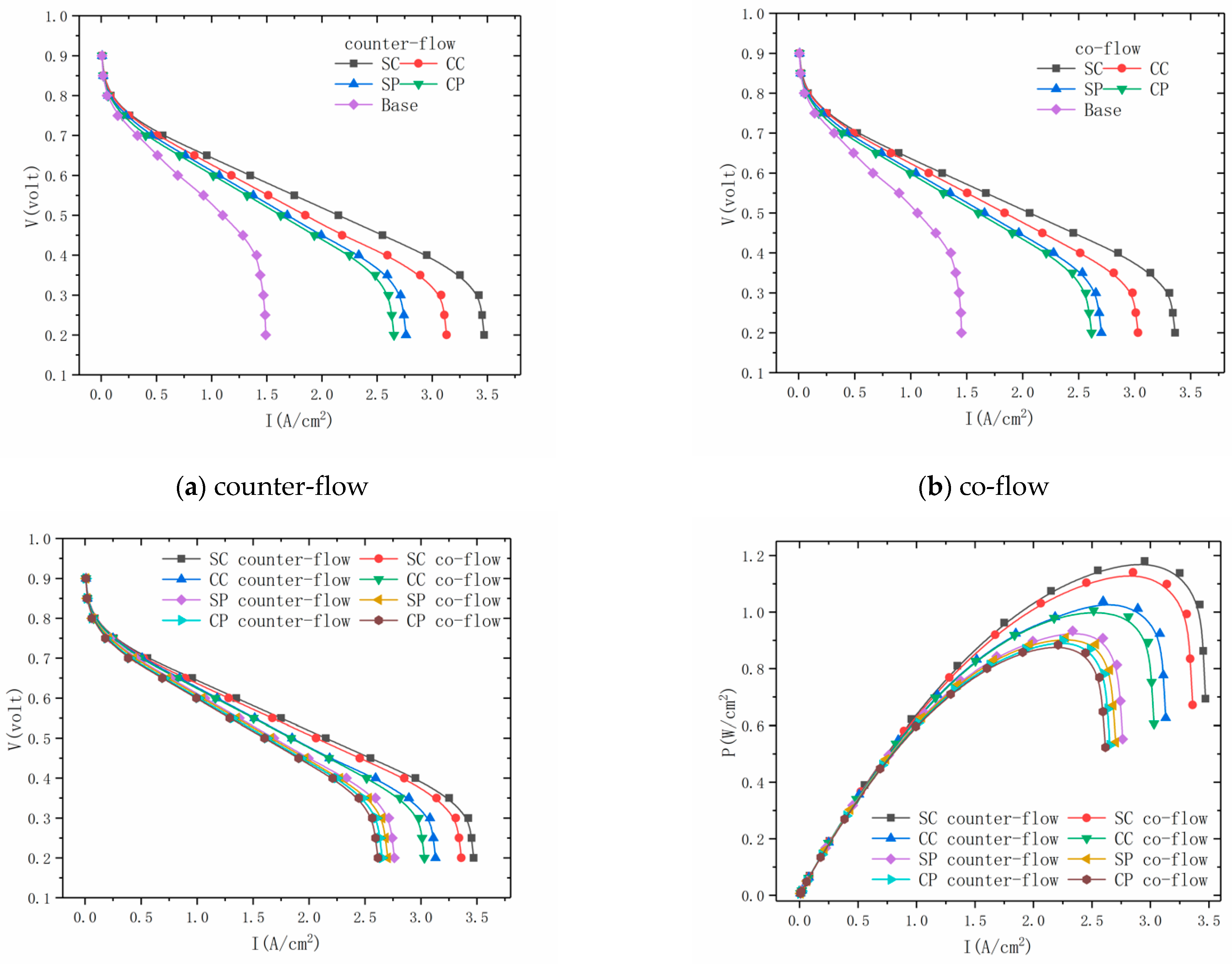
3.1. PEMFC Polarization Curve
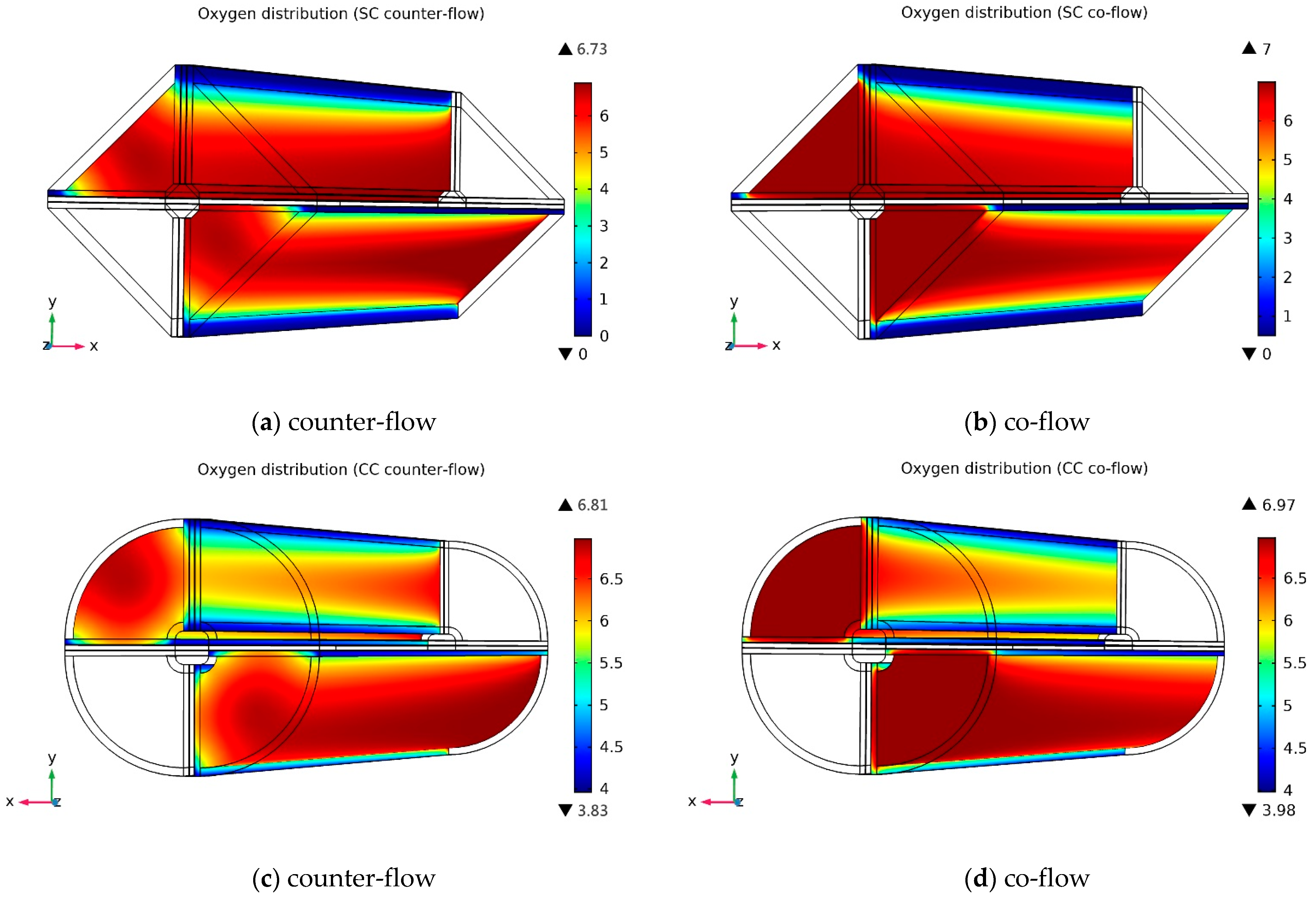
3.2. Species Concentration Distribution
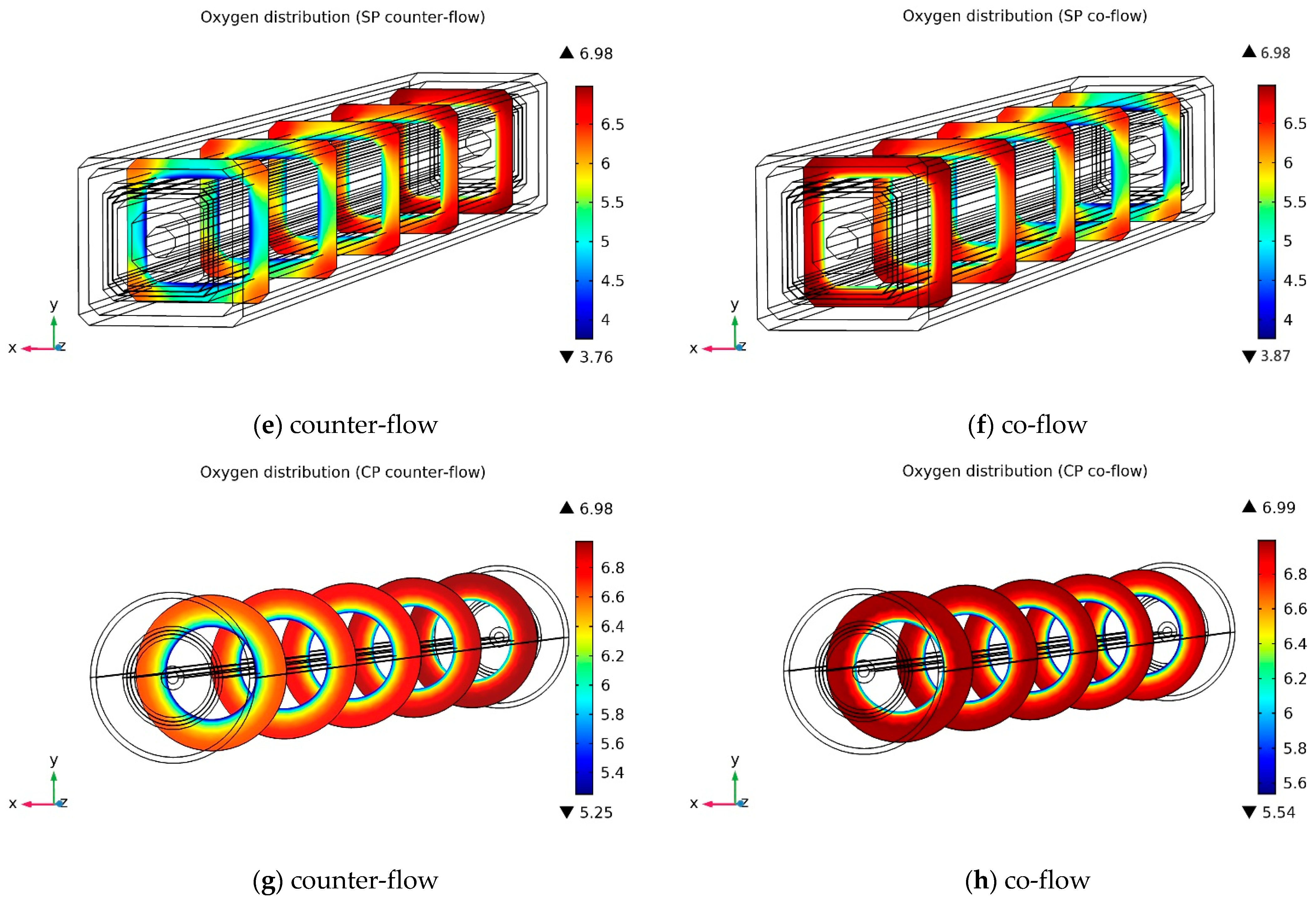
3.2.1. Oxygen Molar Concentration Distribution
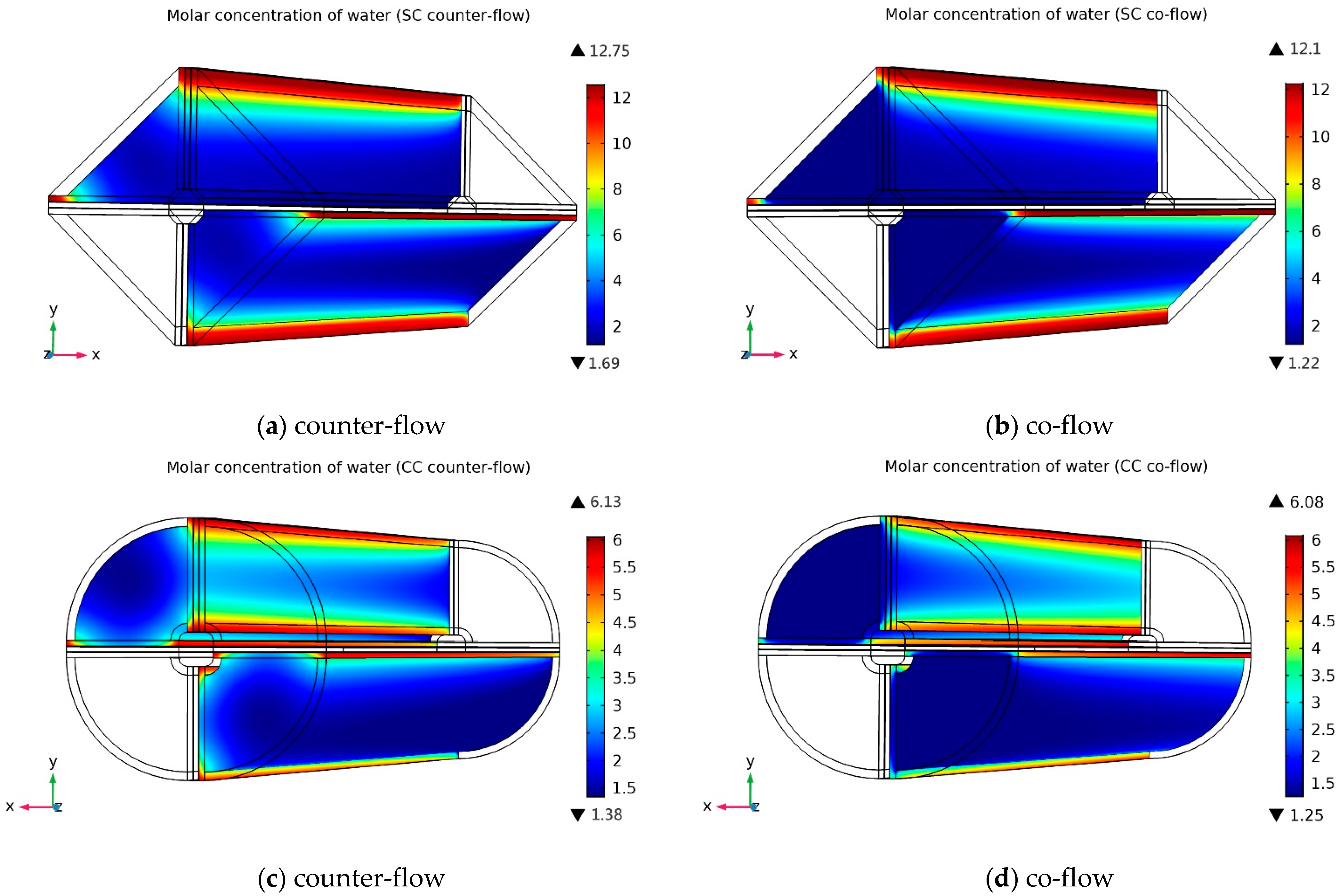
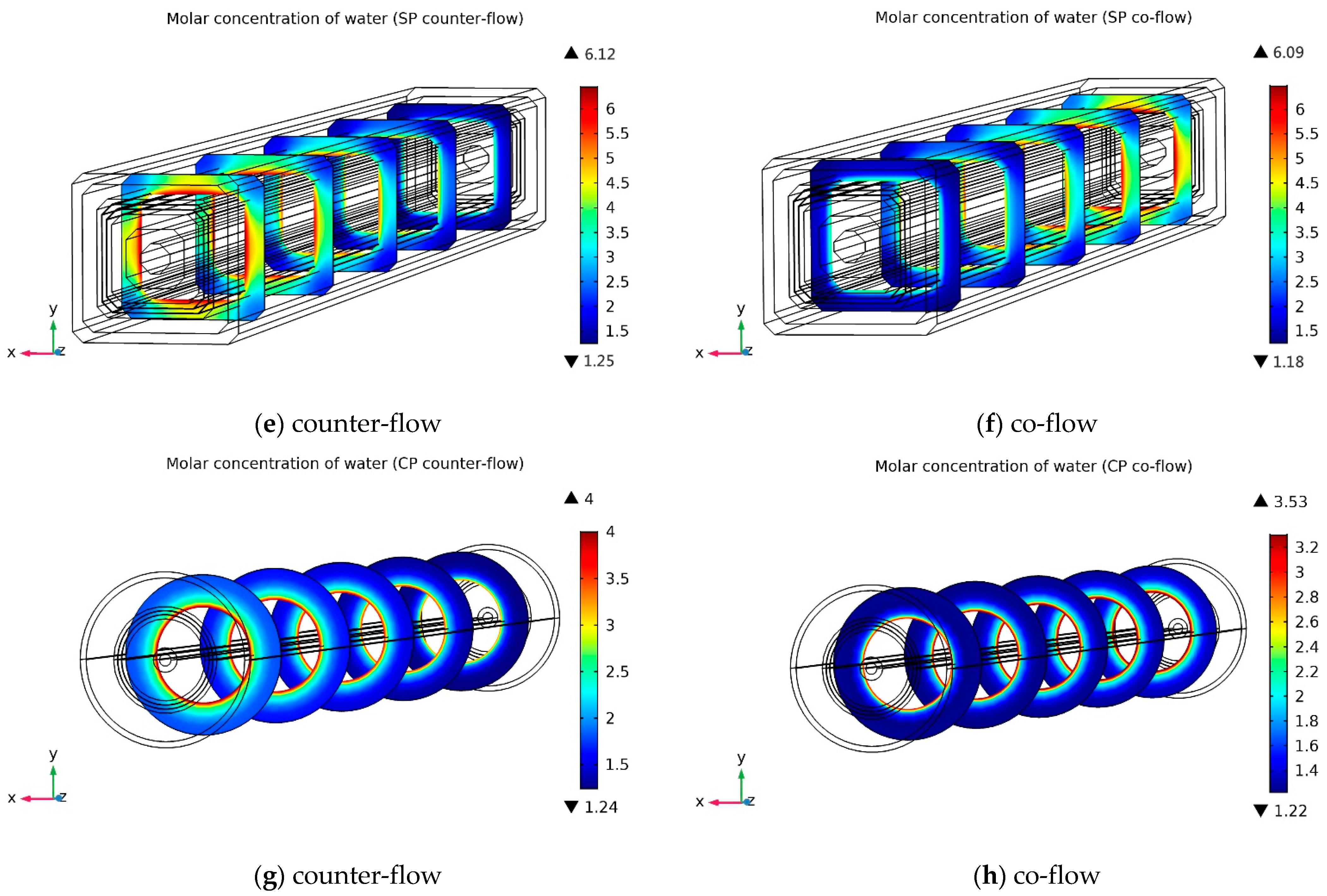
3.2.2. Cathode Water Molar Concentration Distribution
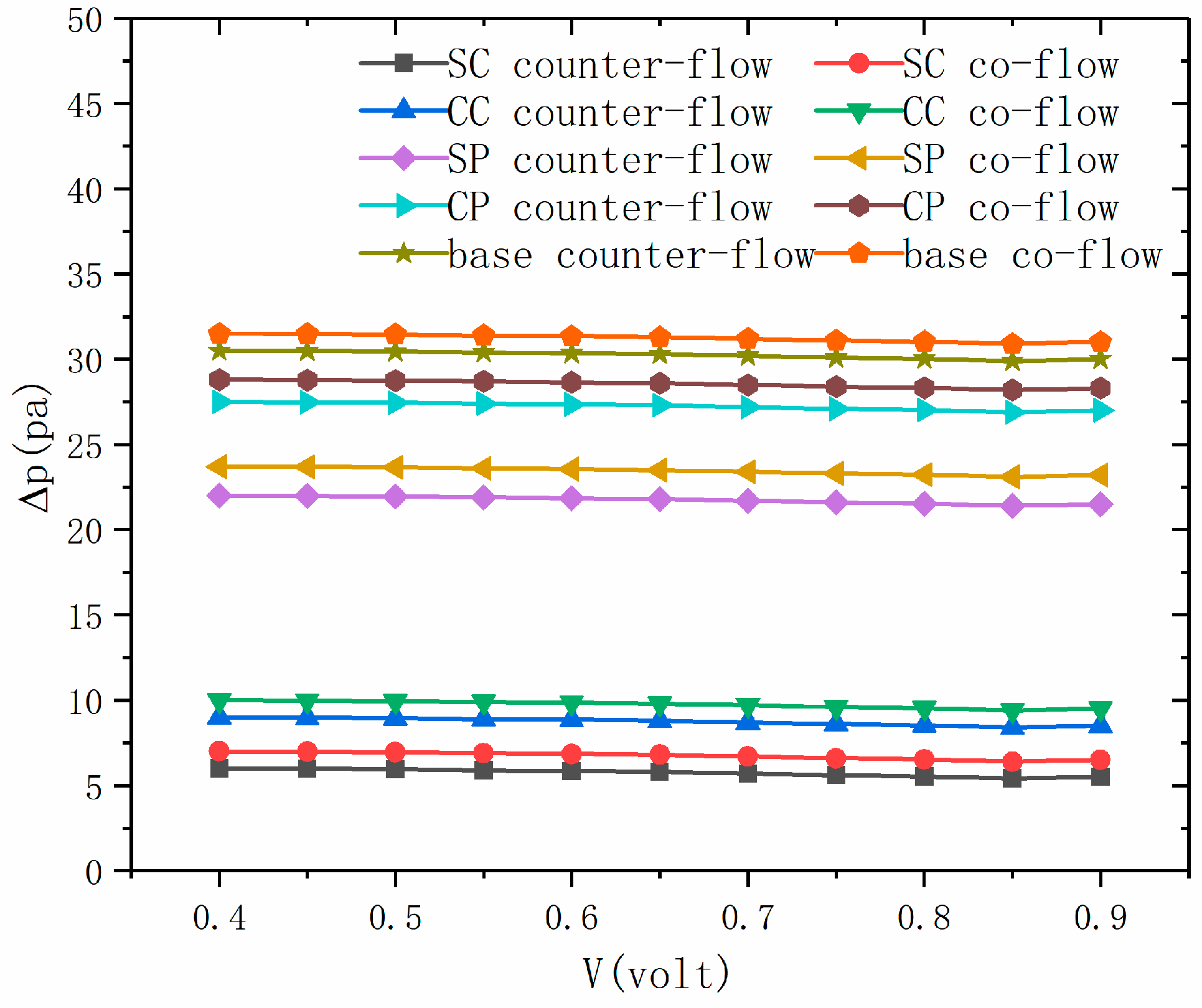
3.3. Pressure Drop
3.4. Cell Net Power
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Aan:ca | area cross section channel for anode and cathode (m2) |
| Amem | active cell area (m2) |
| a | water activity (-) |
| D | mass diffusion coefficient (m2/s) |
| F | Faraday’s constant (C/mol) |
| K | Permeability (m2) |
| k | thermal conductivity (W/m·K) |
| M | molecular weight (g/mol) |
| P | Pressure (Pa) |
| R | universal gas constant (J/mol·K) |
| T | Temperature (K) |
| VOC | open circuit voltage (V) |
| X | mole fraction (-) |
| Subscripts and Superscripts | |
| an | Anode (-) |
| ca | Cathode (-) |
| eff | Effective (-) |
| mem | Membrane (-) |
| m | Mass (-) |
| i | individual specie in the reference condition (-) |
| ref | reference value (-) |
| Greek Symbols | |
| α | water transfer coefficient (-) |
| ε | Porosity (-) |
| ρ | Density (kg/m3) |
| γ | concentration coefficient (-) |
| μ | Viscosity (kg/m·s) |
| σ | membrane conductivity |
| λ | water content (-) |
| ξ | stoichiometric ratio (-) |
References
- Nguyen, T.-T.; Fushinobu, K. Effect of operating conditions and geometric structure on the gas crossover in PEM fuel cell. Sustain. Energy Technol. Assess. 2020, 37, 100584. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Laribi, S.; Mammar, K.; Sahli, Y.; Koussa, K. Analysis and diagnosis of PEM fuel cell failure modes (flooding & drying) across the physical parameters of electrochemical impedance model: Using neural networks method. Sustain. Energy Technol. Assess. 2019, 34, 35–42. [Google Scholar]
- Ijaodola, O.S.; Hassan, Z.E.; Ogungbemi, E.; Khatib, F.N.; Wilberforce, T.; Thompson, J.; Olabi, A.G. Energy efficiency improvements by investigating the water flooding management on proton exchange membrane fuel cell (PEMFC). Energy 2019, 179, 246–267. [Google Scholar] [CrossRef]
- Sayed, E.T.; Eisa, T.; Mohamed, H.O.; Abdelkareem, M.A.; Allagui, A.; Alawadhi, H.; Chae, K.-J. Direct urea fuel cells: Challenges and opportunities. J. Power Sources 2019, 417, 159–175. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; el Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel cell membranes-Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Priya, K.; Babu, T.S.; Balasubramanian, K.; Kumar, K.S.; Rajasekar, N. A novel approach for fuel cell parameter estimation using simple Genetic Algorithm. Sustain. Energy Technol. Assess. 2015, 12, 46–52. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Al Haj, Y.; Alajami, M.; Alawadhi, H.; Barakat, N.A.M. Ni-Cd carbon nanofibers as an effective catalyst for urea fuel cell. J. Environ. Chem. Eng. 2018, 6, 332–337. [Google Scholar] [CrossRef]
- Olabi, A.G.; Mahmoud, M.; Soudan, B.; Wilberforce, T.; Ramadan, M. Geothermal based hybrid energy systems, toward eco-friendly energy approaches. Renewable Energy 2020, 147 Pt 1, 2003–2012. [Google Scholar] [CrossRef]
- Subin, K.; Jithesh, P.K. Experimental study on self-humidified operation in PEM fuel cells. Sustain. Energy Technol. Assess. 2018, 27, 17–22. [Google Scholar] [CrossRef]
- Verma, A.; Pitchumani, R. Influence of transient operating parameters on the mechanical behavior of fuel cells. Int. J. Hydrogen Energy 2015, 40, 8442–8453. [Google Scholar] [CrossRef]
- Jeon, Y.; Na, H.; Hwang, H.; Park, J.; Hwang, H.; Shul, Y. Accelerated life-time test protocols for polymer electrolyte membrane fuel cells operated at high temperature. Int. J. Hydrogen Energy 2015, 40, 3057–3067. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.I.; Lee, N.W.; Kim, M.S. Study on a purge method using pressure reduction for effective water removal in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2015, 40, 9473–9484. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Gao, Y. Understanding and approaches for the durability issues of Pt-based catalysts for PEM fuel cell. J. Power Sources 2007, 171, 558–566. [Google Scholar] [CrossRef]
- Goltsov, V.A.; Veziroglu, T.N. From hydrogen economy to hydrogen civilization. Int. J. Hydrogen Energy 2001, 26, 909–915. [Google Scholar] [CrossRef]
- Sherif, S.A.; Barbir, F.; Veziroglu, T.N. Wind energy and the hydrogen economy-review of the technology. Sol. Energy 2005, 78, 647–660. [Google Scholar] [CrossRef]
- Wei, Z.; Su, K.; Sui, S.; He, A.; Du, S. High performance polymer electrolyte membrane fuel cells (PEMFCs) with gradient Pt nanowire cathodes prepared by decal transfer method. Int. J. Hydrogen Energy 2015, 40, 3068–3074. [Google Scholar] [CrossRef]
- Mert, S.O.; Ozcelik, Z.; Dincer, I. Comparative assessment and optimization of fuel cells. Int. J. Hydrogen Energy 2015, 40, 7835–7845. [Google Scholar] [CrossRef]
- Yuan, W.; Tang, Y.; Pan, M.; Li, Z.; Tang, B. Model prediction of effects of operating parameters on proton exchange membrane fuel cell performance. Renew. Energy 2010, 35, 656–666. [Google Scholar] [CrossRef]
- Larbi, B.; Alimi, W.; Chouikh, R.; Guizani, A. Effect of porosity and pressure on the PEM fuel cell performance. Int. J. Hydrogen Energy 2013, 38, 8542–8549. [Google Scholar] [CrossRef]
- Jeon, D.H.; Kim, K.N.; Baek, S.M.; Nam, J.H. The effect of relative humidity of the cathode on the performance and the uniformity of PEM fuel cell. Int. J. Hydrogen Energy 2011, 36, 12499–12511. [Google Scholar] [CrossRef]
- Ismail, M.S.; Hughes, K.J.; Ingham, D.B. Effects of anisotropic permeability and electrical conductivity of gas diffusion layers on the performance of proton exchange membrane fuel cells. Appl. Energy 2012, 95, 50–63. [Google Scholar] [CrossRef]
- Ahmed, A.; Mangi, F.H.; Kashif, M.; Chachar, F.A.; Ullah, Z. Parametric Analysis of a Serpentine Flow Pattern Proton Exchange Membrane Fuel Cell for Optimized Performance. Int. J. Heat Technol. 2020, 38, 69–76. [Google Scholar] [CrossRef]
- Khazaee, I.; Ghazikhani, M. Performance improvement of proton exchange membrane fuel cell by using annular shaped geometry. J. Power Sources 2011, 196, 2661–2668. [Google Scholar] [CrossRef]
- Perng, S.W.; Wu, H.W. A three-dimensional numerical investigation of trapezoid baffles effect on non-isothermal reactant transport and cell net power in a PEMFC. Appl. Energy 2015, 143, 81–95. [Google Scholar] [CrossRef]
- Fontana, É.; Mancusi, E.; Da Silva, A.; Mariani, V.C.; De Souza, A.A.U.; de Souza, S.M.G.U. Study of the effects of flow channel with non-uniform cross-sectional area on PEMFC species and heat transfer. Int. J. Heat Mass Transf. 2011, 54, 4462–4472. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.; Genc, O.; Toros, S. Numerical optimization of channel to land width ratio for PEM fuel cell. Int. J. Hydrogen Energy 2018, 43, 10798–10809. [Google Scholar] [CrossRef]
- Khazaee, I.; Sabadbafan, H. Numerical study of changing the geometry of the flow field of a PEM fuel cell. Heat Mass Transf. 2016, 52, 993–1003. [Google Scholar] [CrossRef]
- Al-Baghdadi, M.A. Studying the effect of material parameters on cell performance of tubular-shaped PEM fuel cell. Energy Convers Manag. 2008, 49, 2986–2996. [Google Scholar] [CrossRef]
- Torkavannejad, A.; Sadeghifar, H.; Pourmahmoud, N.; Ramin, F. Novel architectures of polymer electrolyte membrane fuel cells: Efficiency enhancement and cost reduction. Int. J. Hydrogen Energy 2015, 40, 12466–12477. [Google Scholar] [CrossRef]
- Sierra, J.M.; Figueroa-Ramírez, S.J.; Díaz, S.E.; Vargas, J.; Sebastian, P.J. Numerical evaluation of a PEM fuel cell with conventional flow fields adapted to tubular plates. Int. J. Hydrogen Energy 2014, 39, 16694–16705. [Google Scholar] [CrossRef]
- Osanloo, B.; Mohammadi-Ahmar, A.; Solati, A. A numerical analysis on the effect of different architectures of membrane, CL and GDL layers on the power and reactant transportation in the square tubular PEMFC. Int. J. Hydrogen Energy 2016, 41, 10844–10853. [Google Scholar] [CrossRef]
- Mohammadi-Ahmar, A.; Osanloo, B.; Solati, A.; Ghasemi, J. Performance improvement of the circular tubular PEMFC by using different architectures and number of layers. Energy Convers Manag. 2016, 128, 238–249. [Google Scholar] [CrossRef]
- Mohammadi-Ahmar, A.; Solati, A.; Osanloo, B.; Hatami, M. Effect of number and arrangement of separator electrode assembly(SEA) on the performance of square tubular PEM fuel cells. Energy 2017, 137, 302–313. [Google Scholar] [CrossRef]
- Wang, L.; Husar, A.; Zhou, T.; Liu, H. A parametric study of PEM fuel cell performances. Int. J. Hydrogen Energy 2003, 28, 1263–1272. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zawodzinski, T.A., Jr.; Springer, T.E.; Davey, J.; Jestel, R.; Lopez, C.; Valerio, J.; Gottesfeld, S. A Comparative Study of Water Uptake By and Transport Through Ionomeric Fuel Cell Membranes. J. Electrochem. Soc. 1993, 140, 1981–1985. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Transportation Fuel Cell Power Systems. 2000 Annual Progress Report; U.S. Department of Energy: Washington, DC, USA, 2000.
- COMSOL (COMSOL Multiphysics). Multiphysics 5.5 Documentation; COMSOL Inc.: Burlington, MA, USA, 2019. [Google Scholar]
- Um, S.; Wang, C.Y.; Chen, K.S. Computational fluid dynamics modeling of proton exchange membrane fuel cells. J. Electrochem. Soc. 2000, 147, 4485–4493. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Yi, J.S.; Van Nguyen, T. Multicomponent transport in porous electrodes of proton exchange membrane fuel cells using the interdigitated gas distributors. J. Electrochem. Soc. 1999, 146, 38–45. [Google Scholar] [CrossRef]
- Nam, J.H.; Kaviany, M. Effective diffusivity and water-saturation distribution in single- and two-layer PEMFC diffusion medium. Int. J. Heat Mass Transf. 2003, 46, 4595–4611. [Google Scholar] [CrossRef]
- Chippar, P.; Kyeongmin, O.; Kang, K.; Ju, H. A numerical investigation of the effects of GDL compression and intrusion in polymer electrolyte fuel cells (PEFCs). Int. J. Hydrogen Energy 2012, 37, 6326–6338. [Google Scholar] [CrossRef]
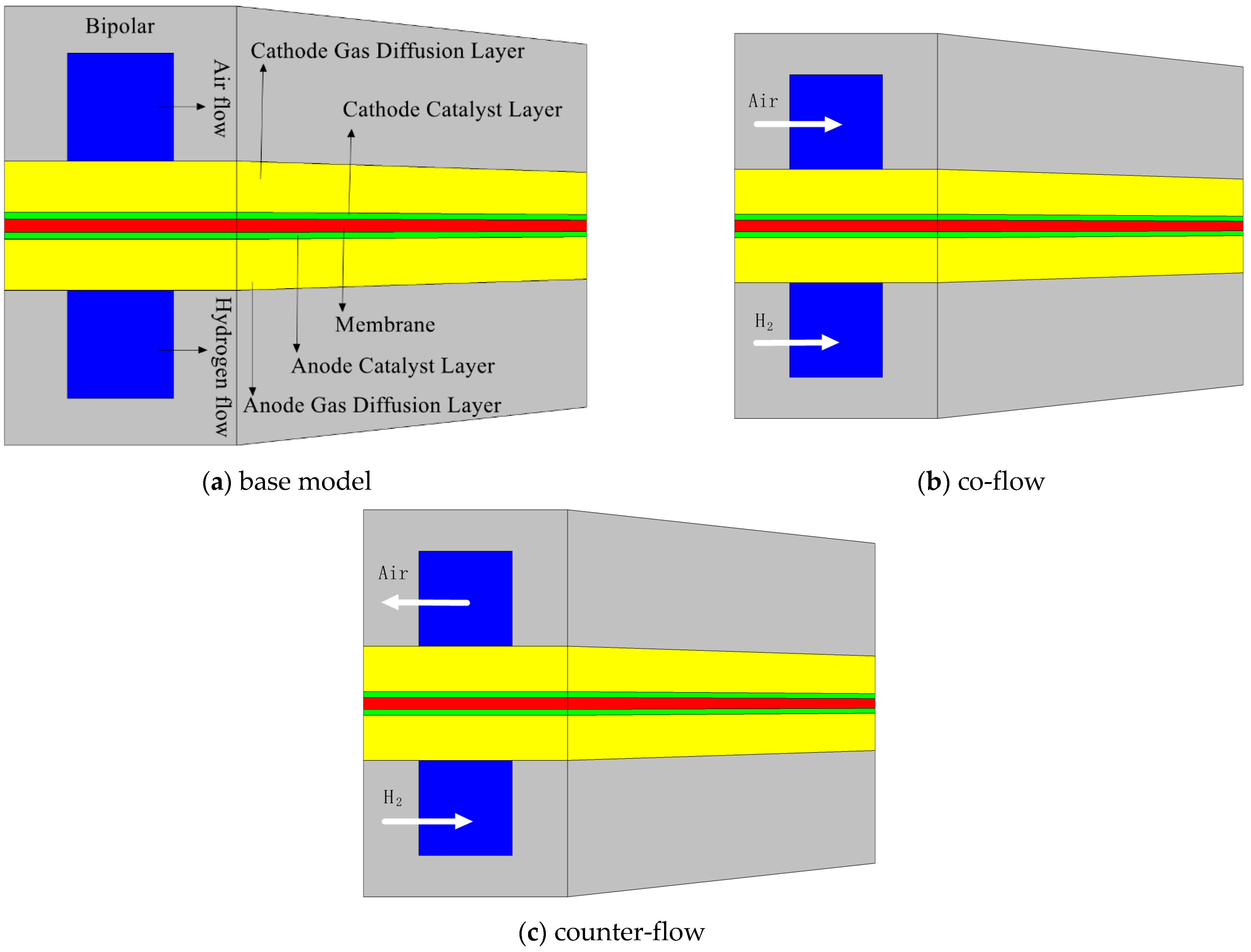

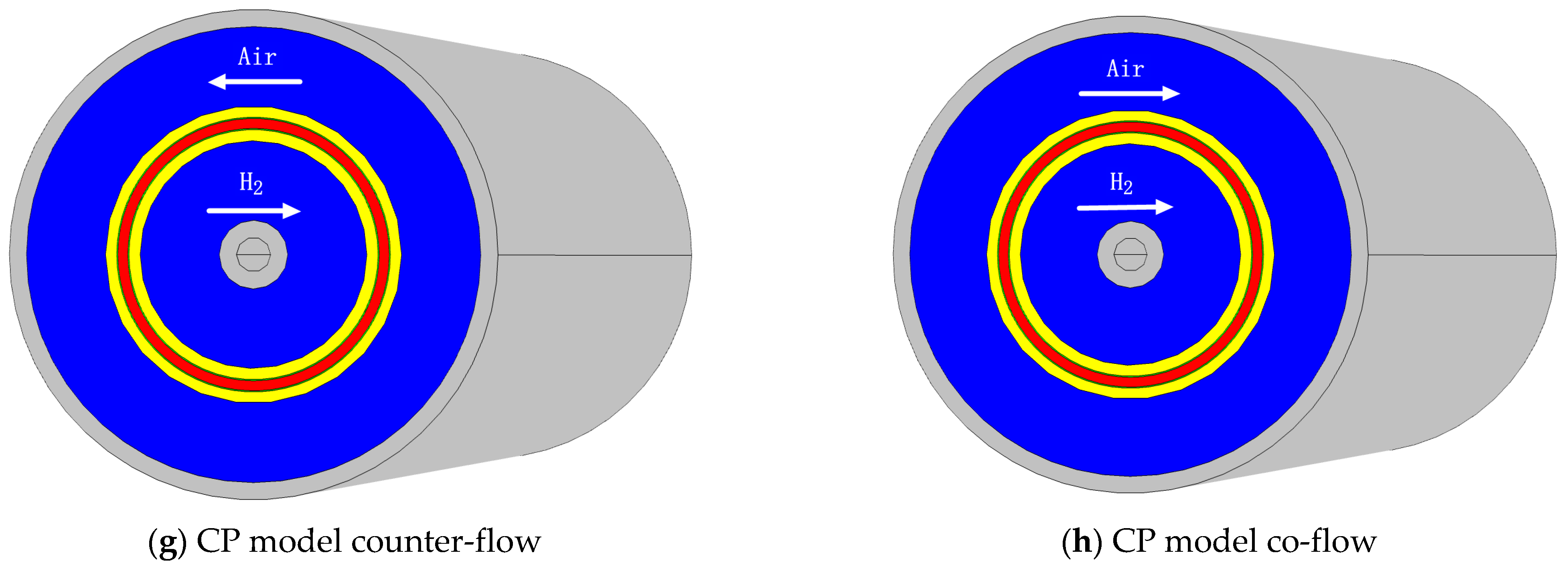


| Parameter | Symbol | Units | Planar | SC | SP | CC | CP |
|---|---|---|---|---|---|---|---|
| Channel length | L | mm | 100 | 50 | 50 | 50 | 21.8 |
| GDL thickness | δGDL | mm | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| CL thickness | δCL | mm | 0.028 | 0.028 | 0.028 | 0.028 | 0.028 |
| Membrane thickness | δM | mm | 0.183 | 0.183 | 0.183 | 0.183 | 0.183 |
| GDL porosity | εGDL | - | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| CL porosity | εCL | - | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Parameter | Symbol | Value | Units |
|---|---|---|---|
| Anode pressure | pa | 3 | atm |
| Cathode pressure | pc | 3 | atm |
| Faraday constant | F | 96,485 | C/mol |
| Inlet fuel and air temperature | Tin | 353 | K |
| Electrode electronic conductivity | λe | 100 | S/m |
| Membrane ionic conductivity (humidified Nafion117) | λm | 8.3 | S/m |
| Relative humidity of inlet fuel and air (fully humidified conditions) | Rh | 100% | - |
| Fuel/air stoichiometric ratio | ξa/ξc | 2/2 | - |
| Electrode thermal conductivity | ke | 1.3 | W/m·K |
| Membrane thermal conductivity | km | 0.455 | W/m·K |
| Transfer coefficient, anode side | αa | 0.5 | - |
| Transfer coefficient, cathode side | αc | 1 | - |
| H2 diffusivity | DH2-H2O | 1.1 × 10−4 | m2/s |
| O2 diffusivity | DO2-N2 | 3.23 × 10−5 | m2/s |
| H2O diffusivity at anode | DH2O-O2 | 7.35 × 10−5 | m2/s |
| H2O diffusivity at cathode | DH2O-N2 | 7.35 × 10−5 | m2/s |
| Membrane equivalent weigh | - | 1100 | g/mol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Jin, Z.; Yang, P.; Yang, Y.; Wang, D.; Chen, X. Numerical Analysis of the Influence of Different Flow Patterns on Power and Reactant Transmission in Tubular-Shaped PEMFC. Energies 2021, 14, 2127. https://doi.org/10.3390/en14082127
Yuan L, Jin Z, Yang P, Yang Y, Wang D, Chen X. Numerical Analysis of the Influence of Different Flow Patterns on Power and Reactant Transmission in Tubular-Shaped PEMFC. Energies. 2021; 14(8):2127. https://doi.org/10.3390/en14082127
Chicago/Turabian StyleYuan, Lei, Zunlong Jin, Penghui Yang, Youchen Yang, Dingbiao Wang, and Xiaotang Chen. 2021. "Numerical Analysis of the Influence of Different Flow Patterns on Power and Reactant Transmission in Tubular-Shaped PEMFC" Energies 14, no. 8: 2127. https://doi.org/10.3390/en14082127






