Acid Treatment as a Way to Reduce Shale Rock Mechanical Strength and to Create a Material Prone to the Formation of Permanent Well Barrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Exposure
2.2. X-ray Computed Tomography
2.3. Powder X-ray Diffraction (XRD)
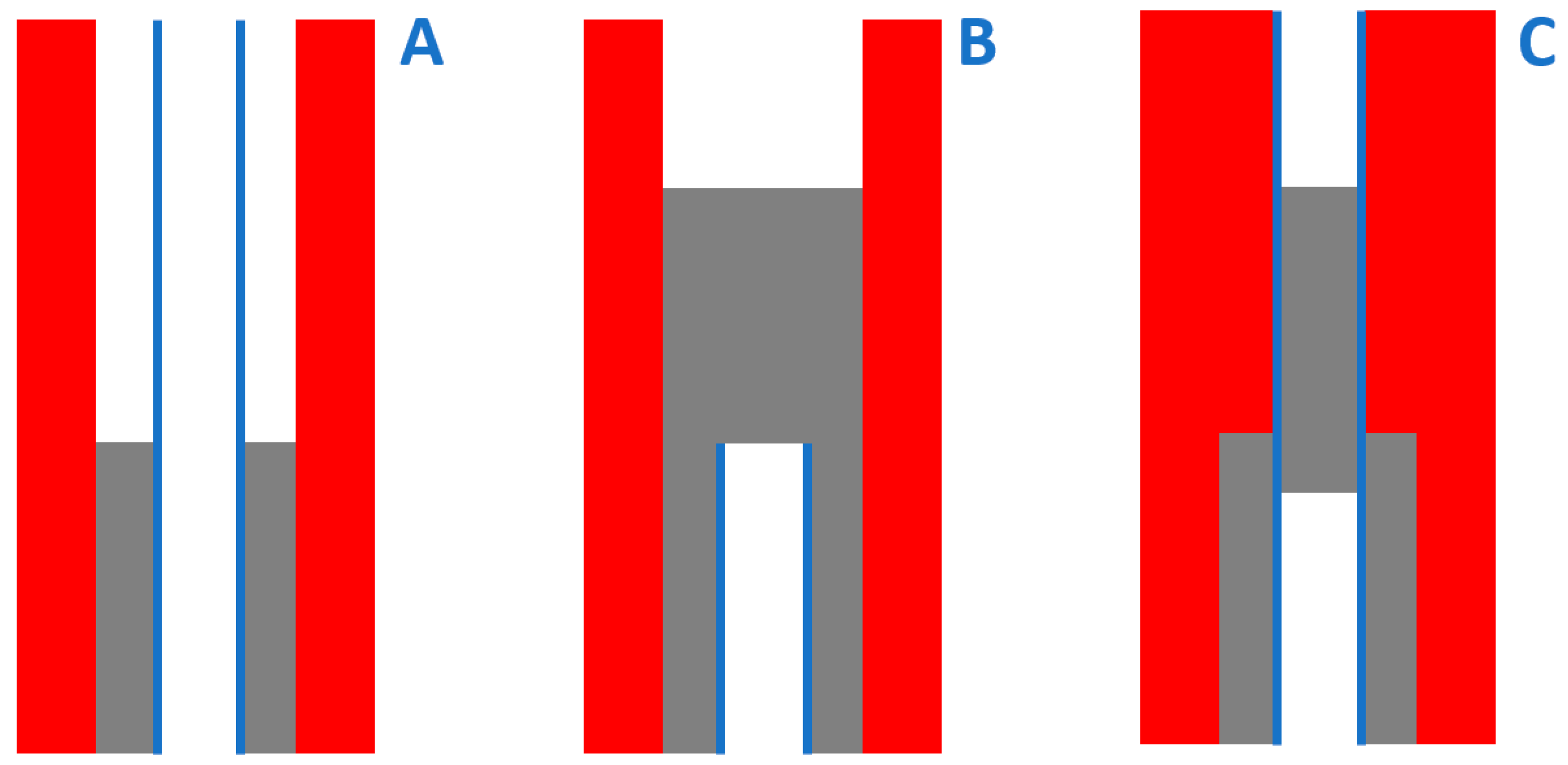
2.4. Shear Strength Measurements
2.5. P-Wave Velocity Measurements
2.6. Scanning Electron Microscopy/Energy Dispersive Spectroscopy (SEM/EDX)
3. Results and Discussion
Limitations
4. Conclusions
- (1)
- removal of around 4–5 wt% of cementation material resulted in 43% reduction in Pierre shale shear strength compared to the non-etched shale exposed to sodium chloride solution for the same time.
- (2)
- the removed material was mainly dolomite
- (3)
- leaching rate was dependent on geometry in which shale was exposed to acid, or more specifically, on volume staying in direct contact with the exposed shale surface. It has been suggested that leaching rate can be enhanced by inducing fluid flow.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| QZ | Quartz |
| K-f | K-feldspar |
| Pl | Plagioclase |
| Ch | Chlorite |
| Ka | Kaolinite |
| Mi/Il | Mica/illite |
| Mix | Mixed layer |
| Sm | Smectite |
| Dol | Dolomite |
| Py | Pyrite |
| P&A | plugging and abandonment |
| µ-CT, CT | X-ray micro-computed tomography |
| XRD | powder X-ray diffraction |
| EDX | energy dispersive X-ray spectroscopy |
References
- Vrålstad, T.; Saasen, A.; Fjær, E.; Øia, T.; Ytrehus, J.D.; Khalifeh, M. Plug & abandonment of offshore wells: Ensuring long-term well integrity and cost-efficiency. J. Pet. Sci. Eng. 2019, 173, 478–491. [Google Scholar] [CrossRef]
- Scanlon, E.; Garfield, G.L.; Brobak, S. New Technologies to Enhance Performance of Section Milling Operations that Reduces Rig Time for P&A Campaign in Norway. In Proceedings of the SPE/IADC Drilling Conference and Exhibition, Amsterdam, The Netherlands, 1–3 March 2011. [Google Scholar]
- D-010, Well Integrity in Drilling and Well Operations, Standards Norway. Available online: https://www.standard.no/en/sectors/energi-og-klima/Petroleum/NORSOK-Standard-Categories/D-Drilling/D-0102/ (accessed on 25 February 2021).
- Williams, S.M.; Carlsen, T.; Constable, K.C.; Guldahl, A.C. Identification and Qualification of Shale Annular Barriers Using Wireline Logs During Plug and Abandonment Operations. In Proceedings of the All Days; Society of Petroleum Engineers (SPE), San Antonio, TX, USA, 17 March 2009; p. 15. [Google Scholar]
- Kristiansen, T.G.; Dyngeland, T.; Kinn, S.; Flatebø, R.; Aarseth, N.A. Activating Shale to Form Well Barriers: Theory and Field Examples. In Proceedings of the SPE Annual Technical Conference and Exhibition 2018, Dallas, TX, USA, 24–26 September 2018; p. 23. [Google Scholar]
- van Oort, E.; Juenger, M.; Aldin, M.; Thombare, A.; McDonald, M. Simplifying Well Abandonments Using Shale as a Barrier. In Proceedings of the IADC/SPE International Drilling Conference and Exhibition, Galveston, TX, USA, 25 February 2020. [Google Scholar]
- Fjær, E.; Larsen, I. Shale as a Sealing Barrier around Deep Wells. In Proceedings of the ASME 2018 37th International Conference on Ocean, Offshore and Arctic Engineering, Madrid, Spain, 17–22 June 2018. [Google Scholar]
- Bauer, A.; Stenebråten, J.F.; Li, L.; Fjær, E. Can heating-induced creep result in shale barriers for P&A applications? In Proceedings of the 51st US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 25–28 June 2017.
- Fjær, E.; Folstad, J.S.; Li, L. How creeping shale may form a sealing barrier around a well. In Proceedings of the 50th US Rock Mechanics/Geomechanics Symposium, Houston, TX, USA, 5–29 June 2016. [Google Scholar]
- Farrokhrouz, M. Mechanics and Mechanisms; Taylor & Francis Group: Oxfordshire, UK, 2013. [Google Scholar]
- Guo, T.; Li, Y.; Ding, Y.; Qu, Z.; Gai, N.; Rui, Z. Evaluation of Acid Fracturing Treatments in Shale Formation. Energy Fuels 2017, 31, 10479–10489. [Google Scholar] [CrossRef]
- Rabbani, E.; Davarpanah, A.; Memariani, M. An experimental study of acidizing operation performances on the wellbore productivity index enhancement. J. Pet. Explor. Prod. Technol. 2018, 8, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, R.; Esfandyari, H.; Pari, M.N.; Davarpanah, A. Conventional diverting techniques and novel fibr-assisted self-diverting system in carbonate reservoir acidizing with successful case studies. Pet. Res. 2021. [Google Scholar] [CrossRef]
- Hu, X.; Xie, J.; Cai, W.; Wang, R.; Davarpanah, A. Thermodynamic effects of cycling carbon dioxide injectivity in shale reservoirs. J. Pet. Sci. Eng. 2020, 195, 107717. [Google Scholar] [CrossRef]
- Cerasi, P.; Lund, E.; Kleiven, M.L.; Stroisz, A.; Pradhan, S.; Kjøller, C.; Frykman, P.; Fjær, E. Shale Creep as Leakage Healing Mechanism in CO2 Sequestration. Energy Procedia 2017, 114, 3096–3112. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.; Agofack, N.; Gawel, K.M.; Cerasi, P.R. Micro- and Macroscale Consequences of Interactions between CO2 and Shale Rocks. Energies 2020, 13, 1167. [Google Scholar] [CrossRef] [Green Version]
- Morsy, S.; Hetherington, C.J.; Sheng, J.J. Effect of Low-Concentration HCl on the Mineralogical, Mechanical, and Physical Properties of Shale Rocks. In Proceedings of the SPE Eastern Regional Meeting, Pittsburgh, PA, USA, 20–22 August 2013. [Google Scholar]
- Stenebraten, J.F.; Sonstebo, E.F.; Lavrov, A.V.; Fjaer, E.; Haaland, S. The shale puncher-a compact tool for fast testing of small shale samples. In Proceedings of the 42nd US Rock Mechanics Symposium (USRMS), San Francisco, CA, USA, 1 January 2008. [Google Scholar]
- Nes, O.; Horsrud, P.; Sonstebo, E.; Holt, R.; Ese, A.; Okland, D.; Kjorholt, H. Rig Site and Laboratory Use of CWT Acoustic Velocity Measurements on Cuttings. SPE Reserv. Eval. Eng. 1998, 1, 282–287. [Google Scholar] [CrossRef]
- Du, J.; Hu, L.; Meegoda, J.N.; Zhang, G. Shale softening: Observations, phenomenological behavior, and mechanisms. Appl. Clay Sci. 2018, 161, 290–300. [Google Scholar] [CrossRef]
- Santarelli, F.J.; Carminati, S. Do Shales Swell? A Critical Review of Available Evidence. In Proceedings of the SPE/IADC Drilling Conference, Amsterdam, The Netherlands, 28 February–2 March 1995; p. 16. [Google Scholar]
- Feng, G.; Kang, Y.; Sun, Z.-D.; Wang, X.-C.; Hu, Y.-Q. Effects of supercritical CO2 adsorption on the mechanical characteristics and failure mechanisms of shale. Energy 2019, 173, 870–882. [Google Scholar] [CrossRef]
- Ao, X.; Lu, Y.; Tang, J.; Chen, Y.; Li, H. Investigation on the physics structure and chemical properties of the shale treated by supercritical CO2. J. CO2 Util. 2017, 20, 274–281. [Google Scholar] [CrossRef]
- Zou, Y.; Li, S.; Ma, X.; Zhang, S.; Li, N.; Chen, M. Effects of CO 2 –brine–rock interaction on porosity/permeability and mechanical properties during supercritical-CO2 fracturing in shale reservoirs. J. Nat. Gas Sci. Eng. 2018, 49, 157–168. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control. 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Golubev, S.V.; Bénézeth, P.; Schott, J.; Dandurand, J.L.; Castillo, A. Siderite dissolution kinetics in acidic aqueous solutions from 25 to 100 °C and 0 to 50 atm pCO2. Chem. Geol. 2009, 265, 13–19. [Google Scholar] [CrossRef]
- Al Moajil, A.; Al-Khaldi, M.; Hazzazi, H.; Caliskan, S. Acidizing Highly Permeable Sandstone Stringers: Drill-in Fluid Damage and Compatibility with Rock Minerals. In Proceedings of the SPE Trinidad and Tobago Section Energy Resources Conference, San Francisco, CA, USA, 22 June 2018. [Google Scholar]
- Descostes, M.; Vitorge, P.; Beaucaire, C. Pyrite dissolution in acidic media. Geochim. Cosmochim. Acta 2004, 68, 4559–4569. [Google Scholar] [CrossRef]
- Negara, A.; Salama, A.; Sun, S.; Elgassier, M.; Wu, Y.-S. Numerical Simulation of Natural Gas Flow in Anisotropic Shale Reservoirs. In Proceedings of the 2015 Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAB, 9–12 November 2015. [Google Scholar]
- Yang, Y.; Lu, X.; Wang, Q.; Song, D.; Chen, Y.; Hong, Y. Study on the anisotropy of mass transfer for oxygen in the ash layer of extremely low calorific oil shale semi-coke. Appl. Therm. Eng. 2018, 128, 1494–1501. [Google Scholar] [CrossRef]
- Sato, M.; Panaghi, K.; Takada, N.; Takeda, M. Effect of Bedding Planes on the Permeability and Diffusivity Anisotropies of Berea Sandstone. Transp. Porous Media 2019, 127, 587–603. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, E.; Xu, M.; Dehghanpour, H.; Bearinger, D. Advances in Understanding Liquid Flow in Gas Shales. In Proceedings of the SPE/CSUR Unconventional Resources Conference, Calgary, AB, Canada, 30 September 2014; pp. 1030–1049. [Google Scholar]
- Panduro, E.A.C.; Torsæter, M.; Gawel, K.; Bjørge, R.; Gibaud, A.; Yang, Y.; Bruns, S.; Zheng, Y.; Sørensen, H.O.; Breiby, D.W. In-Situ X-ray Tomography Study of Cement Exposed to CO2 Saturated Brine. Environ. Sci. Technol. 2017, 51, 9344–9351. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.-P.L.; Li, L.; Karpyn, Z.T.; Huerta, N.J. Fracture opening or self-sealing: Critical residence time as a unifying parameter for cement–CO2–brine interactions. Int. J. Greenh. Gas Control. 2016, 47, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Guidelines on Qualification of Materials for the Abandonment of Wells, Issue 2; The UK Oil and Gas Industry Association Limited: London, UK, 2015.
- Raaen, A.M.; Fjær, E. Pressure Testing of Barrier Integrity. In Proceedings of the ASME 2020 39th International Conference on Ocean, Offshore and Arctic Engineering, Fort Lauderdale, FL, USA, 3–7 August 2020. [Google Scholar]









| QZ | K-f | Pl | Ch | Ka | Mi/Il | Mix | Sm | Dol | Py | |
|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 30 | 5 | 11 | 1 | 7 | 30 | 8 | 2 | 4 | 1 |
| QZ | K-f | Pl | Ch | Ka | Mi/Il | Mix | Sm | Si | Dol | Py | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oil | 30 | 5 | 11 | 1 | 7 | 30 | 8 | 2 | 0 | 4 | 1 |
| H2O | 27 | 6 | 12 | 0 | 8 | 29 | 3 | 10 | 1 | 4 | 1 |
| HCl 2 h | 29 | 7 | 9 | 0 | 7 | 25 | 3 | 15 | 1 | 3 | 1 |
| HCl 21 h | 29 | 8 | 12 | 0 | 6 | 23 | 3 | 18 | 0 | 0 | 1 |
| HCl 46 h | 32 | 8 | 12 | 0 | 4 | 28 | 4 | 10 | 0 | 0 | 2 |
| HCl 96 h | 32 | 7 | 15 | 0 | 6 | 25 | 3 | 9 | 1 | 0 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawel, K.; Lozovyi, M.; Bhuiyan, M.H.; Bjørge, R.; Fjær, E. Acid Treatment as a Way to Reduce Shale Rock Mechanical Strength and to Create a Material Prone to the Formation of Permanent Well Barrier. Energies 2021, 14, 2342. https://doi.org/10.3390/en14092342
Gawel K, Lozovyi M, Bhuiyan MH, Bjørge R, Fjær E. Acid Treatment as a Way to Reduce Shale Rock Mechanical Strength and to Create a Material Prone to the Formation of Permanent Well Barrier. Energies. 2021; 14(9):2342. https://doi.org/10.3390/en14092342
Chicago/Turabian StyleGawel, Kamila, Maksym Lozovyi, Mohammad Hossain Bhuiyan, Ruben Bjørge, and Erling Fjær. 2021. "Acid Treatment as a Way to Reduce Shale Rock Mechanical Strength and to Create a Material Prone to the Formation of Permanent Well Barrier" Energies 14, no. 9: 2342. https://doi.org/10.3390/en14092342






