Ab initio Study of Hydrogen Adsorption on Metal-Decorated Borophene-Graphene Bilayer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Atomistic Model of the Borophene-Graphene Heterostructure
2.2. Details of the Density Functional Calculations
3. Results and Discussion
3.1. Interaction of Metals Atoms with the Borophene-Graphene Bilayer
3.2. Interaction of Hydrogen Atom with the Metal-Decorated Borophene-Graphene Bilayer
3.3. Effect of Mechanical Strain of Cu-Decorated Borophene-Graphene Bilayer on HER Overpotential Value
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for hydrogen evolution in alkaline electrolytes: Mechanisms, challenges, and prospective solutions. Adv. Sci. 2017, 5, 1700464. [Google Scholar] [CrossRef] [PubMed]
- Toh, R.J.; Poh, H.L.; Sofer, Z.; Pumera, M. Transition metal (Mn, Fe, Co, Ni)-doped graphene hybrids for electrocatalysis. Chem. Asian J. 2013, 8, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.; Feng, W.; Chen, Y. Single-atom catalysts in catalytic biomedicine. Adv. Mater. 2020, 32, 1905994. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Zhang, L.; Doyle-Davis, K.; Sun, X. Single-atom catalysts: From design to application. Electrochem. Energ. Rev. 2019, 2, 539–573. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Lin, C.; Gao, Z. Boosting alkaline hydrogen evolution activity with Ni-doped MoS2/reduced graphene oxide hybrid aerogel. ChemSusChem. 2018, 11, 457–466. [Google Scholar]
- Fung, V.; Hu, G.; Wu, Z.; Jiang, D. Descriptors for hydrogen evolution on single atom catalysts in nitrogen-doped graphene. J. Phys. Chem. C 2020, 124, 19571–19578. [Google Scholar] [CrossRef]
- Baby, A.; Trovato, L.; Di Valentin, C. Single atom catalysts (SAC) trapped in defective and nitrogen-doped graphene supported on metal substrates. Carbon 2021, 174, 772–788. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, X.; Song, E.; Hou, X.; Yang, X.; Mi, J.; Huang, J.; Stampfl, C. Transition metal-doped α-borophene as potential oxygen and hydrogen evolution electrocatalyst: A density functional theory study. Catal. Commun. 2020, 144, 106090. [Google Scholar] [CrossRef]
- Xu, X.; Hou, X.; Lu, J.; Zhang, P.; Xiao, B.; Mi, J. Metal-doped two-dimensional borophene nanosheets for the carbon dioxide electrochemical reduction reaction. J. Phys. Chem. C 2020, 124, 24156–24163. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, A.; Wang, Z. Transition metal atom (Ti, V, Mn, Fe, and Co) anchored silicene for hydrogen evolution reaction. RSC Adv. 2019, 9, 26321–26326. [Google Scholar] [CrossRef] [Green Version]
- Hunter, M.; Fischer, J.M.T.A.; Hankel, M.; Yuan, Q.; Searles, D.J. Doping effects on the performance of paired metal catalysts for the hydrogen evolution reaction. J. Chem. Inf. Model. 2019, 59, 2242–2247. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Ke, Y.; Shao, Y.; Chen, W.; Kwok, C.T.; Shi, X.-Q.; Pan, H. Effect of curvature on the hydrogen evolution reaction of graphene. J. Phys. Chem. C 2018, 122, 25331–25338. [Google Scholar] [CrossRef]
- Gao, G.; Sun, Q.; Du, A. Activating catalytic inert basal plane of molybdenum disulfide to optimize hydrogen evolution activity via defect doping and strain engineering. J. Phys. Chem. C 2016, 120, 16761–16766. [Google Scholar] [CrossRef] [Green Version]
- Kistanov, A.A.; Nikitenko, V.R.; Prezhdo, O.V. Point defects in two-dimensional γ-phosphorus carbide. J. Phys. Chem. Lett. 2021, 12, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Kistanov, A.A.; Korznikova, E.A.; Huttula, M.; Cao, W. The interaction of two-dimensional α- and β-phosphorus carbide with environmental molecules: A DFT study. Phys. Chem. Chem. Phys. 2020, 22, 11307–11313. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, Z.; Zhang, X.; Lu, Z.; Xiao, S.; Xiao, B.; Singh, C.V. Exploring single atom catalysts of transition-metal doped phosphorus carbide monolayer for HER: A first-principles study. J. Eng. Chem. 2021, 52, 155–162. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, Y.; Ao, Z. Nitrogen fixation on a single Mo atom embedded stanene monolayer: A computational study. Phys. Chem. Chem. Phys. 2020, 22, 13981–13988. [Google Scholar] [CrossRef] [PubMed]
- Yanga, J.; Eng, K.; Gohbc, J.; Gen, Z.; Rui, Y.; Wongd, E.; Zhang, Y.-W. A first-principles study on strain engineering of monolayer stanene for enhanced catalysis of CO2 reduction. Chemosphere 2021, 268, 129317. [Google Scholar] [CrossRef]
- Annin, B.D.; Baimova, Y.A.; Mulyukov, R.R. Mechanical properties, stability and buckling of graphene sheets and carbon nanotubes (review). J. Appl. Mech.Tech. Phys. 2020, 61, 834–846. [Google Scholar] [CrossRef]
- Baimova, J.A. Property control by elastic strain engineering: Application to graphene. J. Micromech. Mol. Phys. 2017, 2, 1750001. [Google Scholar] [CrossRef] [Green Version]
- Yankowitz, M.; Ma, Q.; Jarillo-Herrero, P.; LeRoy, B.J. Van der Waals heterostructures combining graphene and hexagonal boron nitride. Nat. Rev. Phys. 2019, 1, 112–125. [Google Scholar] [CrossRef]
- Padilha, J.E.; Fazzio, A.; da Silva, A.J.R. Van der Waals heterostructure of phosphorene and graphene: Tuning the schottky barrier and doping by electrostatic gating. Phys. Rev. Lett. 2015, 114, 066803. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, L.; Xu, W.; Pan, J.; Song, S.; Zhang, Y.; Zhou, H.; Wang, Y.; Bao, L.; Zhang, Y.Y.; et al. Stable silicene in graphene/silicene van der Waals heterostructures. Adv. Mat. 2018, 2018, 1804650. [Google Scholar] [CrossRef] [PubMed]
- Romanov, R.I.; Slavich, A.S.; Kozodaev, M.G.; Myakota, D.I..; Lebedinskii, Y.Y.; Novikov, S.M.; Markeev, A.M. Band Alignment in As-Transferred and Annealed Graphene/MoS2 Heterostructures. Phys. Status Solidi (RRL) 2019, 14, 1900406. [Google Scholar] [CrossRef]
- Rahman, M.H.; Chowdhury, E.H.; Redwan, D.A.; Mitra, S.; Hong, S. Characterization of the mechanical properties of van der Waals heterostructures of stanene adsorbed on graphene, hexagonal boron–nitride and silicon carbide. Phys. Chem. Chem. Phys. 2021, 23, 5244–5253. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hersam, M.C. Borophene-graphene heterostructures. Sci. Adv. 2019, 5, eaax6444. [Google Scholar] [CrossRef] [Green Version]
- Kochaev, A.; Katin, K.P.; Maslov, M.M.; Meftakhutdinov, R. AA-stacked borophene-graphene bilayer with covalent bonding: Ab initio investigation of structural, electronic and elastic properties. J. Phys. Chem. Lett. 2020, 11, 5668–5673. [Google Scholar] [CrossRef]
- Hou, C.; Tai, G.; Liu, B.; Wu, Z.; Yin, Y. Borophene-graphene heterostructure: Preparation and ultrasensitive humidity sensing. Nano Res. 2020. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Cond. Matt. 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Cond. Matt. 2017, 29, 465901. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comp. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Wella, S.A.; Hamamoto, Y.; Morikawa, Y.; Hamada, I. Platinum single-atom adsorption on graphene: A density functional theory study. Nanoscale Adv. 2019, 1, 1165–1174. [Google Scholar] [CrossRef] [Green Version]
- Kano, E.; Hashimoto, A.; Kaneko, T.; Tajima, N.; Ohno, T.; Takeguchi, M. Interactions between C and Cu atoms in single-layer graphene: Direct observation and modelling. Nanoscale 2016, 8, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zheng, Y.; Zhang, X.; Wang, Z.; Gong, Y.; Du, C.; Banis, M.N.; Yiu, Y.M.; Sham, T.K.; Gu, L.; et al. High loading single-atom Cu dispersed on graphene for efficient oxygen reduction reaction. Nano Energy 2019, 66, 104088. [Google Scholar] [CrossRef]
- Galashev, A.Y.; Katin, K.P.; Maslov, M.M. Morse parameters for the interaction of metals with graphene and silicene. Phys. Lett. A 2019, 383, 252–258. [Google Scholar] [CrossRef]
- Katin, K.P.; Prudkovskiy, V.S.; Maslov, M.M. Chemisorption of hydrogen atoms and hydroxyl groups on stretched graphene: A coupled QM/QM study. Phys. Lett. A 2017, 381, 2686–2690. [Google Scholar] [CrossRef]
- Kistanov, A.A.; Cai, Y.; Zhang, Y.W.; Dmitriev, S.V.; Zhou, K. Strain and water effects on the electronic structure and chemical activity of in-plane graphene/silicene heterostructure. J. Phys. Condens. Matter 2017, 29, 095302. [Google Scholar] [CrossRef] [PubMed]
- Kistanov, A.A.; Shcherbinin, S.A.; Ustiuzhanina, S.V.; Huttula, M.; Cao, W.; Nikitenko, V.R.; Prezhdo, O.V. First-Principles Prediction of Two-Dimensional B3C2P3 and B2C4P2: Structural Stability, Fundamental Properties, and Renewable Energy Applications. J. Phys. Chem. Lett. 2021, 12, 3436–3442. [Google Scholar] [CrossRef] [PubMed]
- Baimova, J.A.; Korznikova, E.A.; Dmitriev, S.V.; Liu, B.; Zhou, K. Wrinkles and wrinklons in graphene and graphene nanoribbons under strain. Curr. Nanosci. 2016, 12, 184–191. [Google Scholar] [CrossRef]
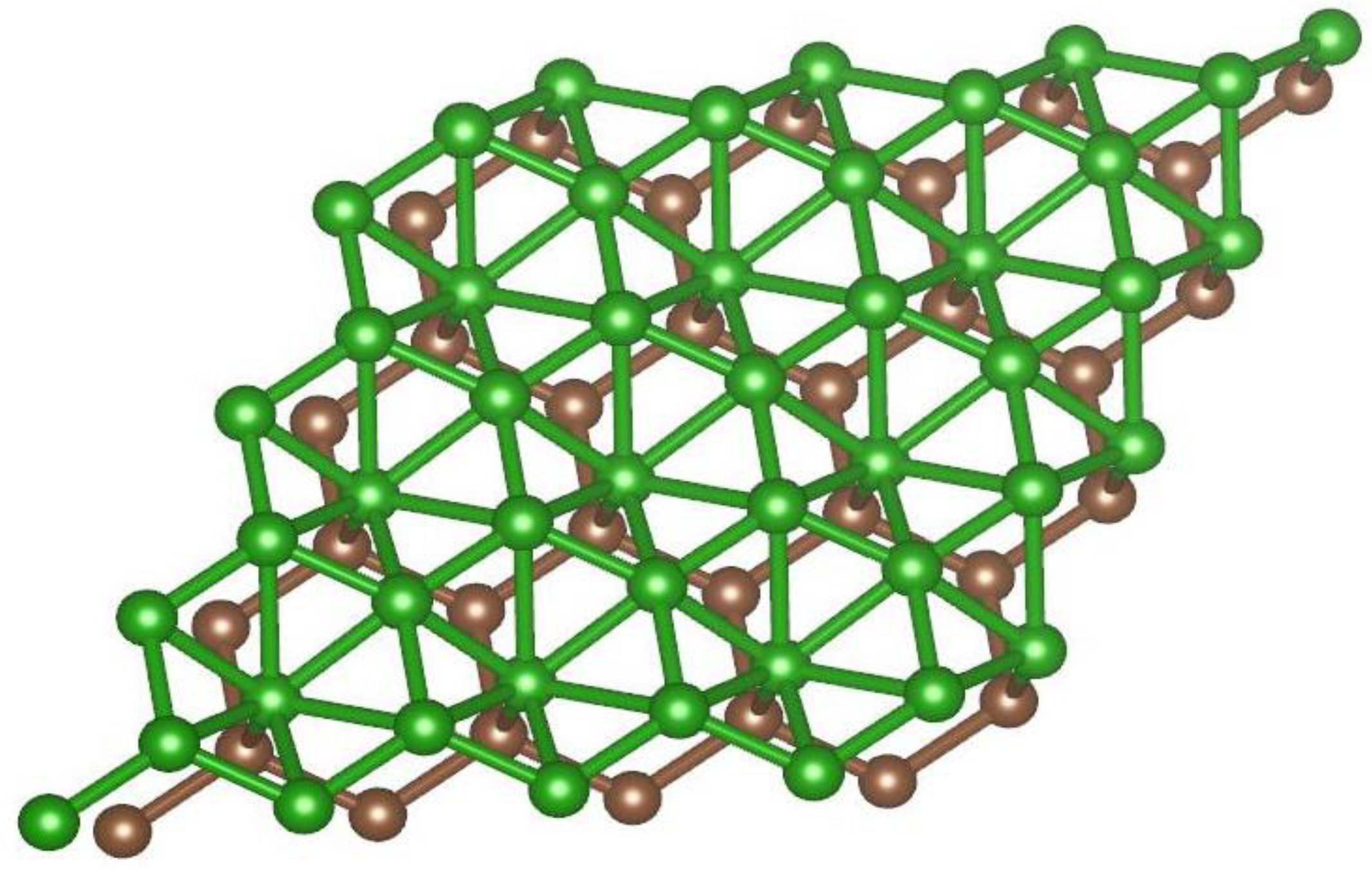
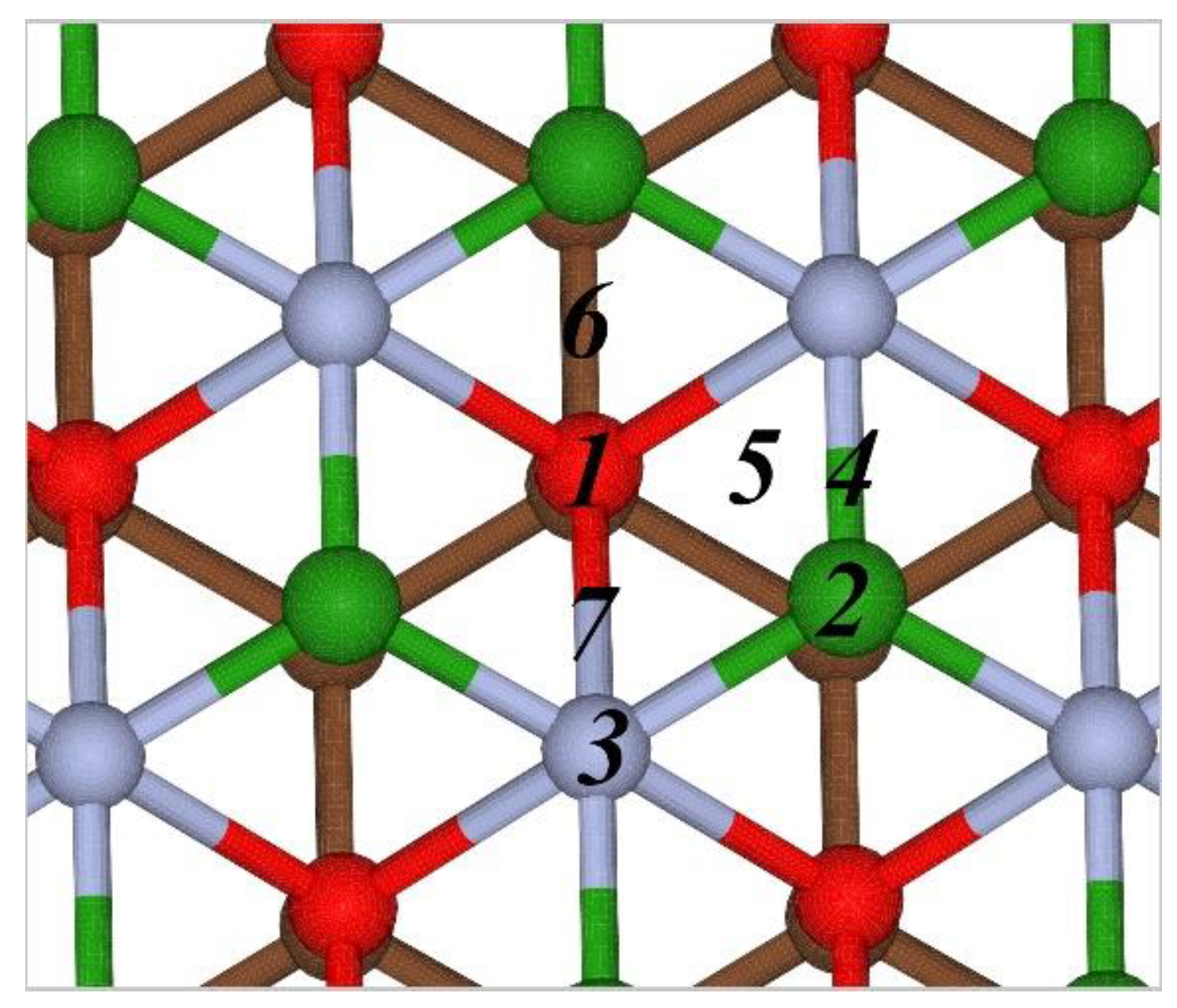

| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| ECu (eV) | 2.51 | 3.05 | 2.35 | to 3 | to 3 | to 1 | to 3 |
| lB–Cu (Å) | 2.138 ÷ 2.219 | 2.142 | 2.174 | - | - | - | - |
| EAg (eV) | 1.42 | 1.98 | 1.55 | 1.53 | 1.55 | to 2 | 1.55 |
| lB–Ag (Å) | 2.468 ÷ 2.616 | 2.045 | 2.471 ÷ 2.474 | 2.220 ÷ 2.356 | 2.310 ÷ 2.371 | - | 2.310 ÷ 2.371 |
| EPt (eV) | to 2 | 5.93 | to 2 | to 2 | to 2 | to 2 | to 2 |
| lB–Pt (Å) | - | 2.093 | - | - | - | - | - |
| ENi (eV) | to 2 | 4.84 | to 2 | to 2 | to 2 | to 2 | to 2 |
| lB–Ni (Å) | - | 2.002 | - | - | - | - | - |
| Metal | EH (eV) | lM–H (Å) | η (V) | qM (e) | qH (e) |
|---|---|---|---|---|---|
| Cu | 0.01 | 1.528 | −0.28 | 10.89 | 1.17 |
| Ag | −0.35 | 1.651 | −0.64 | 10.84 | 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grishakov, K.S.; Katin, K.P.; Kochaev, A.I.; Kaya, S.; Gimaldinova, M.A.; Maslov, M.M. Ab initio Study of Hydrogen Adsorption on Metal-Decorated Borophene-Graphene Bilayer. Energies 2021, 14, 2473. https://doi.org/10.3390/en14092473
Grishakov KS, Katin KP, Kochaev AI, Kaya S, Gimaldinova MA, Maslov MM. Ab initio Study of Hydrogen Adsorption on Metal-Decorated Borophene-Graphene Bilayer. Energies. 2021; 14(9):2473. https://doi.org/10.3390/en14092473
Chicago/Turabian StyleGrishakov, Konstantin S., Konstantin P. Katin, Alexey I. Kochaev, Savas Kaya, Margarita A. Gimaldinova, and Mikhail M. Maslov. 2021. "Ab initio Study of Hydrogen Adsorption on Metal-Decorated Borophene-Graphene Bilayer" Energies 14, no. 9: 2473. https://doi.org/10.3390/en14092473
APA StyleGrishakov, K. S., Katin, K. P., Kochaev, A. I., Kaya, S., Gimaldinova, M. A., & Maslov, M. M. (2021). Ab initio Study of Hydrogen Adsorption on Metal-Decorated Borophene-Graphene Bilayer. Energies, 14(9), 2473. https://doi.org/10.3390/en14092473









