Gasification of Psidium guajava L. Waste Using Supercritical Water: Evaluation of Feed Ratio and Moderate Temperatures
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Biomass Characterization
3.2. Supercritical Water Gasification
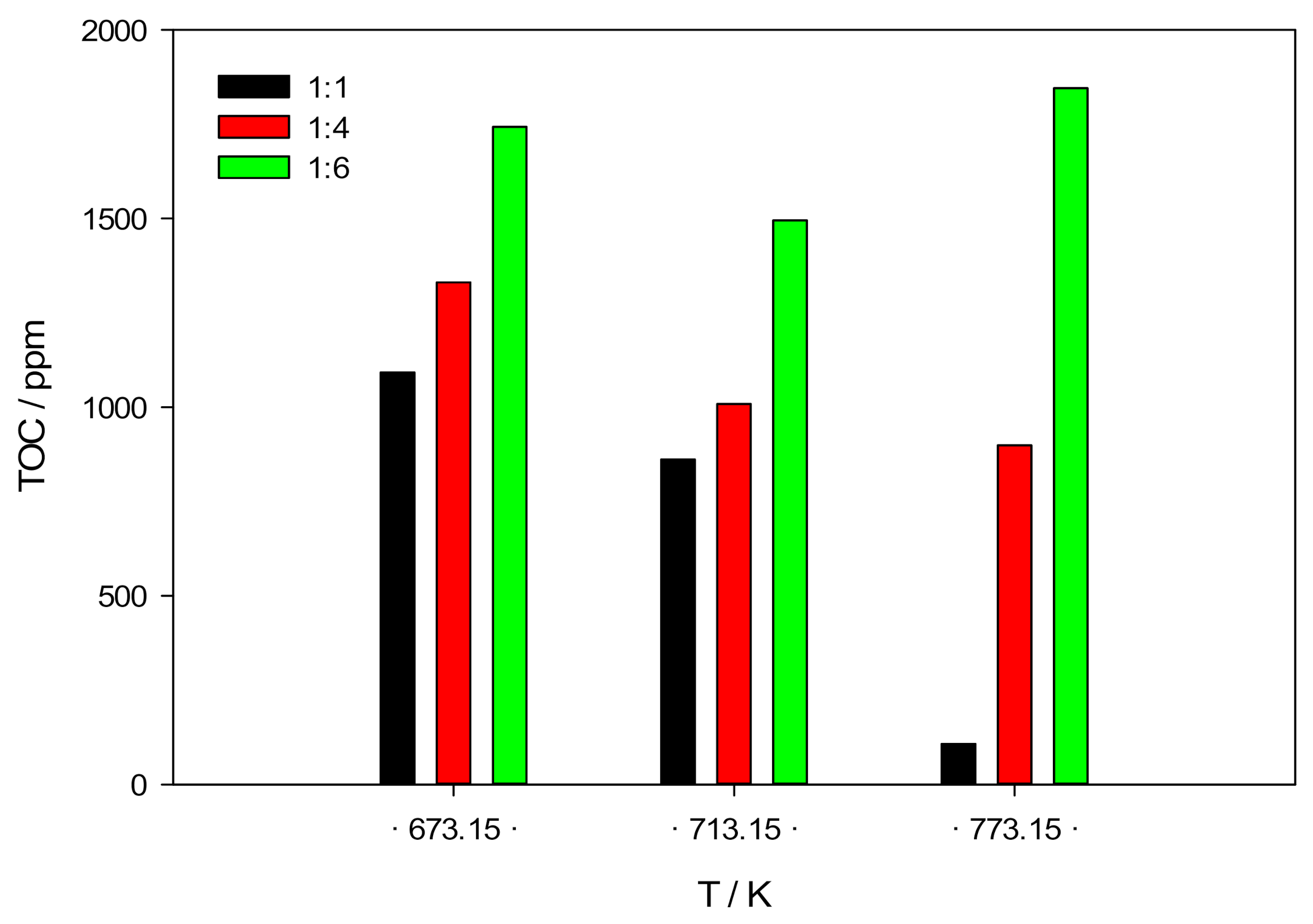
3.3. Liquid and Solid Products
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcus, Y. Supercritical Water: A Green Solvent: Properties and Uses; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Desperdicio de Alimentos en México. Available online: http://www.sedesol.gob.mx/boletinesSinHambre/Informativo_02/infografia.html (accessed on 9 April 2021).
- Guo, Y.; Wang, S.Z.; Xu, D.H.; Gong, Y.M.; Ma, H.H.; Tang, X.Y. Review of catalytic supercritical water gasification for hydrogen production from biomass. Renew. Sust. Energ. Rev. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Kruse, A. Supercritical water gasification of biomass for hydrogen production—Review. J. Supercrit. Fluids 2018, 133, 573–590. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Casademont, P.; García-Jarana, M.B.; Sánchez-Oneto, J.; Portela, J.R.; Martínez de la Ossa, E.J. Supercritical water gasification: A patents review. Rev. Chem. Eng. 2017, 33, 237–261. [Google Scholar] [CrossRef]
- Vargas-Mira, A.; Zuluaga-García, C.; González-Delgado, A.D. A technical and environmental evaluation of six routes for industrial hydrogen production from empty palm fruit bunches. ACS Omega 2019, 4, 15457–15470. [Google Scholar] [CrossRef]
- Loppinet-Serani, A.; Reverte, C.; Cansell, F.; Aymonier, C. Supercritical water biomass gasification process as a successful solution to valorize wine distillery wastewaters. ACS Sustain. Chem. Eng. 2013, 1, 110–117. [Google Scholar] [CrossRef]
- Ruya, P.M.; Lim, S.S.; Purwadi, R.; Zunita, M. Sustainable hydrogen production from oil palm derived wastes through autothermal operation of supercritical water gasification system. Energy 2020, 208, 118280. [Google Scholar] [CrossRef]
- Shenbagaraj, S.; Sharma, P.K.; Sharma, A.K.; Raghav, G.; Kota, K.B.; Ashokkumar, V. Gasification of food waste in supercritical water: An innovative synthesis gas composition prediction model based on Artificial Neural Networks. Int. J. Hydrogen Energy 2021, 46, 12739–12757. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Gorman, D.J.; Tiwari, K.; Rasmussen, E.G.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Supercritical water gasification: Practical design strategies and operational challenges for lab-scale, continuous flow reactors. Heliyon 2019, 5, e01269. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, K.; Fan, J. CFD-DEM coupled with thermochemical sub-models for biomass gasification: Validation and sensitivity analysis. Chem. Eng. Sci. 2020, 217, 115550. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, Y. Hydrogen production by biomass gasification in a supercritical water fluidized bed reactor: A CFD-DEM study. J. Supercrit. Fluids 2018, 131, 26–36. [Google Scholar] [CrossRef]
- Tushar, M.S.H.K.; Dutta, A.; Xu, C. Simulation and kinetic modeling of supercritical water gasification of biomass. Int. J. Hydrogen Energy 2015, 40, 4481–4493. [Google Scholar] [CrossRef]
- Xu, C.; Donald, J. Upgrading peat to gas and liquid fuels in supercritical water with catalysts. Fuel 2012, 102, 16–25. [Google Scholar] [CrossRef]
- Okolie, J.A.; Rana, R.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass: A state-of-the-art review of process parameters, reaction mechanisms and catalysis. Sustain. Energ. Fuels 2019, 5, 578–598. [Google Scholar] [CrossRef]
- Safari, F.; Salimi, M.; Tavasoli, A.; Ataei, A. Non-catalytic conversion of wheat straw, walnut shell and almond shell into hydrogen rich gas in supercritical water media. Chin. J. Chem. Eng. 2016, 24, 1097–1103. [Google Scholar] [CrossRef]
- Nanda, S.; Isen, J.; Dalai, A.K.; Kozinski, J.A. Gasification of fruit wastes and agro-food residues in supercritical water. Energy Convers. Manag. 2016, 110, 296–306. [Google Scholar] [CrossRef]
- Elif, D.; Nezihe, A. Hydrogen production by supercritical water gasification of fruit pulp in the presence of Ru/C. Int. J. Hydrogen Energy 2016, 41, 8073–8083. [Google Scholar] [CrossRef]
- Sheikhdavoodi, M.J.; Almassi, M.; Ebrahimi-Nik, M.; Kruse, A.; Bahrami, H. Gasification of sugarcane bagasse in supercritical water; evaluation of alkali catalysts for maximum hydrogen production. J. Energy Inst. 2015, 88, 450–458. [Google Scholar] [CrossRef]
- Castello, D.; Rolli, B.; Kruse, A.; Fiori, L. Supercritical water gasification of biomass in a ceramic reactor: Long-time batch experiments. Energies 2017, 10, 1734. [Google Scholar] [CrossRef]
- Moghaddam, E.M.; Goel, A.; Siedlecki, M.; Michalska, K.; Yakaboylu, O.; de Jong, W. Supercritical water gasification of wet biomass residues from farming and food production practices: Lab-scale experiments and comparison of different modelling approaches. Sustain. Energ. Fuels 2021, 5, 1521–1537. [Google Scholar] [CrossRef]
- Rashidi, M.; Tavasoli, A. Hydrogen rich gas production via supercritical water gasification of sugarcane bagasse using unpromoted and copper promoted Ni/CNT nanocatalysts. J. Supercrit. Fluids 2015, 98, 111–118. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Yang, J.; Xu, D.; Li, Y.; Li, J.; Zhang, Y. Hydrothermal liquefaction and gasification of biomass and model compounds: A review. Green Chem. 2020, 22, 8210–8232. [Google Scholar] [CrossRef]
- Chen, J.; Fan, Y.; Jiaqiang, E.; Cao, W.; Zhang, F.; Gong, J.; Liu, G.; Xu, W. Effects analysis on the gasification kinetic characteristics of food waste in supercritical water. Fuel 2019, 241, 94–104. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Valerio, V.; Martino, M.; Marino, T.; Rimauro, J.; Casella, P. Biofuels and bio-based production via supercritical water gasification of peach scraps. Energy Fuels 2016, 30, 10443–10447. [Google Scholar] [CrossRef]
- Chen, J.; Fan, Y.; Zhao, X.; Jiaqiang, E.; Xu, W.; Zhang, F.; Liao, G.; Leng, E.; Liu, S. Experimental investigation on gasification characteristic of food waste using supercritical water for combustible gas production: Exploring the way to complete gasification. Fuel 2020, 263, 116735. [Google Scholar] [CrossRef]
- Su, H.; Kanchanatip, E.; Wang, D.; Zheng, R.; Huang, Z.; Chen, Y.; Mubeen, I.; Yan, M. Production of H2-rich syngas from gasification of unsorted food waste in supercritical water. Waste Manag. 2020, 102, 520–527. [Google Scholar] [CrossRef]
- Yan, M.; Su, H.; Hantoko, D.; Kanchanatip, E.; Hamid, F.B.S.; Zhang, S.; Wang, G.; Xu, Z. Experimental study on the energy conversion of food waste via supercritical water gasification: Improvement of hydrogen production. Int. J. Hydrogen Energy 2019, 44, 4664–4673. [Google Scholar] [CrossRef]
- Silveira-Junior, E.G.; Perez, V.H.; Rodriguez Justo, O.; Ferreira David, G.; Simionatto, E.; Silva de Oliveira, L.C. Valorization of guava (Psidium guajava L.) seeds for levoglucosan production by fast pyrolysis. Cellulose 2021, 28, 71–79. [Google Scholar] [CrossRef]
- ASABE Standards, S319.4. Method of Determining and Expressing Fineness of Feed Materials by Sieving; ASABE: St. Joseph, MI, USA, 2008. [Google Scholar]
- Claye, S.S.; Idouraine, A.; Weber, C.W. Extraction and fractionation of insoluble fiber from five fiber sources. Food Chem. 1996, 57, 305–310. [Google Scholar] [CrossRef]
- Rani, A.; Kawatra, A. Fiber constituents of some foods. Plant Foods Hum. Nutr. 1994, 45, 343–347. [Google Scholar] [CrossRef]
- Osorio, C.; Forero, D.P.; Carriazo, J.G. Characterisation and performance assessment of guava (Psidium guajava L.) microencapsulates obtained by spray-drying. Food Res. Int. 2011, 44, 1174–1181. [Google Scholar] [CrossRef]
- Long, Y.; Ruan, L.; Lv, X.; Lv, Y.; Su, J.; Wen, Y. TG–FTIR analysis of pyrolusite reduction by major biomass components. Chin. J. Chem. Eng. 2015, 23, 1691–1697. [Google Scholar] [CrossRef]
- Kumar, M.; Shukla, S.K.; Upadhyay, S.N.; Mishra, P.K. Analysis of thermal degradation of banana (Musa balbisiana) trunk biomass waste using iso-conversional models. Bioresour. Technol. 2020, 310, 123393. [Google Scholar] [CrossRef]
- Ridout, A.J.; Carrier, M.; Görgens, J. Fast pyrolysis of low and high ash paper waste sludge: Influence of reactor temperature and pellet size. J. Anal. Appl. Pyrolysis 2015, 111, 64–75. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- El Moustaqim, M.; El Kaihal, A.; El Marouani, M.; Men-La Yakhaf, S.; Taibi, M.; Sebbahi, S.; El Hajjaji, S.; Kifani-Sahban, F. Thermal and thermomechanical analyses of lignin. Sustain. Chem. Pharm. 2018, 9, 63–68. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG–FTIR and Py–GC–FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Chen, W.H.; Eng, C.F.; Lin, Y.Y.; Bach, Q.V. Independent parallel pyrolysis kinetics of cellulose, hemicelluloses and lignin at various heating rates analyzed by evolutionary computation. Energy Convers. Manag. 2020, 221, 113165. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Y.; Yang, H.; Xin, S.; Zhang, X.; Wang, X.; Chen, H. Effect of volatiles interaction during pyrolysis of cellulose, hemicellulose, and lignin at different temperatures. Fuel 2019, 248, 1–7. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Athmaselvi, K.A.; Kumar, C.; Balasubramanian, M.; Roy, I. Thermal, structural, and physical properties of freeze dried tropical fruit powder. J. Food Process. 2014, 2014, 524705. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Canteri, M.H.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef]
- Samy, A.M.; Gopalrao, M. Physiochemical characterization of mucilage obtained from the fresh fruits of Psidium guajava L. Int. J. Phytopharm. 2015, 5, 30–36. [Google Scholar] [CrossRef]
- Fitri, R.A.; Wirakusuma, A.; Fahrina, A.; Roil Bilad, M.; Arahman, N. Adsorption performance of low-cost java plum leaves and guava fruits as natural adsorbents for removal of free fatty acids from coconut oil. Int. J. Eng. 2019, 32, 1372–1378. [Google Scholar] [CrossRef]
- Bilba, K.; Ouensanga, A. Fourier transform infrared spectroscopic study of thermal degradation of sugar cane bagasse. J. Anal. Appl. Pyrolysis 1996, 38, 61–73. [Google Scholar] [CrossRef]
- Lazzari, E.; Schena, T.; Marcelo, M.C.A.; Primaz, C.T.; Silva, A.N.; Ferrão, M.F.; Bjerk, T.; Caramão, E.B. Classification of biomass through their pyrolytic bio-oil composition using FTIR and PCA analysis. Ind. Crops. Prod. 2018, 111, 856–864. [Google Scholar] [CrossRef]
- Bilba, K.; Arsene, M.A.; Ouensanga, A. Study of banana and coconut fibers: Botanical composition, thermal degradation and textural observations. Bioresour. Technol. 2007, 98, 58–68. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Fahey, L.M.; Nieuwoudt, M.K.; Harris, P.J. Predicting the cell-wall compositions of Pinus radiata (radiata pine) wood using ATR and transmission FTIR spectroscopies. Cellulose 2017, 24, 5275–5293. [Google Scholar] [CrossRef]
- Tian, Z.; Zong, L.; Niu, R.; Wang, X.; Li, Y.; Ai, S. Recovery and characterization of lignin from alkaline straw pulping black liquor: As feedstock for bio-oil research. J. Appl. Polym. Sci. 2015, 132, 42057. [Google Scholar] [CrossRef]
- Armynah, B.; Tahir, D.; Tandilayuk, M.; Djafar, Z.; Piarah, W.H. Potentials of biochars derived from bamboo leaf biomass as energy sources: Effect of temperature and time of heating. Int. J. Biomater. 2019, 2019, 3526145. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sawatari, C. A Fourier transform infra-red spectroscopic analysis of the character of hydrogen bonds in amorphous cellulose. Polymer 1996, 37, 393–399. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F. Crystallinity changes in lyocell and viscose-type fibres by caustic treatment. Eur. Polym. J. 2002, 38, 2225–2230. [Google Scholar] [CrossRef]
- Toribio Cuaya, H.; Pedraza Segura, L.; Macías Bravo, S.; Gonzalez García, I.; Vasquez Medrano, R.; Favela Torres, E. Characterization of lignocellulosic biomass using five simple steps. J. Chem. Biol. Phys. Sci. 2014, 4, 28–47, E-ISSN: 2249–1929. [Google Scholar]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR spectra and PCA to the bulk characterization of cell wall residues of fruits and vegetables along a fraction process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Martínez Cortizas, A. Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci. Tech. 2018, 52, 487–504. [Google Scholar] [CrossRef]
- Moosavinejad, S.M.; Madhoushi, M.; Vakili, M.; Rasouli, D. Evaluation of degradation in chemical compounds of wood in historical buildings using FT-IR and FT-Raman vibrational spectroscopy. Maderas, Cienc. Tecnol. 2019, 21, 381–392. [Google Scholar] [CrossRef]
- Horikawa, Y.; Hirano, S.; Mihashi, A.; Kobayashi, Y.; Zhai, S.; Sugiyama, J. Prediction of lignin contents from infrared spectroscopy: Chemical digestion and lignin/biomass ratios of Cryptomeria japonica. Appl. Biochem. Biotechnol. 2019, 188, 1066–1076. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Sun, R.C.; Fang, J.M.; Tomkinson, J.; Jones, G.L. Acetylation of wheat straw hemicelluloses in N,N-dimethylacetamide/LiCl solvent system. Ind. Crops. Prod. 1999, 10, 209–218. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, D.; Zhang, Y.; Huang, Y.; Sun, S. Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar. Fuel Process. Technol. 2016, 141, 54–60. [Google Scholar] [CrossRef]









| Ultimate Analysis/wt% | Moisture 1/wt% | gmd/mm | ||||
| C | H | N | S | O 2 | ||
| 42.19 | 6.19 | 0.89 | 0.02 | 50.71 | 82 | 0.624 |
| Structural Analysis/wt% | Ash/wt% | HHV/kJ·kg−1 | TOC/wt% | |||
| Cellulose | Hemicellulose | Lignin | Extracts | |||
| 2.70 | 64.60 | 26.10 | 3.68 | 2.92 | 17250.6 | 33.5 |
| Wavenumber/cm−1 | Functional Group | Polymer | Reference |
|---|---|---|---|
| 3273 | OH, NH | Carbohydrates, lignin, hemicellulose, cellulose | [50,51,52,53,54] |
| 2921 | CH2 aliphatic | Carbohydrates, lignin | [47,52,53] |
| 2850 | CH2 aliphatic | Pectin, protein | [47,52] |
| 1730 | C=O ester group | Hemicellulose | [47,50,55,56] |
| 1600 | C=O, aromatic | Lignin | [55,56,57,58] |
| 1420 | Aromatic, C–H | Cellulose, hemicellulose, lignin | [56,57,59,60,61] |
| 1371 | C–H | Cellulose, hemicellulose | [56,59,60,61,62] |
| 1335 | CH–, OH | Cellulose, hemicellulose, lignin | [55,56,63] |
| 1237 | C–O, C–O–C | Carbohydrates | [53,64,65] |
| 1143 | Aromatic C–H, OH, C=O | Lignin | [56,65] |
| 1098 | C–O–H, C–O | Cellulose, hemicellulose, lignin | [58,66] |
| 1047 | Cellulose, hemicellulose | [56,62,65,67] | |
| 1028 | C3–O3H, C–O | Cellulose, hemicellulose, lignin | [50,52,55,65] |
| 923 | C6–O6H, C–O, C=C, C–C–O | Lignin | [56,68] |
| 869 | Aromatic, C–H | Hemicellulose | [56,65,66] |
| 820 | C2–H | Lignin | [56,65,66] |
| 776 | C–H | Carbohydrates and lignin | [53,69] |
| Run | B:W 1 | T/K | P/MPa | yCH4 | yCO2 | yC2H6 | yC3H8 | yC4H10 | yH2 | yCO |
|---|---|---|---|---|---|---|---|---|---|---|
| R1 | 1:1 | 673.15 | 27.8 | 9.0 | 72.6 | 1.4 | 0.3 | 0.2 | 13.4 | 3.0 |
| R2 | 1:4 | 673.15 | 25.0 | 21.5 | 49.7 | 5.4 | 1.8 | 0.4 | 20.5 | 0.7 |
| R3 | 1:6 | 673.15 | 25.5 | 26.3 | 45.0 | 2.1 | 0.3 | – | 20.2 | 6.1 |
| R4 | 1:1 | 713.15 | 43.0 | 20.8 | 51.8 | 3.1 | 0.8 | 0.2 | 20.1 | 3.2 |
| R5 | 1:4 | 713.15 | 45.0 | 19.9 | 50.5 | 2.7 | 0.7 | 0.2 | 23.7 | 2.5 |
| R6 | 1:6 | 713.15 | 38.5 | 20.7 | 48.3 | 3.2 | 0.9 | 0.3 | 25.2 | 1.4 |
| R7 | 1:1 | 773.15 | 44.5 | 12.3 | 58.1 | 3.0 | 0.9 | 0.4 | 21.2 | 4.0 |
| R8 | 1:4 | 773.15 | 40.0 | 26.7 | 38.4 | 3.8 | 0.8 | 0.1 | 28.3 | 1.9 |
| R9 | 1:6 | 773.15 | 42.0 | 21.1 | 34.2 | 2.6 | 0.6 | 0.1 | 39.5 | 1.9 |
| Run | Gas Yield (mol·kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| CH4 | CO2 | C2H6 | C3H8 | C4H10 | H2 | CO | |
| R1 | 0.211 | 1.696 | 0.033 | 0.008 | 0.005 | 0.313 | 0.070 |
| R2 | 0.960 | 2.218 | 0.241 | 0.081 | 0.017 | 0.915 | 0.030 |
| R3 | 1.979 | 3.384 | 0.161 | 0.019 | – | 1.520 | 0.457 |
| R4 | 0.532 | 1.321 | 0.080 | 0.021 | 0.005 | 0.514 | 0.081 |
| R5 | 1.361 | 3.458 | 0.182 | 0.046 | 0.011 | 1.623 | 0.168 |
| R6 | 2.584 | 6.034 | 0.395 | 0.118 | 0.036 | 3.151 | 0.175 |
| R7 | 0.520 | 2.465 | 0.128 | 0.039 | 0.018 | 0.900 | 0.171 |
| R8 | 2.609 | 3.755 | 0.372 | 0.075 | 0.010 | 2.766 | 0.187 |
| R9 | 4.137 | 6.705 | 0.501 | 0.119 | 0.025 | 7.743 | 0.376 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Arias, S.; Zúñiga-Moreno, A.; García-Morales, R.; Elizalde-Solis, O.; Verónico-Sánchez, F.J.; Flores-Valle, S.O. Gasification of Psidium guajava L. Waste Using Supercritical Water: Evaluation of Feed Ratio and Moderate Temperatures. Energies 2021, 14, 2555. https://doi.org/10.3390/en14092555
González-Arias S, Zúñiga-Moreno A, García-Morales R, Elizalde-Solis O, Verónico-Sánchez FJ, Flores-Valle SO. Gasification of Psidium guajava L. Waste Using Supercritical Water: Evaluation of Feed Ratio and Moderate Temperatures. Energies. 2021; 14(9):2555. https://doi.org/10.3390/en14092555
Chicago/Turabian StyleGonzález-Arias, Sandro, Abel Zúñiga-Moreno, Ricardo García-Morales, Octavio Elizalde-Solis, Francisco J. Verónico-Sánchez, and Sergio O. Flores-Valle. 2021. "Gasification of Psidium guajava L. Waste Using Supercritical Water: Evaluation of Feed Ratio and Moderate Temperatures" Energies 14, no. 9: 2555. https://doi.org/10.3390/en14092555
APA StyleGonzález-Arias, S., Zúñiga-Moreno, A., García-Morales, R., Elizalde-Solis, O., Verónico-Sánchez, F. J., & Flores-Valle, S. O. (2021). Gasification of Psidium guajava L. Waste Using Supercritical Water: Evaluation of Feed Ratio and Moderate Temperatures. Energies, 14(9), 2555. https://doi.org/10.3390/en14092555






