Drying of Sisal Fiber: A Numerical Analysis by Finite-Volumes
Abstract
:1. Introduction
2. Methodology
2.1. Mathematical Modeling
2.1.1. Geometry and the Physical Problem
2.1.2. The Governing Equations
Vapor Diffusion Equation
- Initial condition:
- Boundary conditions:
Heat Conduction Equation
- Initial condition:
- Boundary conditions for heat transfer:
Λ Equilibrium Equation
2.2. Numerical Procedure
- (a)
- The solid is homogeneous and isotropic;
- (b)
- The only mechanism of water transport within the solid is diffusion;
- (c)
- The thermophysical parameters are constant along the drying process;
- (d)
- The diffusion coefficient is constant along the drying;
- (e)
- Convective mass and heat transfer coefficients are constant along the drying.
2.3. Experimental Study
2.4. Parameters Estimation
2.4.1. Transport Coefficients
2.4.2. Equilibrium Equation
2.4.3. Estimation of the Fiber and Sample Parameters
2.5. Thermophysical Properties of the Materials
2.5.1. For Drying Air
2.5.2. For Water and Fiber
3. Results and Discussion
3.1. Fiber Morphology
3.2. Analysis of Equilibrium Equation
3.3. Moisture Removal and Heat Transfer Analysis
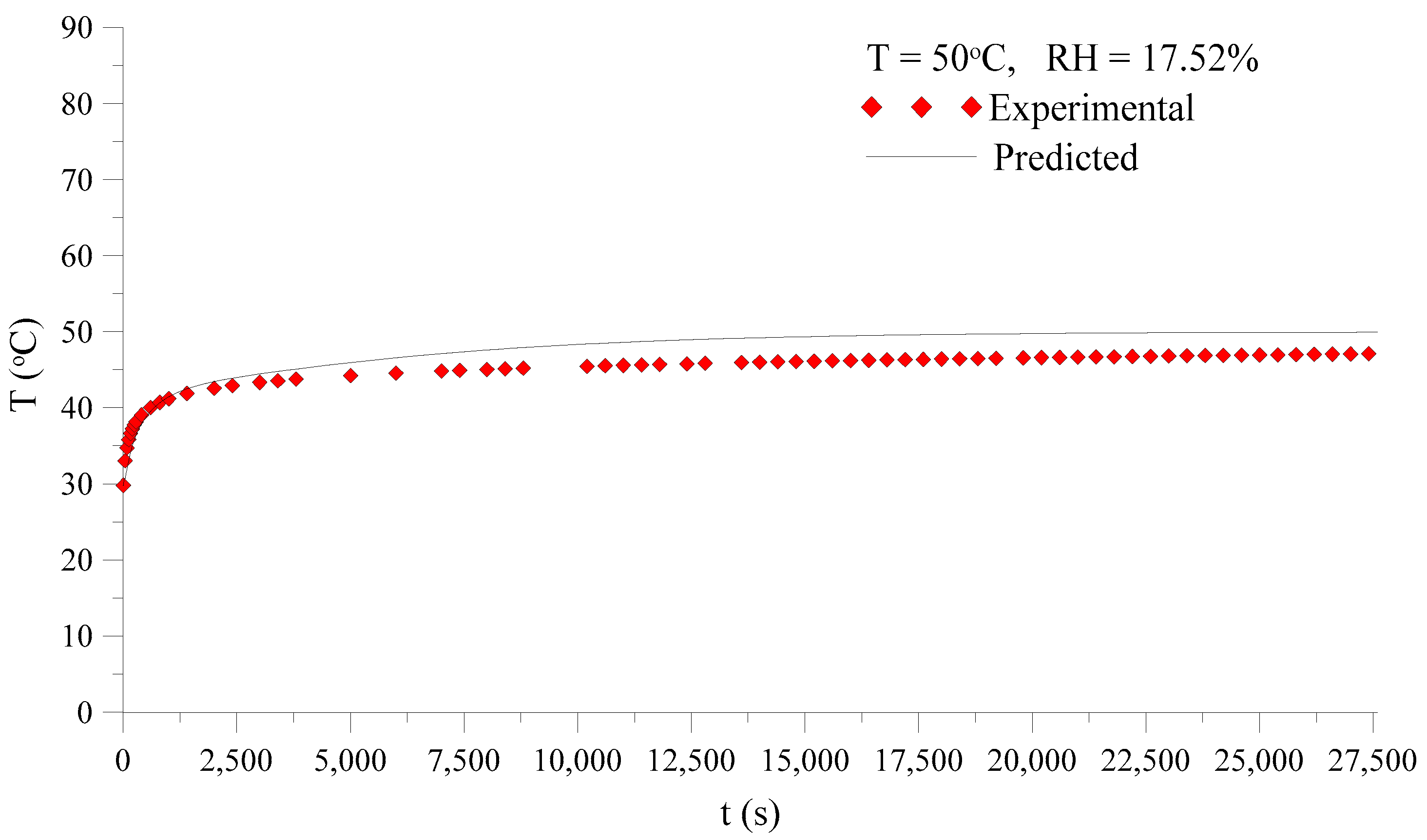
3.3.1. Drying and Heating Kinetics
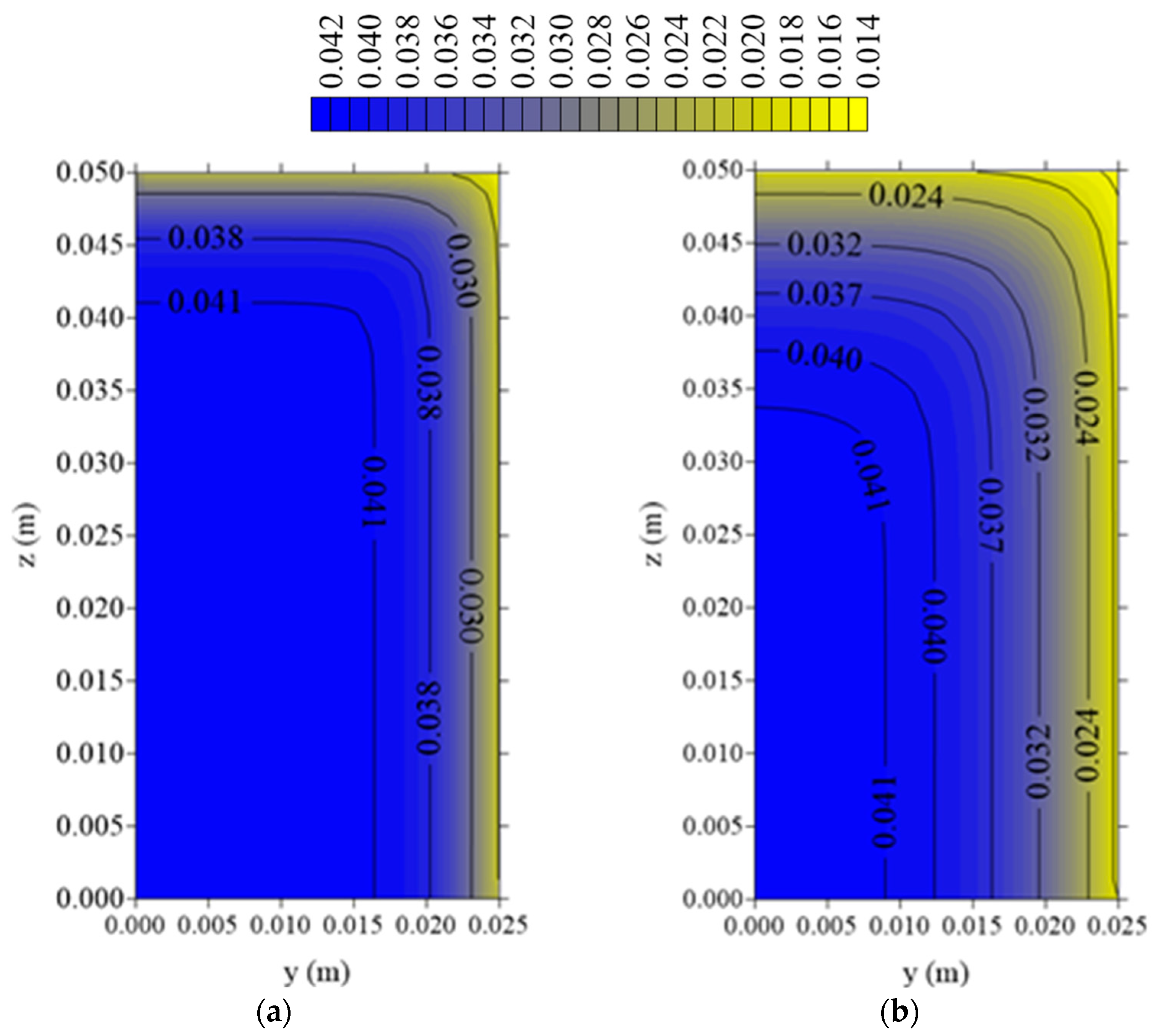
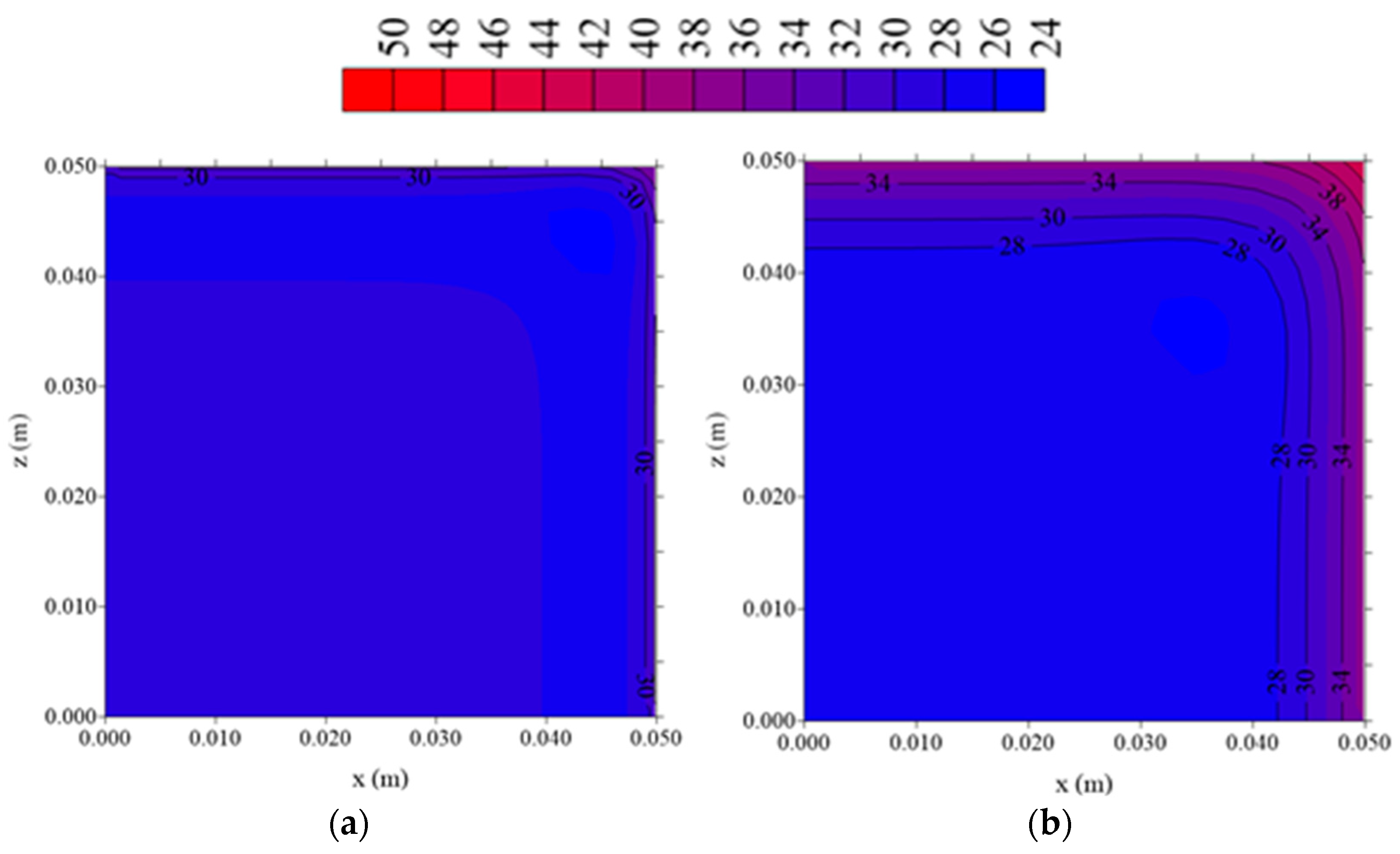
3.3.2. Water Vapor Concentration and Temperature Distributions
3.3.3. Estimation of Transport Coefficients (D, hm and hc)
- Diffusion processes of wetting, drying, heating and cooling, whether coupled or separate;
- Coupled diffusion processes of liquid, vapor and heat in different porous bodies;
- It is possible to study different diffusion problems assuming variable or constant thermophysical properties, and equilibrium or convective boundary conditions;
- Under numerical aspects it is possible to use uniform or nonuniform grids, and to consider changes in the dimensions (dilation and shrinkage) of the bodies in the transient simulations.
- The finite volume method is unconditionally stable even when applied to solving nonlinear partial differential equations;
- No restriction about the nature of the porous body bed (fruits, grains, vegetables, textiles, etc.) is required when it is considered as continuum media;
- Good estimation of process time to dry the product, contributing to reduction in energy consumption and increase in productivity of dried porous bodies;
- Rigorous checkup and understanding of the effect of process variables on product quality during drying.
- (a)
- Chemical transformation, thermal and structure inside the porous body that, in general, are responsible to provoke no uniform distribution of void are not considered;
- (b)
- Inadequacy to be applied in drying processes where high air-drying temperature and effects of pressure are important;
- (c)
- Mass diffusion is the only moisture migration mechanism, and gravity, capillarity, and filtration effects, for example, are not considered;
- (d)
- It is necessary to use estimated parameters after fitting nonlinear regression or other techniques for this purpose.
4. Conclusions
- (a)
- The proposed mathematical model proved to be useful for describing the drying process of sisal fibers, considering the good agreement between the predicted and experimental data of average moisture content and surface temperature of the sisal fibers obtained in all drying conditions;
- (b)
- Despite the highly nonlinear character of the governing equations that represent the mathematical model, the finite-volume method proved to be useful to solve the cited equations and to assist in predicting the phenomenon of heat and mass transfer within the porous sample;
- (c)
- Drying of sisal fibers occurred at a falling drying rate period;
- (d)
- The largest water vapor concentration and temperature gradients are located in the regions near the vertex of the fiber porous bed. Then, these regions are more suitable to deformation and hydric and thermal effects, which are responsible for reducing product quality after drying process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Coefficient of the discretized linear equation | ---- | |
| Coefficient of the discretized linear equation | ---- | |
| Coefficient of the discretized linear equation | ---- | |
| Coefficient of the discretized linear equation | ---- | |
| Coefficient of the discretized linear equation | ---- | |
| Coefficient of the discretized linear equation | ---- | |
| Coefficient of the discretized linear equation | ---- | |
| AHair | Air absolute humidity | kgvapor /kgdry air |
| AHair, sat | Air absolute humidity at the saturation | kgvapor /kgdry air |
| Source term | ---- | |
| Water vapor concentration | kgvapor/m3 | |
| C” | Specific water vapor concentration | kgvapor/m3/m2 |
| Ceq | Equilibrium water vapor concentration | kgvapor/m3 |
| Co | Initial water vapor concentration | kgvapor/m3 |
| Average water vapor concentration | kgvapor/m3 | |
| Water vapor concentration in the nodal point B | kgvapor/m3 | |
| Water vapor concentration in the nodal point E | kgvapor/m3 | |
| Water vapor concentration in the nodal point F | kgvapor/m3 | |
| Water vapor concentration in the nodal point N | kgvapor/m3 | |
| Water vapor concentration in the nodal point P | kgvapor/m3 | |
| Specific heat; | J/kg·K | |
| Old water vapor concentration in the nodal point P | kgvapor/m3 | |
| Water vapor concentration in the nodal point S | kgvapor/m3 | |
| Water vapor concentration in the nodal point W | kgvapor/m3 | |
| Diffusivity of water vapor in the air | ||
| Water vapor diffusion coefficient in the x-direction | m2/s | |
| Water vapor diffusion coefficient in the z-direction | m2/s | |
| Water vapor diffusion coefficient in the y-direction | m2/s | |
| Water vapor diffusion coefficient in the z-direction | m2/s | |
| Water vapor diffusion coefficient in the y-direction | m2/s | |
| Water vapor diffusion coefficient in the x-direction | m2/s | |
| Water vapor diffusion coefficient | m2/s | |
| d | Fiber diameter | m |
| ERMQ | Deviations between experimental and predicted values | ---- |
| Enthalpy | J/kg | |
| Convective heat transfer coefficient | W/m2·K | |
| Convective mass transfer coefficient applied to the air | m/s | |
| hcx | Convective heat transfer coefficient | W/m2·K |
| hcy | Convective heat transfer coefficient | W/m2·K |
| hcz | Convective heat transfer coefficient | W/m2·K |
| hmx | Convective mass transfer coefficient | m/s |
| hmy | Convective mass transfer coefficient | m/s |
| hmz | Convective mass transfer coefficient | m/s |
| K | Thermal conductivity; | W/m·K |
| k | Air thermal conductivity | W/m·K |
| Thermal conductivity in the z-direction | W/m·K | |
| Thermal conductivity in the y-direction | W/m·K | |
| Thermal conductivity in the x-direction | W/m·K | |
| Thermal conductivity in the z-direction | W/m·K | |
| Thermal conductivity in the y-direction | W/m·K | |
| Thermal conductivity in the x-direction | W/m·K | |
| L | Fiber length | m |
| M | Moisture content | kgvapor/kgdry fiber |
| Meq | Equilibrium moisture content | kgvapor/kgdry fiber |
| n | Number of experimental points | ---- |
| Number of fitted parameters | ---- | |
| Average Nusselt number | ---- | |
| P | Atmospheric pressure | Pa |
| Pr | Average Prandtl number | ---- |
| R1 | Half-length of the bed in the x-direction | m |
| R2 | Half-length of the bed in the y-direction | m |
| R3 | Half-length of the bed in the z-direction | m |
| RH | Relative humidity | ---- |
| Particular gas constant (atmospheric air) | J/mol/K | |
| Average Reynolds number | ---- | |
| Average Schmidt number | ---- | |
| Variance | ---- | |
| Average Sherwood number | ---- | |
| Source term | ---- | |
| Source term | ---- | |
| t | Time. | s |
| T | Temperature, | oC |
| Temperature in the nodal point B | oC | |
| Temperature in the nodal point E | oC | |
| Temperature in the nodal point F | oC | |
| Temperature in the nodal point N | oC | |
| Temperature in the nodal point P | oC | |
| Old temperature in the nodal point P | oC | |
| Temperature in the nodal point S | oC | |
| Temperature in the nodal point W | oC | |
| Tabs | Absolute temperature of the drying-air | oC |
| Teq | Equilibrium temperature | °C |
| Tf | Final temperature | °C |
| To | Initial temperature | °C |
| To | Initial temperature | °C |
| V | Volume of the fiber bed | m3 |
| v | Air velocity | m/s |
| x | Cartesian coordinate | m |
| w, e, n, s, f, t | Faces of the control-volume | ---- |
| W, E, N, S, F, T, P | Nodal points | ---- |
| y | Cartesian coordinate | m |
| z | Cartesian coordinate | m |
| Constant of the equilibrium equation | kgvapor/kgdry fiber | |
| Constant of the equilibrium equation | kgvapor/kgdry fiber/oC | |
| Distance between nodal points in the x-direction | m | |
| Distance between nodal points in the y-direction | m | |
| Distance between nodal points in the y-direction | m | |
| Distance between nodal points in the z-direction | m | |
| Distance between nodal points in the z-direction | m | |
| Distance between nodal points in the x-direction | m | |
| Time step | s | |
| Volume of the elemental volume, | m3 | |
| Length of the control-volume in the x-direction | m | |
| Length of the control-volume in the y-direction | m | |
| Length of the control-volume in the z-direction | m | |
| ε | Bed porosity | --- |
| Air viscosity | N·s/m2 | |
| Dry solid density | kg/m3 | |
| Constant of the equilibrium equation | m3/kgdry fiber | |
| Potential of interest | --- | |
| Average value of the potential of interest | ---- |
References
- Silva, R.V. Polyurethane Resin Composite Derived from Castor Oil and Vegetable Fibers. Ph.D. Thesis, University of São Paulo, São Carlos, Brazil, 2003. Available online: https://www.teses.usp.br/teses/disponiveis/88/88131/tde-29082003-105440/pt-br.php (accessed on 10 April 2020). (In Portuguese).
- Martin, A.R.; Martins, M.A.; Mattoso, L.H.C.; Silva, O.R.R.F. Chemical and structural characterization of sisal fibers from Agave Sisalana variety. Polímeros Ciência e. Tecnol. 2009, 19, 40–46. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Meyer, C. Improving degradation resistance of sisal fiber in concrete through fiber surface treatment. Appl. Surface Sci. 2014, 289, 511–523. [Google Scholar] [CrossRef]
- Cruz, V.C.A.; Nóbrega, M.M.S.; Silva, W.P.; Carvalho, L.H.; Lima, A.G.B. An experimental study of water absorption in polyester composites reinforced with macambira natural fiber. Mater. Werkst. 2011, 42, 979–984. [Google Scholar] [CrossRef]
- Melo Filho, J.A.; Silva, F.A.; Toledo Filho, R.D. Degradation kinetics and aging mechanisms on sisal fiber cement composite systems. Cem. Concr. Compos. 2013, 40, 30–39. [Google Scholar] [CrossRef]
- Nóbrega, M.M.S.; Cavalcanti, W.S.; Carvalho, L.H.; Lima, A.G.B. Water absorption in unsaturated polyester composites reinforced with caroá fiber fabrics: Modeling and simulation. Mater. Werkst. 2010, 41, 300–305. [Google Scholar] [CrossRef]
- Zhou, F.; Cheng, G.; Jiang, B. Effect of silane treatment on microstructure of sisal fibers. Appl. Surf. Sci. 2014, 292, 806–812. [Google Scholar] [CrossRef]
- Lima, A.G.B.; Silva, J.B.; Almeida, G.S.; Nascimento, J.J.S.; Tavares, F.V.S.; Silva, V.S. Clay products convective drying: Foundations, modeling and applications. In Drying and Energy Technologies. Advanced Structured Materials; Delgado, J.M.P.Q., Lima, A.G.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 63, pp. 43–70. [Google Scholar] [CrossRef]
- Ferreira, S.R.; Lima, P.R.L.; Silva, F.A.; Toledo Filho, R.D. Effect of sisal fiber humidification on the adhesion with portland cement matrices. Matéria 2012, 17, 1024–1034. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Santos, D.G.; Lima, A.G.B.; Costa, P.S. The Effect of the Drying Temperature on the Moisture Removal and Mechanical Properties of Sisal Fibers. Defect Diffus. Forum 2017, 380, 66–71. [Google Scholar] [CrossRef]
- Diniz, J.F.B.; Lima, E.S.; Magalhães, H.L.F.; Lima, W.M.P.B.; Porto, T.R.N.; Gomez, R.S.; Moreira, G.; Lima, A.G.B. Drying of sisal fibers in oven with forced air circulation: An experimental study. Res. Soc. Dev. 2020, 9, e8639109342. [Google Scholar] [CrossRef]
- Ghosh, B.N. Studies on the High Temperature Drying and Discoloration of Sisal Fibre. J. Agric. Engng. Res. 1966, 11, 69–75. [Google Scholar] [CrossRef]
- Diniz, J.F.B.; Magalhães, H.L.F.; Lima, E.S.; Gomez, R.S.; Porto, T.R.N.; Moreira, G.; Lima, W.M.P.B.; Lima, A.G.B. Drying of sisal fibers in fixed bed: A predictive analysis using lumped models. Res. Soc. Dev. 2020, 9. (In Portuguese) [Google Scholar] [CrossRef]
- Diniz, J.F.B.; Lima, H.G.G.M.; Lima, A.G.B.; Nascimento, J.J.S.; Ramos, A.D.O. Drying of Sisal Fiber: A Theoretical and Experimental Investigation. Defect Diffus. Forum 2019, 391, 36–41. [Google Scholar] [CrossRef]
- Santos, D.G. Thermo-Hydric Study and Mechanical Characterization of Polymeric Matrix Composites Reinforced with Vegetable Fiber: 3D Simulation and Experimentation. Ph.D. Thesis, Federal University of Campina Grande, Campina Grande, Brazil, 2017. Available online: http://dspace.sti.ufcg.edu.br:8080/jspui/handle/riufcg/946 (accessed on 15 April 2020). (In Portuguese).
- Diniz, J.F.B.; Lima, A.R.C.; Oliveira, I.R.; Farias, R.P.; Batista, F.A.; Lima, A.G.B.; Andrade, R.O. Vegetable Fiber Drying: Theory, Advanced Modeling and Application. In Transport Process and Separation Technologies. Advanced Structured Materials, 1st ed.; Delgado, J.M.P.Q., Lima, A.G.B., Eds.; Springer: Cham, Switzerland, 2021; Volume 133, pp. 31–60. [Google Scholar] [CrossRef]
- Nordon, P.; David, H.G. Coupled diffusion of moisture and heat in hygroscopic textile materials. Int. J. Heat Mass Transf. 1967, 10, 853–866. [Google Scholar] [CrossRef]
- Haghi, A.K. Simultaneous moisture and heat transfer in porous systems. J. Comput. Appl. Mech. 2001, 2, 195–204. [Google Scholar]
- Xiao, B.; Huang, Q.; Chen, H.; Chen, X.; Long, G. A fractal model for capillary flow through a single tortuous capillary with roughened surfaces in fibrous porous media. Fractals 2021, 29, 2150017. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Y.; Wang, Y.; Jiang, G.; Liang, M.; Chen, X.; Long, G. A fractal model for kozeny–carman constant and dimensionless permeability of fibrous porous media with roughened surfaces. Fractals 2019, 27, 1950116. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975. [Google Scholar]
- Maliska, C.R. Computational Heat Transfer and Fluid Mechanics, 2nd ed.; LTC: Rio de Janeiro, Brazil, 2004. [Google Scholar]
- Patankar, S.V. Numerical Heat Transfer and Fluid Flow; CRC Press: Boca Raton, FL, USA, 1980. [Google Scholar]
- Versteeg, H.K.; Malalasekera, W. An Introduction to Computational Fluid Dynamics: The Finite Volume Method, 2nd ed.; Pearson Education Limited: Harlow, UK, 2007. [Google Scholar]
- Figliola, R.S.; Beasley, D.E. Theory and Design for Mechanical Measurements; John Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Diniz, J.F.B.; Moreira, G.; Nascimento, J.J.S.; Farias, R.P.; Magalhães, H.L.F.; Barbalho, G.H.A.; Lima, A.G.B. Drying of Sisal Fiber: Three-Dimensional Mathematical Modeling and Simulation. Defect Diffus. Forum 2020, 399, 202–207. [Google Scholar] [CrossRef]
- Diniz., J.F.B. Heat and Mass Transfer in Porous Solids with Parallelepiped Shape. Case Study: Drying of Sisal Fibers. Ph.D. Thesis, Federal University of Campina Grande, Campina Grande, Brazil, 2018. Available online: http://dspace.sti.ufcg.edu.br:8080/jspui/handle/riufcg/2424 (accessed on 21 April 2020). (In Portuguese).
















| Air | Fibrous Medium | Process Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RH (%) | T (°C) | v (m/s) | 2R1 (m) | 2R2 (m) | 2R3 (m) | Mo (d.b.) | Meq (d.b.) | To (°C) | Tf (°C) | t (h) |
| 17.52 | 50 | 0.05 | 0.1 | 0.05 | 0.1 | 0.11327 | 0.03837 | 29.8 | 46.3 | 7.7 |
| 11.04 | 60 | 0.06 | 0.1 | 0.05 | 0.1 | 0.11118 | 0.02606 | 29.8 | 56.6 | 6.7 |
| 6.89 | 70 | 0.07 | 0.1 | 0.05 | 0.1 | 0.11148 | 0.02015 | 31.5 | 67.3 | 5.7 |
| 4.19 | 80 | 0.08 | 0.1 | 0.05 | 0.1 | 0.11030 | 0.01390 | 29.6 | 76.3 | 5.0 |
| 3.28 | 90 | 0.09 | 0.1 | 0.05 | 0.1 | 0.11342 | 0.00525 | 30.4 | 87.4 | 4.7 |
| T (°C) | Fiber Length (m) | * Fiber Diameter (µm) | ** Fiber Volume (nm3) | Numbers of Fibers/Sample (---) | Vfiber (m3) | Vsample (m3) | ε (---) |
|---|---|---|---|---|---|---|---|
| 50 | 0.1 | 247 | 4.789 | 8539 | 40.895 × 10−6 | 5.00 × 10−4 | 0.918 |
| 60 | 0.1 | 247 | 4.789 | 8190 | 39.224 × 10−6 | 5.00 × 10−4 | 0.922 |
| 70 | 0.1 | 247 | 4.789 | 8490 | 40.660 × 10−6 | 5.00 × 10−4 | 0.919 |
| 80 | 0.1 | 247 | 4.789 | 8627 | 41.316 × 10−6 | 5.00 × 10−4 | 0.917 |
| 90 | 0.1 | 247 | 4.789 | 8869 | 42.475 × 10−6 | 5.00 × 10−4 | 0.915 |
| T (°C) | Parameter | ||||
|---|---|---|---|---|---|
| AHair (kgvapor /kgdry air) | AHair, sat (kgvapor /kgdry air) | ||||
| 50 | 1.0835 | 0.0269 | 19.054 × 10−6 | 0.01363 | 0.0863 |
| 60 | 1.0508 | 0.0276 | 19.509 × 10−6 | 0.01387 | 0.1524 |
| 70 | 1.0204 | 0.0283 | 19.966 × 10−6 | 0.01354 | 0.2765 |
| 80 | 0.9921 | 0.0290 | 20.427 × 10−6 | 0.01247 | 0.5464 |
| 90 | 0.9637 | 0.0296 | 20.847 × 10−6 | 0.01449 | 1.3990 |
| hs (kJ/kg) | Pr (---) | Sc (---) | |||
| 50 | 28.06 × 10−6 | 1.0331 | 2382.90 | 0.73210 | 0.62671 |
| 60 | 29.60 × 10−6 | 1.0335 | 2358.34 | 0.73123 | 0.62726 |
| 70 | 31.17 × 10−6 | 1.0335 | 2333.26 | 0.73016 | 0.62774 |
| 80 | 32.78 × 10−6 | 1.0328 | 2307.62 | 0.72884 | 0.62818 |
| 90 | 34.42 × 10−6 | 1.0348 | 2281.39 | 0.72877 | 0.62855 |
| Dimensionless Parameter | T (°C) | ||||
|---|---|---|---|---|---|
| 50 | 60 | 70 | 80 | 90 | |
| 142.1625 | 134.6619 | 127.7713 | 121.4225 | 115.5627 | |
| 71.0812 | 67.3309 | 63.8856 | 60.7112 | 57.7813 | |
| 142.1625 | 134.6619 | 127.7713 | 121.4225 | 115.5627 | |
| 7.13539 | 6.94186 | 6.75860 | 6.58460 | 6.42355 | |
| 5.04548 | 4.90864 | 4.77905 | 4.65601 | 4.54213 | |
| 7.13539 | 6.94186 | 6.75860 | 6.58460 | 6.42355 | |
| 6.77511 | 6.59588 | 6.42655 | 6.26630 | 6.11445 | |
| 4.79072 | 4.66399 | 4.54425 | 4.43094 | 4.32357 | |
| 6.77511 | 6.59588 | 6.42655 | 6.26630 | 6.11445 | |
| T (°C) | Parameter | |||
|---|---|---|---|---|
| Co (kgvapor/m3) | Ceq (kgvapor/m3) | (kg/m3) | (kg/m3) | |
| 50 | 0.04146 | 0.01482 | 83.088 | 1450.00 |
| 60 | 0.04052 | 0.01458 | 74.960 | 1450.00 |
| 70 | 0.04125 | 0.01381 | 81.956 | 1450.00 |
| 80 | 0.04005 | 0.01439 | 85.138 | 1450.00 |
| 90 | 0.4174 | 0.01403 | 90.782 | 1450.00 |
(J/kg·K) | (J/kg·K) | (W/m·K) | (W/m·K) | |
| 50 | 960.8454 | 149.65 | 0.03017 | 0.067 |
| 60 | 964.2007 | 149.65 | 0.03067 | 0.067 |
| 70 | 961.6180 | 149.65 | 0.03141 | 0.067 |
| 80 | 959.8464 | 149.65 | 0.03209 | 0.067 |
| 90 | 959.6211 | 149.65 | 0.03278 | 0.067 |
| T (oC) | Parameter | |||||
|---|---|---|---|---|---|---|
| ERMQM (kg/kg)2 | ||||||
| 50 | 3.8022 × 10−4 | 5.3771 × 10−4 | 3.8022 × 10−4 | 0.612 × 10−6 | 0.0001576 | 1.1939 × 10−6 |
| 60 | 4.0436 × 10−4 | 20.2160 × 10−4 | 4.0436 × 10−4 | 1.012 × 10−6 | 0.0004562 | 4.0015 × 10−6 |
| 70 | 5.0622 × 10−4 | 21.6564 × 10−4 | 5.0622 × 10−4 | 1.612 × 10−6 | 0.0000722 | 7.5257 × 10−7 |
| 80 | 7.6108 × 10−4 | 7.7809 × 10−4 | 7.6108 × 10−4 | 2.212 × 10−6 | 0.0002573 | 3.0629 × 10−6 |
| 90 | 4.2089 × 10−4 | 5.9523 × 10−4 | 4.2089 × 10−4 | 2.312 × 10−6 | 0.0003805 | 4.8164 × 10−6 |
| T (oC) | Parameters | |||||
|---|---|---|---|---|---|---|
| ERMQT (°C)2 | ||||||
| 50 | 4.2208 | 5.9692 | 4.2208 | 3.7789 × 10−7 | 989.6026 | 7.4406 |
| 60 | 4.9768 | 7.0382 | 4.9768 | 4.2430 × 10−7 | 1516.8398 | 13.1899 |
| 70 | 5.5389 | 7.8332 | 5.5389 | 3.9856 × 10−7 | 967.74872 | 9.9768 |
| 80 | 5.1463 | 7.2780 | 5.1463 | 3.9270 × 10−7 | 1324.2250 | 15.5791 |
| 90 | 6.6552 | 9.4119 | 6.6552 | 3.7627 × 10−7 | 1273.6510 | 15.9206 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniz, J.F.B.; Delgado, J.M.P.Q.; Vilela, A.F.; Gomez, R.S.; Viana, A.D.; Figueiredo, M.J.; Diniz, D.D.S.; Rodrigues, I.S.; Rolim, F.D.; Santos, I.B.; et al. Drying of Sisal Fiber: A Numerical Analysis by Finite-Volumes. Energies 2021, 14, 2514. https://doi.org/10.3390/en14092514
Diniz JFB, Delgado JMPQ, Vilela AF, Gomez RS, Viana AD, Figueiredo MJ, Diniz DDS, Rodrigues IS, Rolim FD, Santos IB, et al. Drying of Sisal Fiber: A Numerical Analysis by Finite-Volumes. Energies. 2021; 14(9):2514. https://doi.org/10.3390/en14092514
Chicago/Turabian StyleDiniz, Jacqueline F. B., João M. P. Q. Delgado, Anderson F. Vilela, Ricardo S. Gomez, Arianne D. Viana, Maria J. Figueiredo, Diego D. S. Diniz, Isis S. Rodrigues, Fagno D. Rolim, Ivonete B. Santos, and et al. 2021. "Drying of Sisal Fiber: A Numerical Analysis by Finite-Volumes" Energies 14, no. 9: 2514. https://doi.org/10.3390/en14092514
APA StyleDiniz, J. F. B., Delgado, J. M. P. Q., Vilela, A. F., Gomez, R. S., Viana, A. D., Figueiredo, M. J., Diniz, D. D. S., Rodrigues, I. S., Rolim, F. D., Santos, I. B., Carmo, J. E. F., & Lima, A. G. B. (2021). Drying of Sisal Fiber: A Numerical Analysis by Finite-Volumes. Energies, 14(9), 2514. https://doi.org/10.3390/en14092514