Mercury Speciation in Various Coals Based on Sequential Chemical Extraction and Thermal Analysis Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coal Samples with Different Ranks
2.2. Hg Speciation Determined by Sequential Chemical Extraction Method
2.3. Hg Speciation Determined by Temperature Programmed Decomposition Method
2.4. Coal Pyrolysis in TG Coupled with MS
3. Results and Discussion
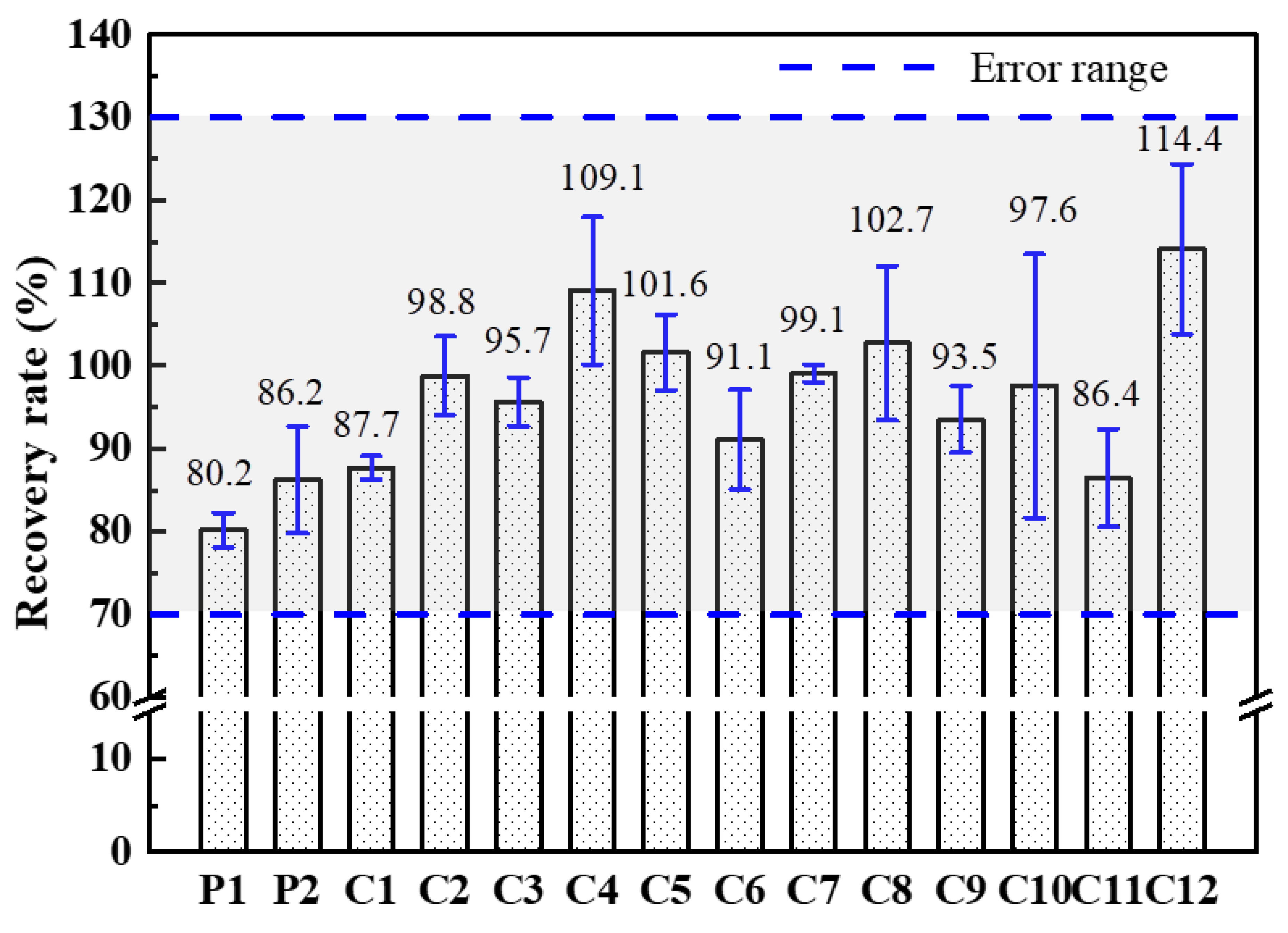
3.1. Hg Content in Coal Samples
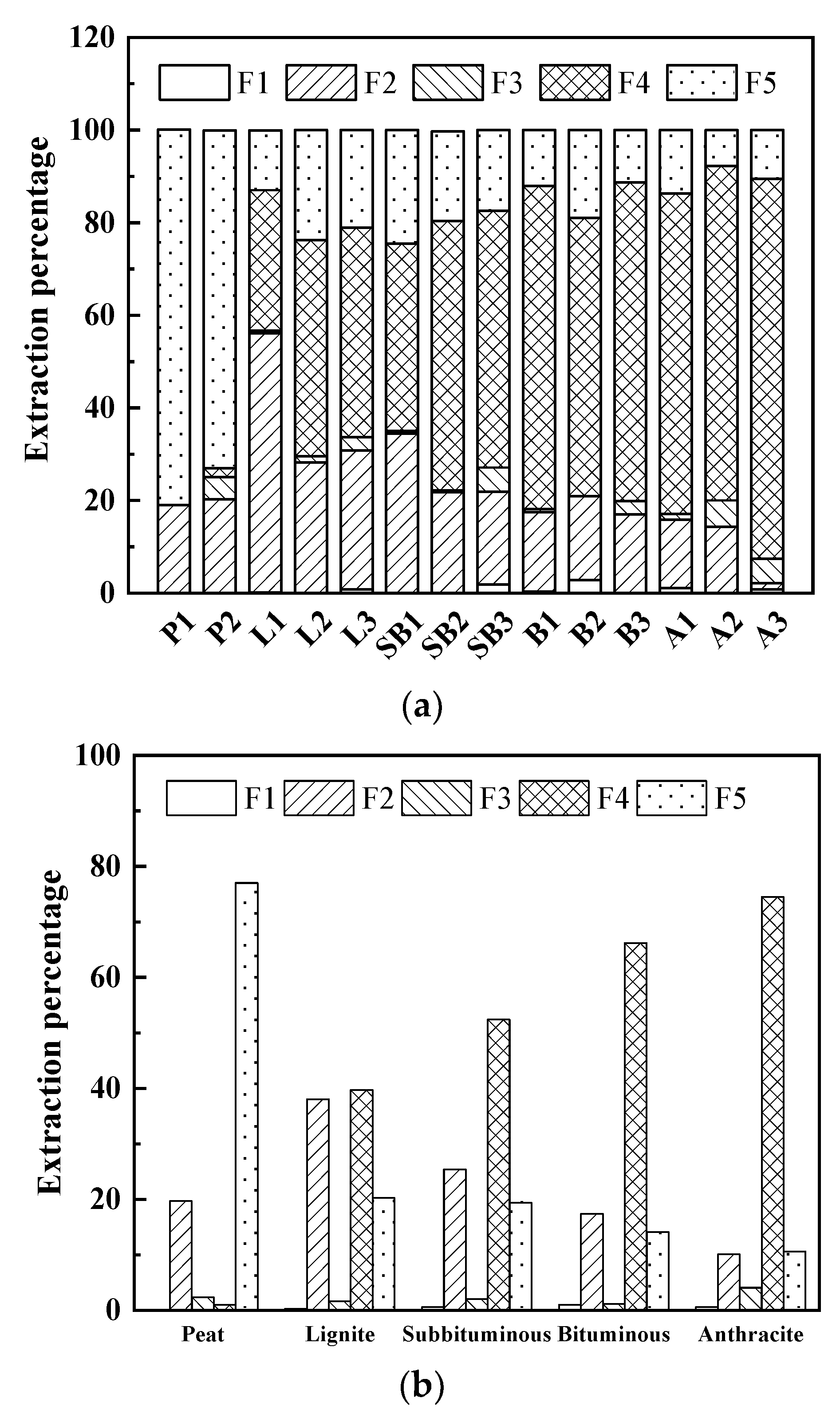
3.2. Hg Speciation in Coal with Different Ranks
3.2.1. Hg Speciation in Coals
3.2.2. Relationship between Hg Speciation and Coal Rank
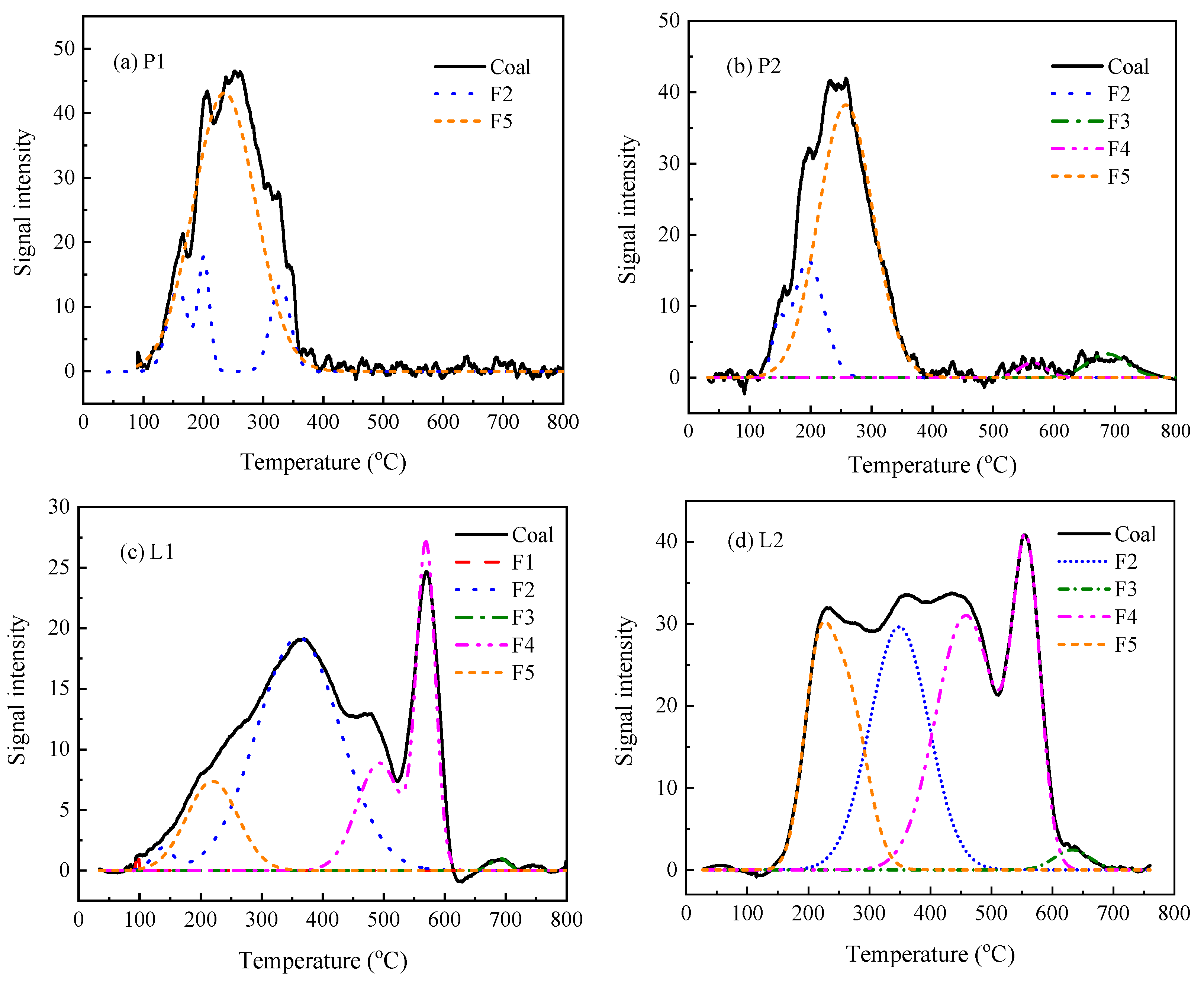
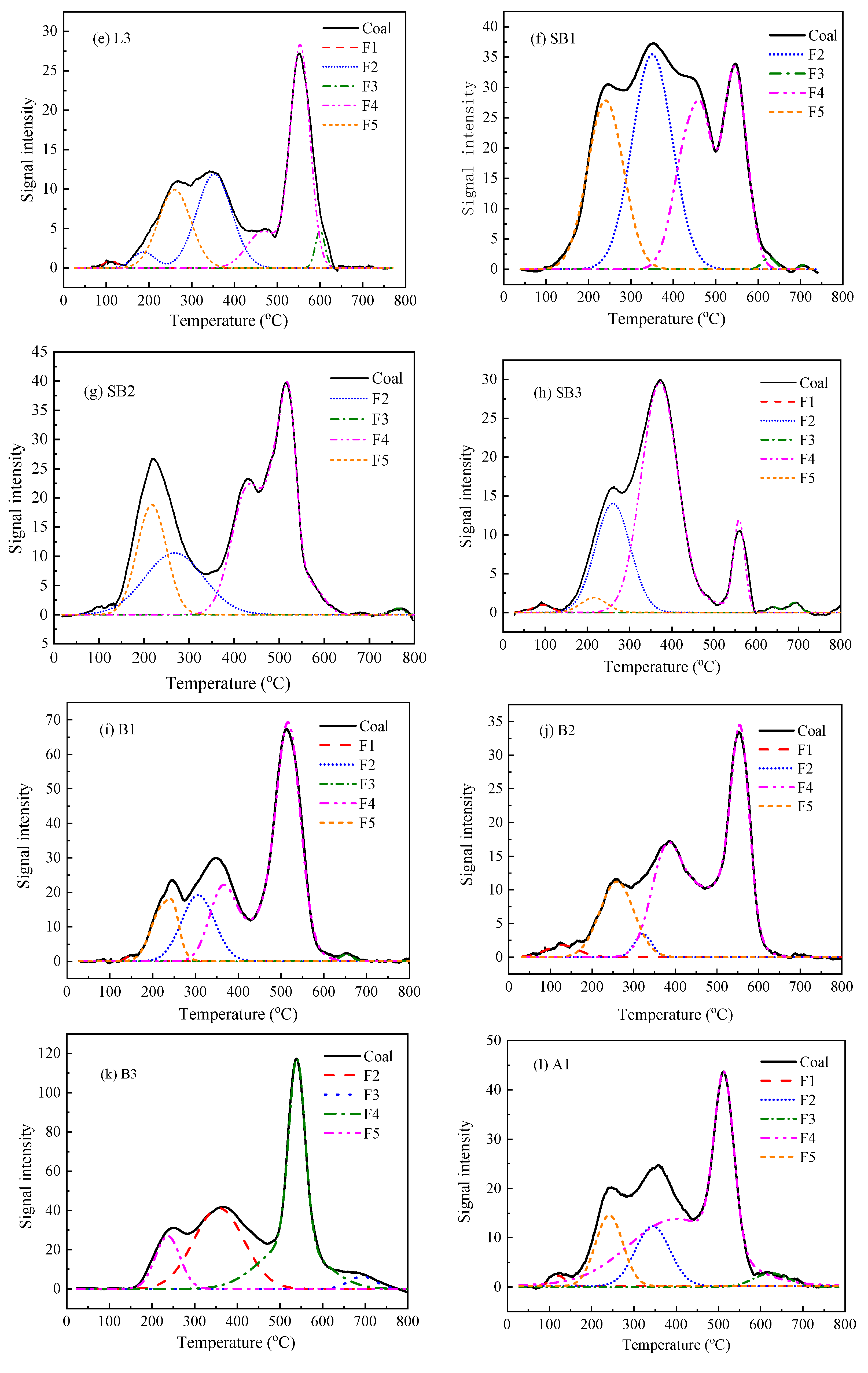
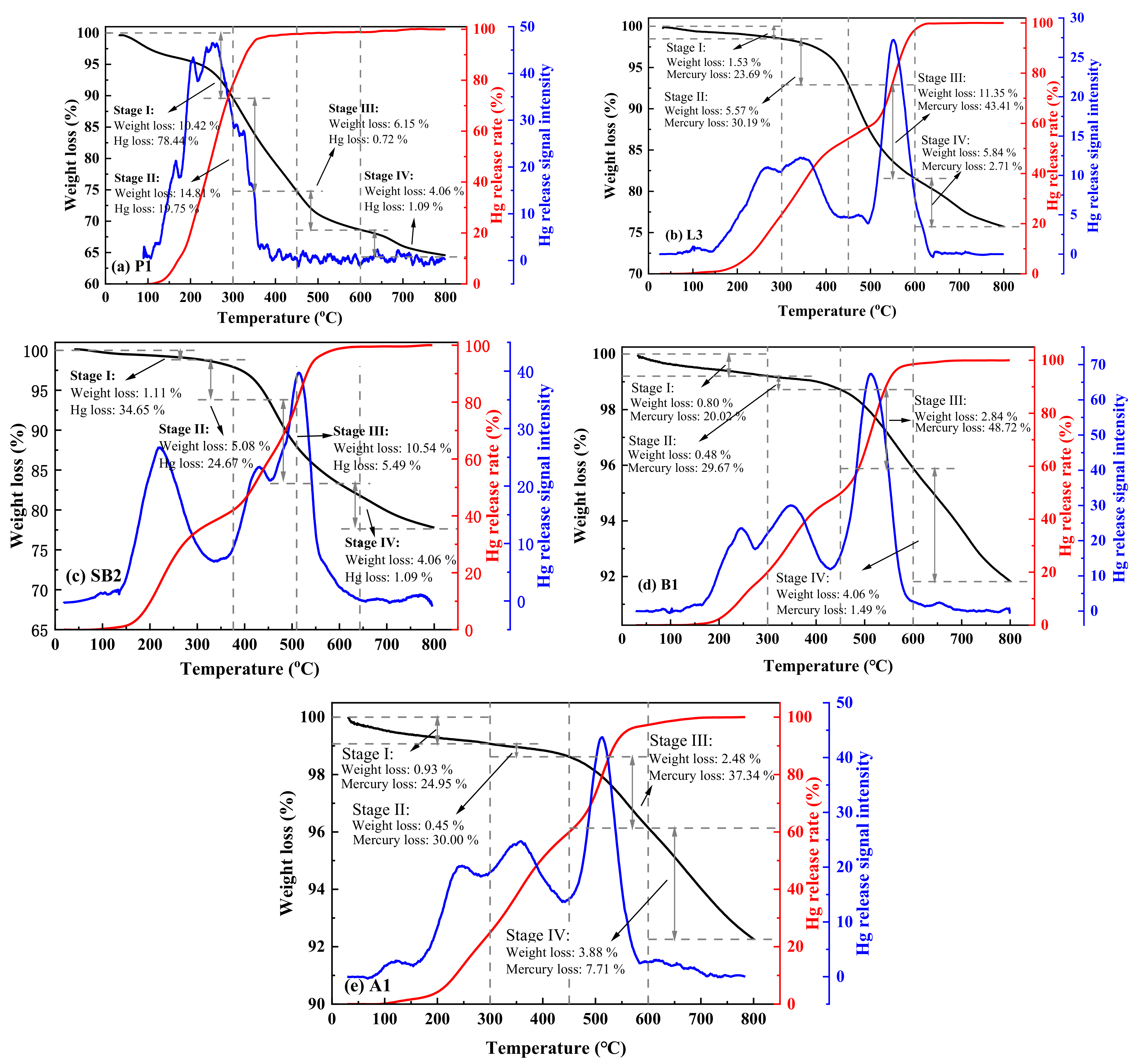
3.3. Hg Thermal Stability Based on Its Speciation
3.4. Relationship between Hg Thermal Release and the Weight Loss of Coal
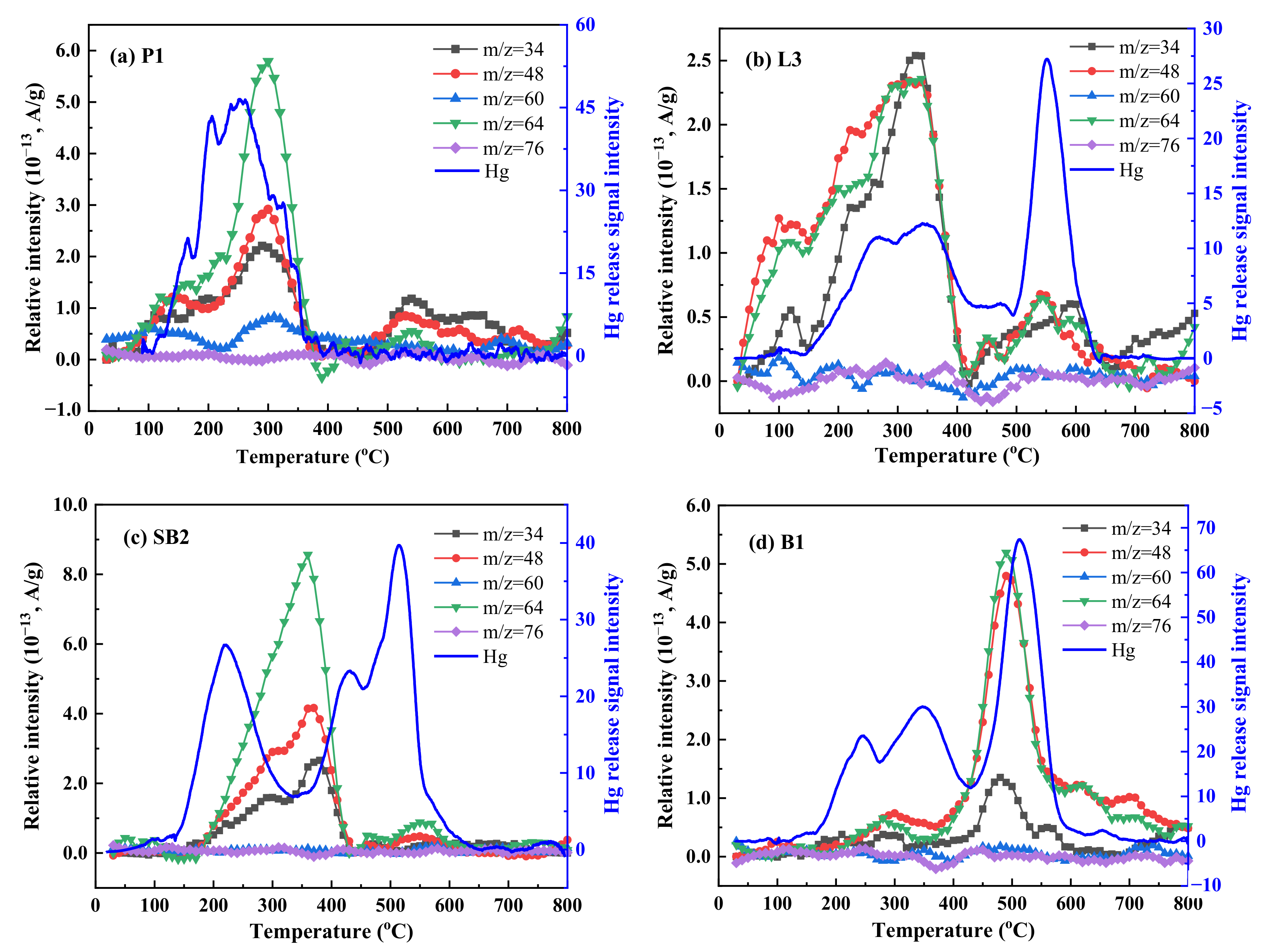
3.5. Relationship between Hg and Sulfur-Containing Gases from Coal Pyrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Teng, Y.; Zhang, K.; Peng, H.; Cheng, F.; Yoshikawa, K. Mercury Migration Behavior from Flue Gas to Fly Ashes in a Commercial Coal-Fired CFB Power Plant. Energies 2020, 13, 1040. [Google Scholar] [CrossRef] [Green Version]
- Yudovich, Y.E.; Ketris, M.P. Mercury in coal: A review: Part 1. Geochemistry. Int. J. Coal. Geol. 2005, 62, 107–134. [Google Scholar] [CrossRef]
- Lopez-Anton, M.A.; Yuan, Y.; Perry, R.; Maroto-Valer, M.M. Analysis of mercury species present during coal combustion by thermal desorption. Fuel 2010, 89, 629–634. [Google Scholar] [CrossRef] [Green Version]
- UNEP. UN Environment Program. Global Mercury Assessment 2018. In UN Environment Program, Chemicals and Health Branch; UNEP: Geneva, Switzerland, 2018. [Google Scholar]
- Luo, G.; Ma, J.; Han, J.; Yao, H.; Xu, M.; Zhang, C. Hg occurrence in coal and its removal before coal utilization. Fuel 2013, 104, 70–76. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, S.; Niu, Q.; Chen, S.; Meng, X.; Zhang, D.; Li, M.; Liang, P. Mercury distribution in Guizhou bituminous coal and its releasing behavior during mild pyrolysis process. Fuel Process Technol. 2019, 185, 38–45. [Google Scholar] [CrossRef]
- Sotiropoulou, R.E.P.; Serafidou, M.; Skodras, G. Thermal mercury removal from coals: Effect of pyrolysis conditions and kinetic analysis. Fuel 2019, 238, 44–50. [Google Scholar] [CrossRef]
- Bool, L.E.; Helble, J.J. A Laboratory Study of the Partitioning of Trace Elements during Pulverized Coal Combustion. Energy Fuel 1995, 9, 880–887. [Google Scholar] [CrossRef]
- Ohki, A.; Sagayama, K.; Tanamachi, S.; Iwashita, A.; Nakajima, T.; Takanashi, H. Release behavior of mercury during mild pyrolysis of coals and nitric acid-treated coals. Powder Technol. 2008, 180, 30–34. [Google Scholar] [CrossRef]
- Liu, L.; Duan, Y.; Wang, Y.; Wang, H.; Yin, J. Experimental study on mercury release behavior and speciation during pyrolysis of two different coals. J. Fuel Chem. Technol. 2010, 38, 134–139. (In Chinese) [Google Scholar] [CrossRef]
- Gao, L.; Wang, Y.; Huang, Q.; Guo, S. Modes of occurrence and thermal stability of mercury in different samples from Guandi coal preparation plant. Fuel 2017, 200, 22–30. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, L.; Niu, X.; Gao, L.; Cao, Y.; Wei, X.; Li, X. Mercury release characteristics during pyrolysis of eight bituminous coals. Fuel 2018, 222, 250–257. [Google Scholar] [CrossRef]
- Strezov, V.; Morrison, A.L.; Nelson, P.F. Pyrolytic Mercury Removal from Coal and Its Adverse Effect on Coal Swelling. Energy Fuel 2007, 21, 496–500. [Google Scholar] [CrossRef]
- Kevin, C.G.; Zygarlicke, C.J. Mercury Speciation in Coal Combustion and Gasification Flue Gases. Environ. Sci. Technol. 1996, 30, 2421–2426. [Google Scholar]
- Diehl, S.F.; Goldhaber, M.B.; Hatch, J.R. Modes of occurrence of mercury and other trace elements in coals from the warrior field, Black Warrior Basin, Northwestern Alabama. Int. J. Coal Geol. 2004, 59, 193–208. [Google Scholar] [CrossRef]
- Brownfield, M. Geologic setting and characterization of coals and the modes of occurrence of selected elements from the Franklin coal zone, Puget Group, John Henry No. 1 mine, King County, Washington, USA. Int. J. Coal Geol. 2006, 63, 247–275. [Google Scholar] [CrossRef]
- Esbrí, J.M.; Bernaus, A.; Avila, M.; Kocman, D.; Radiation, J. XANES speciation of mercury in three mining districts—Almaden, Asturias (Spain), Idria (Slovenia). J. Synchrotron Radiat. 2010, 17, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Mastalerz, M. Modes of occurrence of trace elements in coal. Inter. J. Coal Geol. 2001, 46, 66. [Google Scholar] [CrossRef]
- Iwashita, A.; Tanamachi, S.; Nakajima, T.; Takanashi, H.; Ohki, A. Removal of mercury from coal by mild pyrolysis and leaching behavior of mercury. Fuel 2004, 83, 631–638. [Google Scholar] [CrossRef]
- Wagner, N.J.; Tlotleng, M.T. Distribution of selected trace elements in density fractionated Waterberg coals from South Africa. Inter. J. Coal Geol. 2012, 94, 225–237. [Google Scholar] [CrossRef]
- Finkelman, R.B. Modes of Occurrence of Trace Elements in Coal. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 1980. [Google Scholar]
- Dziok, T.; Strugała, A.; Rozwadowski, A.; Macherzyński, M. Studies of the correlation between mercury content and the content of various forms of sulfur in Polish hard coals. Fuel 2015, 159, 206–213. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, O.; Nakazato, T.; Zhang, Z.; Suzuki, Y.; Sakanishi, K. Statistical analysis of the concentrations of trace elements in a wide diversity of coals and its implications for understanding elemental modes of occurrence. Fuel 2008, 87, 2211–2222. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, G.; Chou, C. Abundance and modes of occurrence of mercury in some low-sulfur coals from China. Inter. J. Coal Geol. 2008, 73, 19–26. [Google Scholar] [CrossRef]
- Tessier, A.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Palmer, C.A.; Krasnow, M.R.; Aruscavage, P.J.; Sellers, G.A.; Dulong, F.T. Combustion and leaching behavior of elements in the Argonne Premium Coal Samples. Energy Fuel 1990, 4, 755–766. [Google Scholar] [CrossRef]
- Bloom, N.S.; Preus, E.; Katon, J.; Hiltner, M. Selective extractions to assess the biogeochemically relevant fractionation of inorganic mercury in sediments and soils. Anal. Chim. Acta 2003, 479, 233–248. [Google Scholar] [CrossRef]
- Issaro, N.; Abi-Ghanem, C.; Bermond, A. Fractionation studies of mercury in soils and sediments: A review of the chemical reagents used for mercury extraction. Anal. Chim. Acta 2009, 631, 1–12. [Google Scholar] [CrossRef]
- Różański, S.Ł.; Castejón, J.M.P.; Fernández, G.G. Bioavailability and mobility of mercury in selected soil profiles. Environ. Earth Sci. 2016, 75, 1065. [Google Scholar] [CrossRef] [Green Version]
- Hoffart, A.; Seames, W.; Kozliak, E.; Riedinger, S.; Francini, J.; Carlson, C. A two-step acid mercury removal process for pulverized coal. Fuel 2006, 85, 1166–1173. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, G.; Qi, C.; Zhang, Y.; Wong, M. The use of sequential extraction to determine the distribution and modes of occurrence of mercury in Permian Huaibei coal, Anhui Province, China. Int. J. Coal Geol. 2008, 73, 139–155. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Li, B.; Wang, G. Study on the fate of As, Hg and Pb in Yima coal via sub-critical water extraction. Fuel 2007, 86, 1822–1830. [Google Scholar] [CrossRef]
- Bombach, G.; Bombach, K.; Klemm, W. Speciation of mercury in soils and sediments by thermal evaporation and cold vapor atomic absorption. Fresenius J. Anal. Chem. 1994, 350, 18–20. [Google Scholar] [CrossRef]
- Windmoller, C.C.; Wilken, R.D.; Jardim, W.D.F. Mercury speciation in contaminated soils by thermal release analysis. Water Air Soil Poll. 1995, 89, 399–416. [Google Scholar] [CrossRef]
- Harald, B.; Mateja, G.; Muller, G. Mercury speciation in tailings of the Idrija mercury mine. J. Geochem. Explor. 1999, 65, 195–204. [Google Scholar]
- Kouichi, M.; Kazuhiro, M.; Makoto, S.; Minami, H. Analysis of Formation Rates of Sulfur-Containing Gases during the Pyrolysis of Various Coals. Energy Fuels 2001, 15, 629–636. [Google Scholar]
- Worasuwannarak, N.; Sonobe, T.; Tanthapanichakoon, W. Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique. J. Anal. Appl. Pyrolysis 2007, 78, 265–271. [Google Scholar] [CrossRef]
- Sonobe, T.; Worasuwannarak, N.; Pipatmanomai, S. Synergies in co-pyrolysis of Thai lignite and corncob. Fuel Process Technol. 2008, 89, 1371–1378. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gökalp, I. Thermal characterization, gasification and kinetic studies of different sized Indian coal and char particles. Int. J. Adv. Eng. Sci. App. Math. 2014, 6, 31–40. [Google Scholar] [CrossRef]
- Goodarzi, F. Mineralogy, elemental composition and modes of occurrence of elements in Canadian feed-coals. Fuel 2002, 81, 1199–1213. [Google Scholar] [CrossRef]
- Guo, S.; Yang, J.; Liu, Z. Characterization of Hg in Coals by Temperature-Programmed Decomposition–Atomic Fluorescence Spectroscopy and Acid-Leaching Techniques. Energy Fuel 2012, 26, 3388–3392. [Google Scholar] [CrossRef]
- Su, Y.; Liu, X.; Li, L.; Li, X.; Jiang, P.; Teng, Y.; Zhang, K. The distribution characteristics of Mercury Speciation in Coals with Three Different Ranks. J. Chem. Ind. Eng. 2019, 70, 1559–1566. (In Chinese) [Google Scholar]
- Air Emission Measurement Center. Method 29—Determination of Metals Emissions from Stationary Sources; United States Environmental Protection Agency: Washington, DC, USA, 2009.
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Palmer, C.A.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Feng, X.; Hong, Y. Modes of occurrence of mercury in coals from Guizhou, People’s Republic of China. Fuel 1999, 78, 1181–1188. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, D.; Zhu, Y.; Chou, C.; Zeng, R.; Zheng, B. Mineral matter and potentially hazardous trace elements in coals from Qianxi Fault Depression Area in southwestern Guizhou, China. Inter. J. Coal. Geol. 2004, 57, 49–61. [Google Scholar] [CrossRef]
- Tewalt, S.J.; Bragg, L.J.; Finkelman, R. Mercury in U.S. Coal—Abundance, Distribution, and Modes of Occurrence; U.S. Geological Survey: Reston, VA, USA, 2004.
- Curtis, A.; Palmer, K.O.; Dennen, A.K.; Robert, B.; Finkelman, J.H.; Bullock, J. Chemical Analysis and Modes of Occurrence of Selected Trace Elements in a Coal Sample from Eastern Kentucky Coal Bed: White Creek Mine, Martin County, Kentucky; Open-File Report; U.S. Geological Survey: Reston, VA, USA, 2002. [Google Scholar]
- Skyllberg, U.; Xia, K.; Bloom, P.R.; Nater, E.A.; Bleam, W.F. Binding of Mercury (II) to Reduced Sulfur in Soil Organic Matter along Upland-Peat Soil Transects. J. Environ. Qual. 2000, 29. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Q.; Lu, X.; Fang, F.; Wang, Y. Distribution and speciation of mercury in the peat bog of Xiaoxing’an Mountain, northeastern China. Environ. Pollut. 2003, 124, 39–46. [Google Scholar] [CrossRef]
- Schoonen, M.A.A. Mechanisms of sedimentary pyrite formation. Geol. Soc. Am. 2004, 379, 117–134. [Google Scholar]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Roos-Barraclough, F.; Martinez-Cortizas, A.; García-Rodeja, E.; Shotyk, W. A 14500-year record of the accumulation of atmospheric mercury in peat: Volcanic signals, anthropogenic influences and a correlation to bromine accumulation. Earth Planet. Sci. Lett. 2002, 202, 435–451. [Google Scholar] [CrossRef]
- Srinivasachar, S.; Boni, A.A. A kinetic model for pyrite transformations in a combustion environment. Fuel 1989, 68, 829–836. [Google Scholar] [CrossRef]
- Huffman, G.P.; Huggins, F.E.; Levasseur, A.A.; Chow, O.; Srinivasachar, S.; Mehta, A.K. Investigation of the transformations of pyrite in a drop-tube furnace. Fuel 1989, 68, 485–490. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, H.; Jin, L.; He, X.; Wu, B. Pyrolysis behavior of vitrinite and inertinite from Chinese Pingshuo coal by TG–MS and in a fixed bed reactor. Fuel Process Technol. 2011, 92, 780–786. [Google Scholar] [CrossRef]








| Sample | Proximate Analysis, wt %, ad | S Content, wt % | Hg Content, ng/g, ad | ||||
|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | ||||
| Peat | P1 | 1.91 | 51.80 | 37.18 | 9.11 | 0.11 | 198.7 |
| P2 | 2.19 | 56.04 | 32.47 | 9.30 | 0.10 | 184.3 | |
| Lignite | L1 | 3.43 | 26.88 | 31.87 | 37.82 | 0.66 | 161.6 |
| L2 | 1.18 | 35.67 | 27.72 | 35.43 | 3.12 | 292.6 | |
| L3 | 3.29 | 28.23 | 28.18 | 40.30 | 2.32 | 228.7 | |
| Sub-bituminous coal | SB1 | 1.09 | 36.86 | 24.16 | 37.89 | 0.85 | 181.9 |
| SB2 | 3.29 | 34.35 | 22.68 | 39.68 | 0.91 | 172.1 | |
| SB3 | 2.70 | 23.29 | 26.02 | 47.99 | 0.64 | 164.0 | |
| Bituminous coal | B1 | 2.94 | 22.57 | 21.23 | 53.26 | 2.24 | 175.3 |
| B2 | 1.33 | 18.24 | 11.52 | 68.91 | 1.16 | 138.5 | |
| B3 | 1.17 | 21.46 | 10.03 | 67.34 | 1.87 | 162.4 | |
| Anthracite | A1 | 1.42 | 18.18 | 10.26 | 70.14 | 2.63 | 186.3 |
| A2 | 0.92 | 29.48 | 8.22 | 61.38 | 1.34 | 176.5 | |
| A3 | 1.43 | 15.50 | 8.49 | 74.58 | 0.45 | 112.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Liu, X.; Teng, Y.; Zhang, K. Mercury Speciation in Various Coals Based on Sequential Chemical Extraction and Thermal Analysis Methods. Energies 2021, 14, 2361. https://doi.org/10.3390/en14092361
Su Y, Liu X, Teng Y, Zhang K. Mercury Speciation in Various Coals Based on Sequential Chemical Extraction and Thermal Analysis Methods. Energies. 2021; 14(9):2361. https://doi.org/10.3390/en14092361
Chicago/Turabian StyleSu, Yinjiao, Xuan Liu, Yang Teng, and Kai Zhang. 2021. "Mercury Speciation in Various Coals Based on Sequential Chemical Extraction and Thermal Analysis Methods" Energies 14, no. 9: 2361. https://doi.org/10.3390/en14092361







