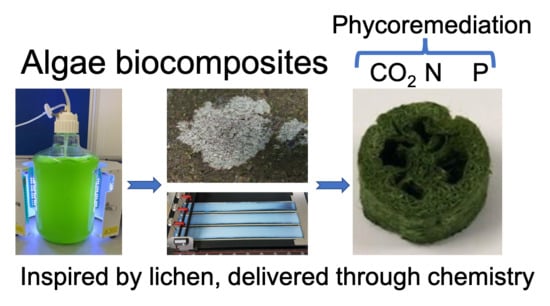
Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing
Abstract
1. Introduction
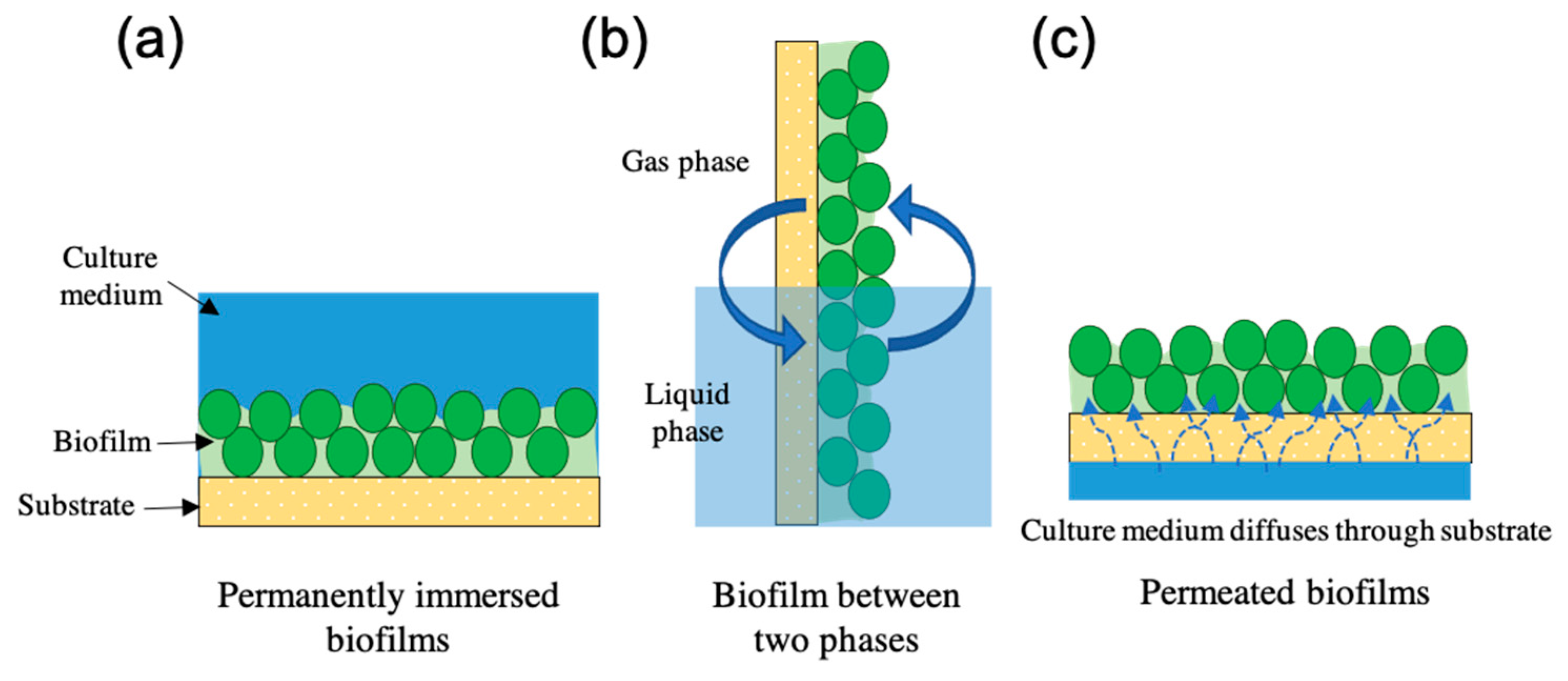
2. Immobilised Cultivation
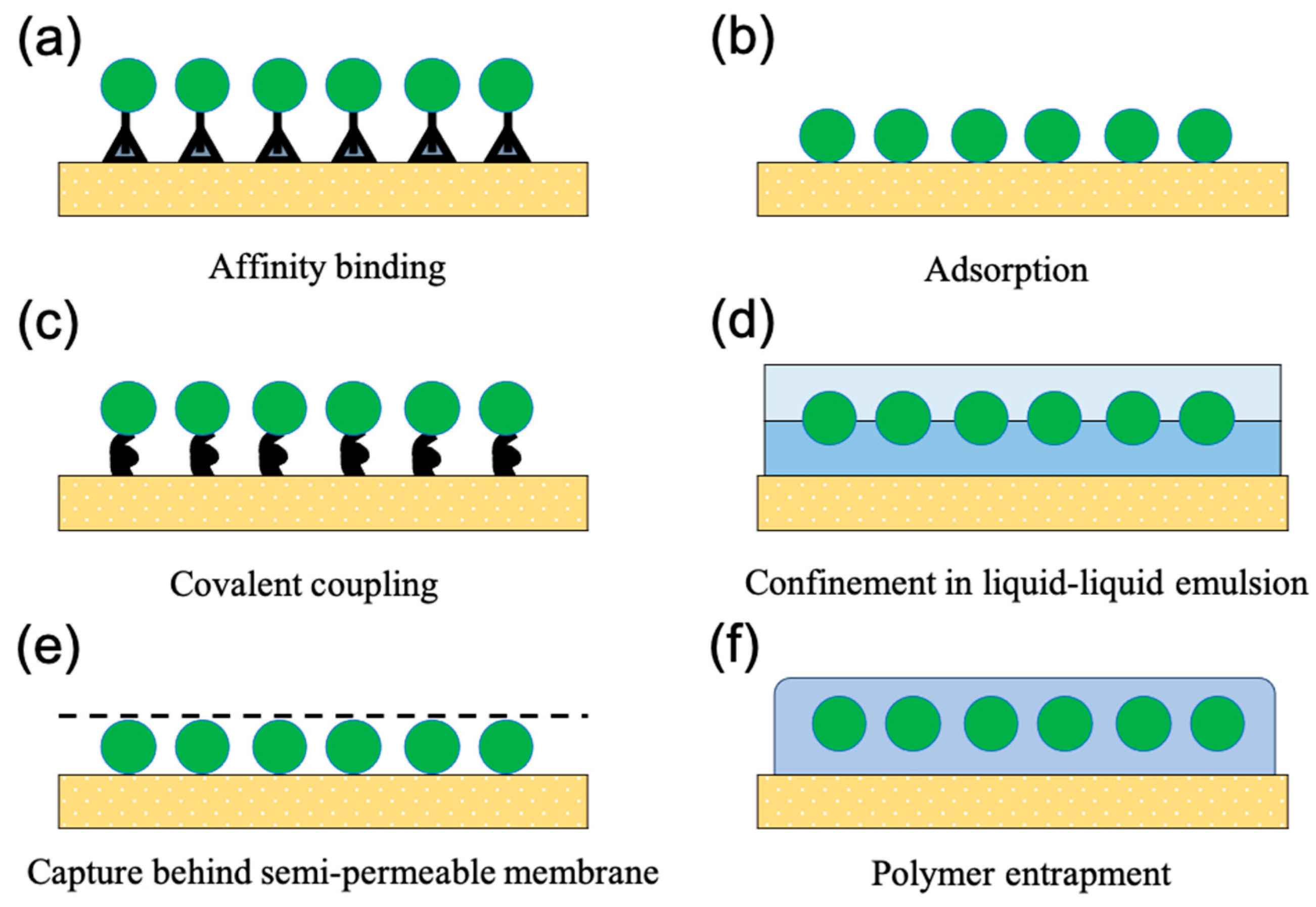
3. Effects of Immobilisation on Microalgae
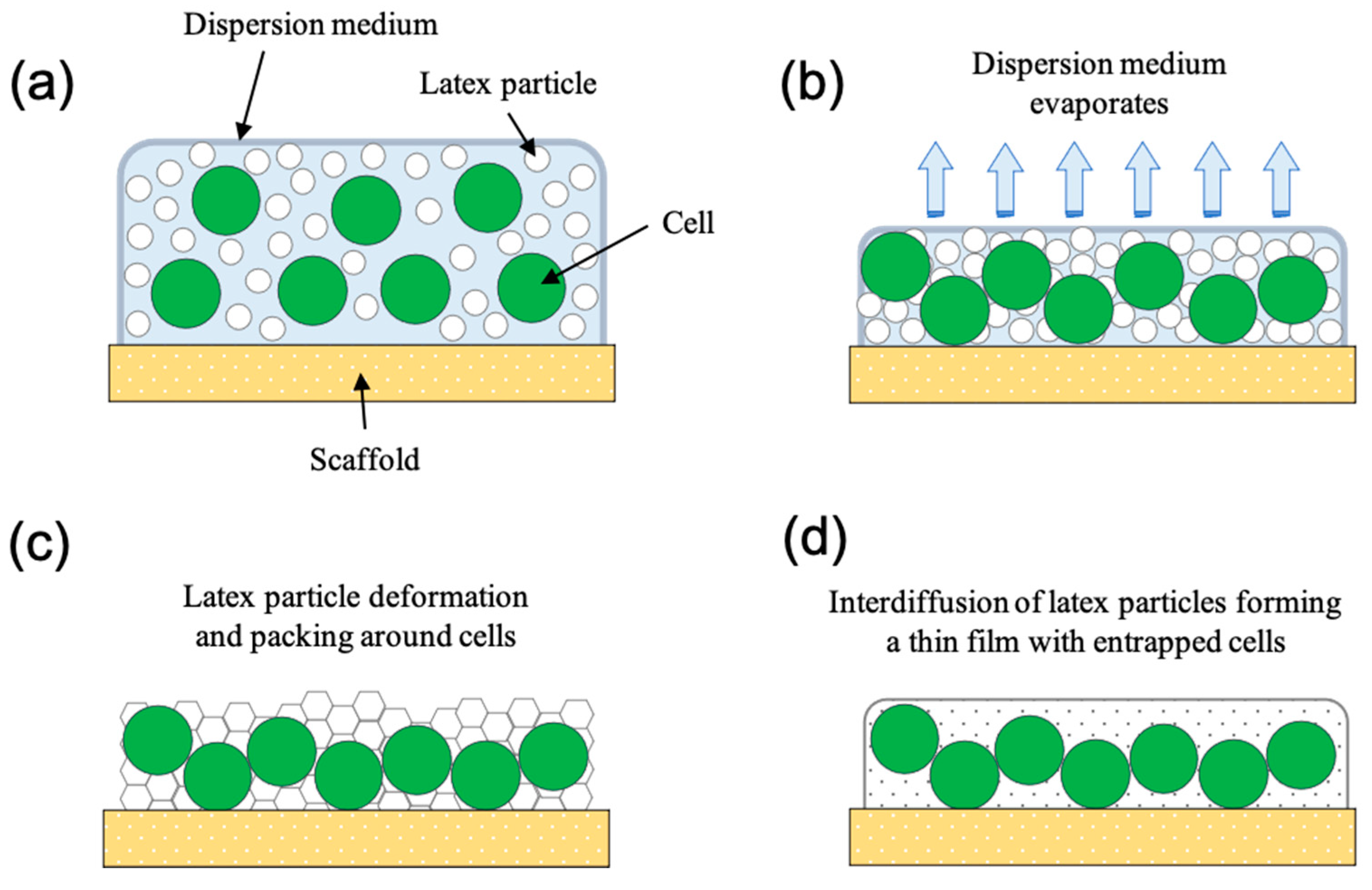
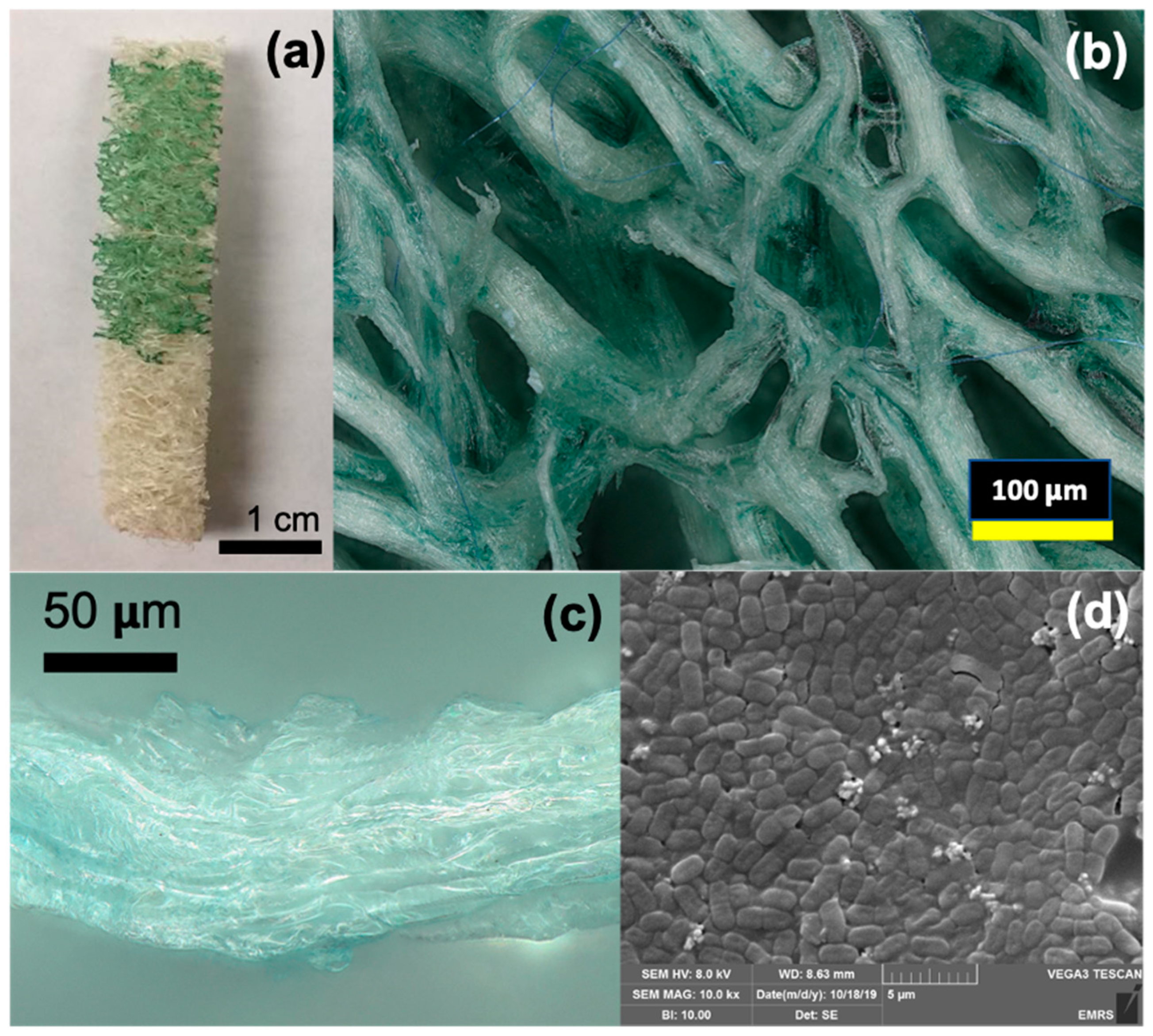
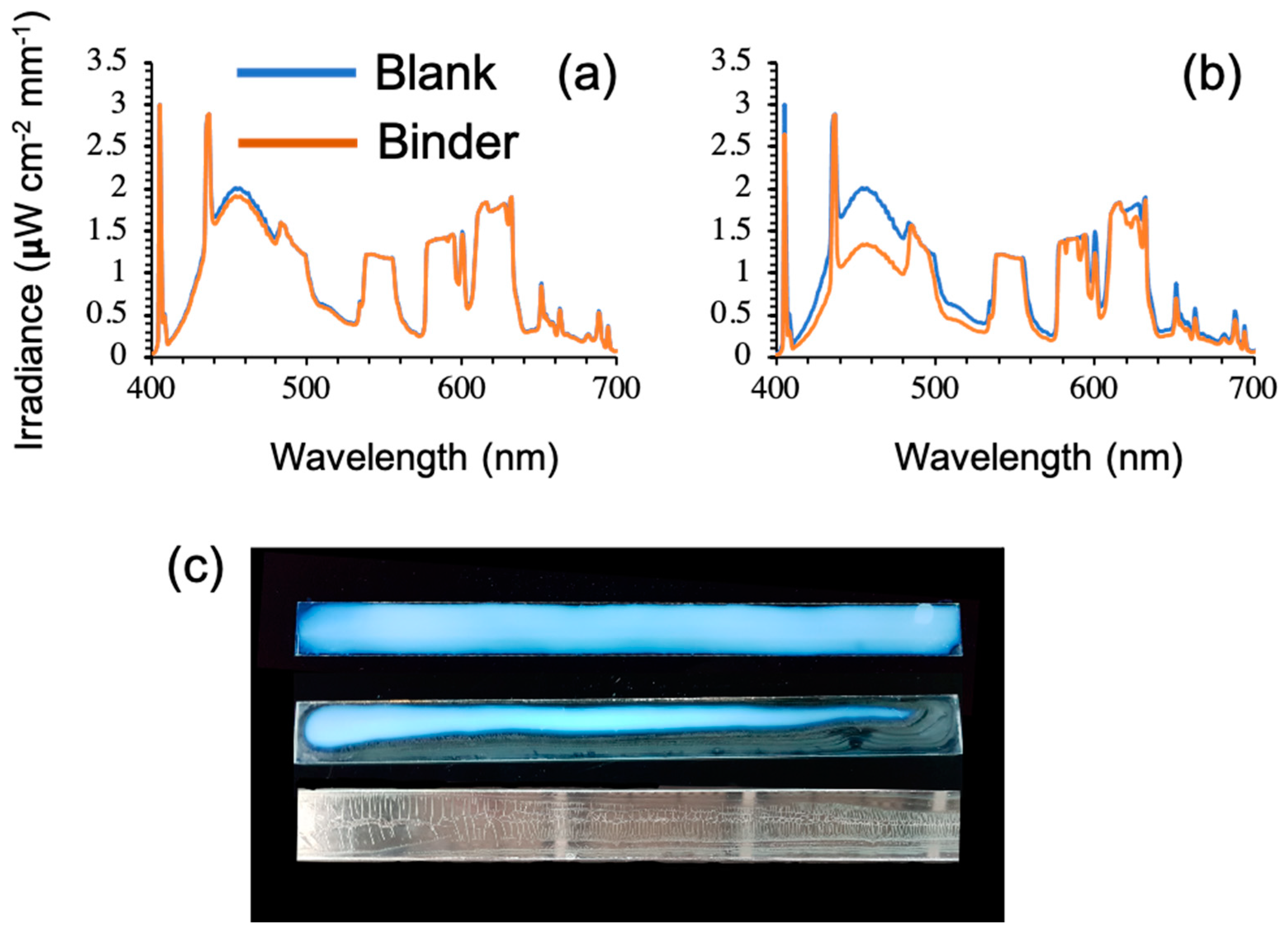
4. Latex Polymer Immobilisation
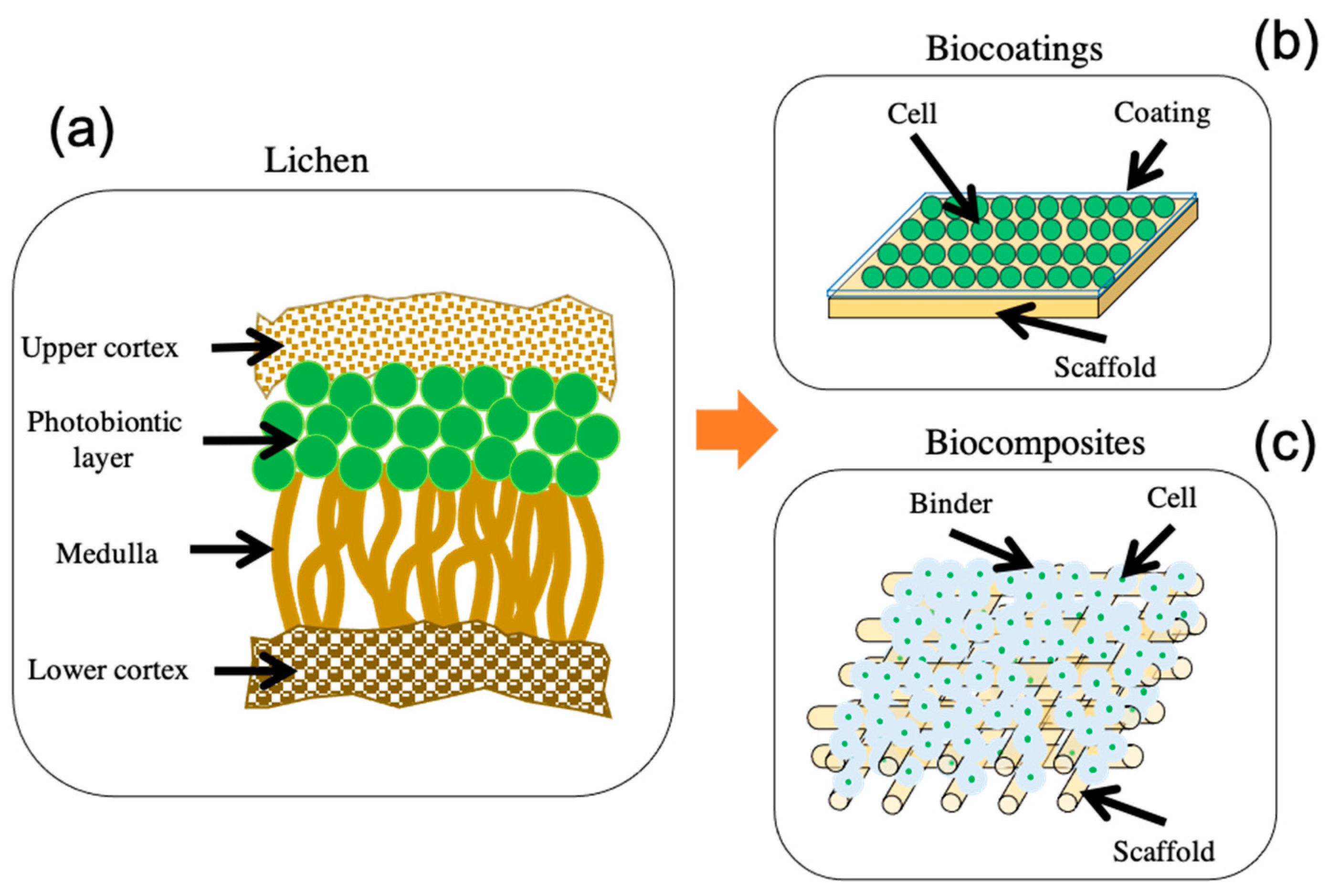
5. Bioinspiration from Lichen
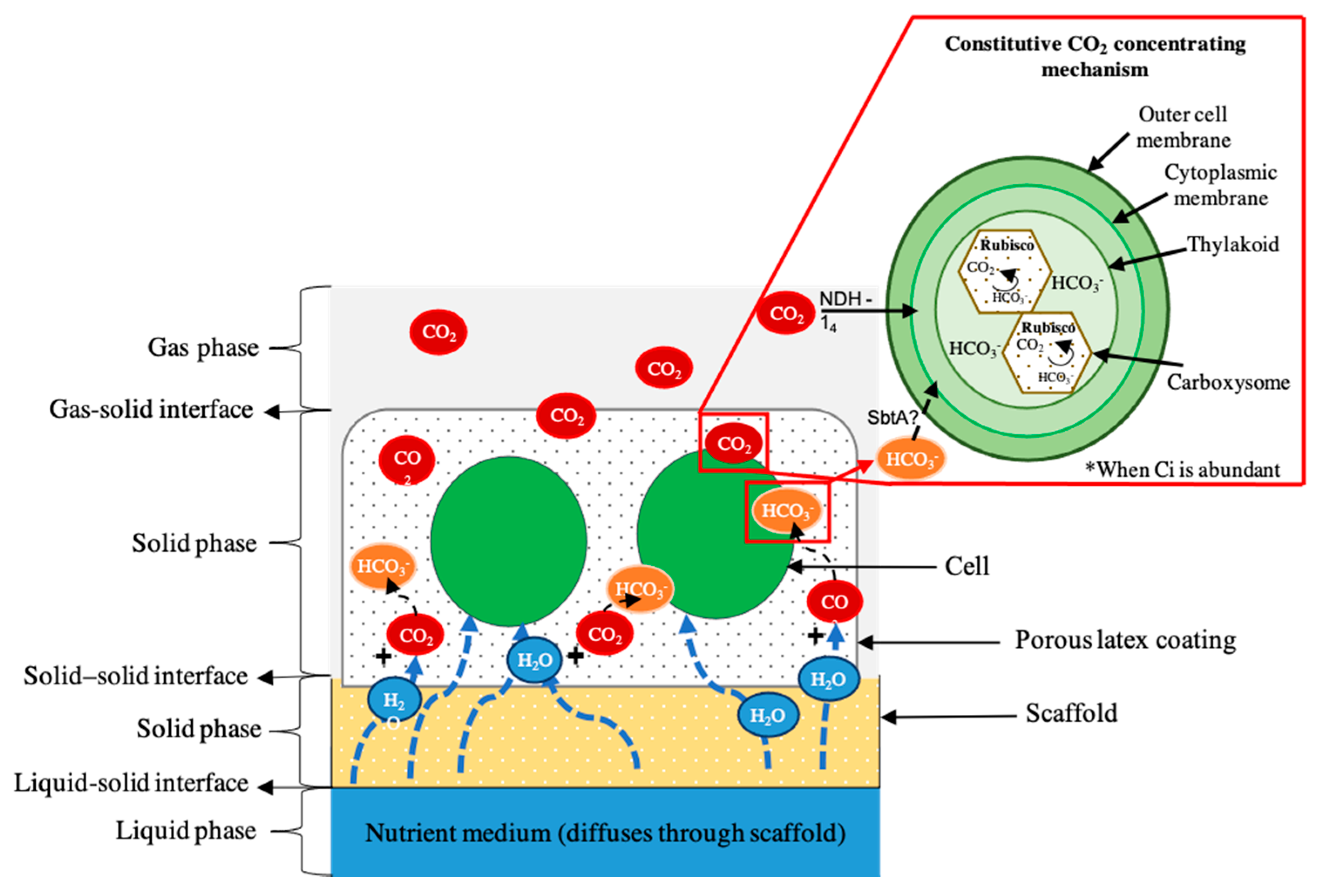
6. Biocomposites as Process Intensification
7. Immobilisation for Synthetic Biology
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Supriyanto Noguchi, R.; Ahamed, T.; Rani, D.S.; Sakurai, K.; Nasution, M.A.; Wibawa, D.S.; Demura, M.; Watanabe, M.M. Artificial neural networks model for estimating growth of polyculture microalgae in an open raceway pond. Biosyst. Eng. 2019, 177, 122–129. [Google Scholar] [CrossRef]
- Chandra, R.; Amit Ghosh, U.K. Effects of various abiotic factors on biomass growth and lipid yield of Chlorella minutissima for sustainable biodiesel production. Environ. Sci. Pollut. Res. 2019, 26, 3848–3861. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.J.; Greenwell, H.C.; Lovitt, R.W.; Shields, R.J. Selection for fitness at the individual or population levels: Modelling effects of genetic modifications in microalgae on productivity and environmental safety. J. Theor. Biol. 2010, 263, 269–280. [Google Scholar] [CrossRef]
- Hidasi, N.; Belay, A. Diurnal variation of various culture and biochemical parameters of Arthrospira platensis in large-scale outdoor raceway ponds. Algal Res. 2018, 29, 121–129. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. Seasonal variation in light utilisation, biomass production and nutrient removal by wastewater microalgae in a full-scale high-rate algal pond. J. Appl. Phycol. 2014, 26, 1317–1329. [Google Scholar] [CrossRef]
- Unnithan, V.V.; Unc, A.; Smith, G.B. Mini-review: A priori considerations for bacteria–algae interactions in algal biofuel systems receiving municipal wastewaters. Algal Res. 2014, 4, 35–40. [Google Scholar] [CrossRef]
- Ugwu, C.; Aoyagi, H.; Uchiyama, H. Photobioreactors for mass cultivation of algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Delrue, F.; Setier, P.-A.; Sahut, C.; Cournac, L.; Roubaud, A.; Peltier, G.; Froment, A.-K. An economic, sustainability, and energetic model of biodiesel production from microalgae. Bioresour. Technol. 2012, 111, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.W.; Johnson, M.D.; Outlaw, J.L. Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res. 2012, 1, 93–100. [Google Scholar] [CrossRef]
- de Godos, I.; Mendoza, J.L.; Acien, F.G.; Molina, E.; Banks, C.J.; Heaven, S.; Rogalla, F. Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresour. Technol. 2014, 153, 307–314. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Liu, T. Biofilm based attached cultivation technology for microalgal biorefineries-A review. Bioresour. Technol. 2017, 244 Pt 2, 1245–1253. [Google Scholar] [CrossRef]
- Mantzorou, A.; Ververidis, F. Microalgal biofilms: A further step over current microalgal cultivation techniques. Sci. Total Environ. 2019, 651 Pt 2, 3187–3201. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, C.; Chen, W.; Xu, X. Attached culture of Nannochloropsis oculata for lipid production. Bioprocess. Biosyst. Eng. 2014, 37, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, J.; Hu, Q.; Cheng, P.; Ji, B.; Liu, J.; Chen, Y.; Zhang, W.; Chen, X.; Chen, L.; et al. Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresour. Technol. 2013, 127, 216–222. [Google Scholar] [CrossRef]
- Rincon, S.M.; Romero, H.M.; Aframehr, W.M.; Beyenal, H. Biomass production in Chlorella vulgaris biofilm cultivated under mixotrophic growth conditions. Algal Res. 2017, 26, 153–160. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Guo, D.; Ye, T.; Xiong, M.; Zhu, L.; Liu, C.; Jin, S.; Hu, Z. Operation of a vertical algal biofilm enhanced raceway pond for nutrient removal and microalgae-based byproducts production under different wastewater loadings. Bioresour. Technol. 2018, 253, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Q.; Wang, J.-H.; Zhang, T.-Y.; Dao, G.-H.; Wu, G.-X.; Hu, H.-Y. Attached microalgae cultivation and nutrients removal in a novel capillary-driven photo-biofilm reactor. Algal Res. 2017, 27, 198–205. [Google Scholar] [CrossRef]
- Martín-Girela, I.; Curt, M.D.; Fernández, J. Flashing light effects on CO2 absorption by microalgae grown on a biofilm photobioreactor. Algal Res. 2017, 25, 421–430. [Google Scholar] [CrossRef]
- Martins, S.C.S.; Martins, C.M.; Fiúza, L.M.C.G.; Santaella, S.T. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol. 2013, 12, 4412–4418. [Google Scholar]
- Hamano, H.; Nakamura, S.; Hayakawa, J.; Miyashita, H.; Harayama, S. Biofilm-based photobioreactor absorbing water and nutrients by capillary action. Bioresour. Technol. 2017, 223, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Schultze, L.K.P.; Simon, M.-V.; Li, T.; Langenbach, D.; Podola, B.; Melkonian, M. High light and carbon dioxide optimize surface productivity in a twin-layer biofilm photobioreactor. Algal Res. 2015, 8, 37–44. [Google Scholar] [CrossRef]
- Travieso, L.; Benitez, F.; Weiland, P.; Sanchez, E.; Dupeyron, R.; Dominguez, A.R. Experiments on immobilization of microalgae for nutrient removal in wastewater treatments. Bioresour. Technol. 1996, 55, 181–186. [Google Scholar] [CrossRef]
- Thepenier, C.; Gudin, C. Immobilization of Porphyridium cruentum in polyurethane foams for the production of polysaccharide. Biomass 1985, 7, 225–240. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.M.; Jo, B.H.; Lee, S.A.; Shin, S.Y.; Kim, H.S.; Lee, S.H.; Ahn, C.Y. Higher biomass productivity of microalgae in an attached growth system, using wastewater. J. Microbiol. Biotechnol. 2014, 24, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, A.; Kinney, K.; Katz, L.; Berberoglu, H. Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour. Technol. 2012, 114, 542–548. [Google Scholar] [CrossRef]
- Hoh, D.; Watson, S.; Kan, E. Algal biofilm reactors for integrated wastewater treatment and biofuel production: A review. Chem. Eng. J. 2016, 287, 466–473. [Google Scholar] [CrossRef]
- Gross, M.; Henry, W.; Michael, C.; Wen, Z. Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour. Technol. 2013, 150, 195–201. [Google Scholar] [CrossRef]
- Christenson, L.B.; Sims, R.C. Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol. Bioeng. 2012, 109, 1674–1684. [Google Scholar] [CrossRef]
- Akhtar, N.; Iqbal, J.; Iqbal, M. Removal and recovery of nickel(II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: Characterization studies. J. Hazard. 2004, 108, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Saeed, A.; Iqbal, M. Chlorella sorokiniana immobilized on the biomatrix of vegetable sponge of Luffa cylindrica: A new system to remove cadmium from contaminated aqueous medium. Bioresour. Technol. 2003, 88, 163–165. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Berner, F.; Heimann, K.; Sheehan, M. Microalgal biofilms for biomass production. J. Appl. Phycol. 2014, 27, 1793–1804. [Google Scholar] [CrossRef]
- Gross, M.; Wen, Z. Yearlong evaluation of performance and durability of a pilot-scale Revolving Algal Biofilm (RAB) cultivation system. Bioresour. Technol. 2014, 171, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Blanken, W.; Janssen, M.; Cuaresma, M.; Libor, Z.; Bhaiji, T.; Wijffels, R.H. Biofilm growth of Chlorella sorokiniana in a rotating biological contactor based photobioreactor. Biotechnol. Bioeng. 2014, 111, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Wen, Z. Development of an attached microalgal growth system for biofuel production. Appl. Microbiol. Biotechnol. 2010, 85, 525–534. [Google Scholar] [CrossRef]
- Ouspensky, P.D. The Fourth Way: A Record of Talks and Answers to Questions Based on the Teaching of G.I. Gurdjieff; Routledge & Kegan Paul: London, UK, 1957. [Google Scholar]
- Pinck, S.; Etienne, M.; Dossot, M.; Jorand, F.P.A. A rapid and simple protocol to prepare a living biocomposite that mimics electroactive biofilms. Bioelectrochemistry 2017, 118, 131–138. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N. Biotechnological potential of immobilized algae for wastewater N, P and metal removal: A review. BioMetals 2002. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G. Biotechnology of flavours—The next generation. Biotechnol. Lett. 2009, 31, 1651. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, J.D.; Quezada, M.A.; Hoyos, P.; Simeó, Y.; Hernaiz, M.J.; Alcantara, A.R.; Sinisterra, J.V. Microbial cells as catalysts for stereoselective red–ox reactions. Biotechnol. Adv. 2009, 27, 686–714. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Biosolutions to the energy problem. J. Ind. Microbiol. Biotechnol. 2009, 36, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.N.; Lütz, S.; Würges, K.; Minör, D. Continuous biocatalytic processes. Org. Process. Res. Dev. 2009, 13, 607–616. [Google Scholar] [CrossRef]
- End, N.; Schöning, K.-U. Immobilized biocatalysts in industrial research and production. In Immobilized Catalysts: Solid Phases, Immobilization and Applications; Kirschning, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 273–317. [Google Scholar]
- Flickinger, M.C.; Fidaleo, M.; Gosse, J.; Polzin, K.; Charaniya, S.; Solheid, C.; Lyngberg, O.K.; Laudon, M.; Ge, H.; Schottel, J.L.; et al. Engineering Nanoporous Bioactive Smart Coatings Containing Microorganisms: Fundamentals and Emerging Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009. [Google Scholar]
- Flickinger, M.C.; Schottel, J.L.; Bond, D.R.; Aksan, A.; Scriven, L.E. Painting and printing living bacteria: Engineering nanoporous biocatalytic coatings to preserve microbial viability and intensify reactivity. Biotechnol. Prog. 2007, 23, 2–17. [Google Scholar] [CrossRef]
- Cohan, Y. Biofiltration—The treatment of fluids by microorganisms immobilized into the filter bedding material: A review. Bioresour. Technol. 2001, 77, 257–274. [Google Scholar] [CrossRef]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef]
- Magdeldin, S.; Moser, A. Affinity Chromatography: Principles and Applications; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Chandramohan, D.; Marimuthu, K. A review on natural fibers. Int. J. Res. Rev. Appl. Sci. 2011, 8, 194–206. [Google Scholar]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Holzmeister, I.; Schamel, M.; Groll, J.; Gbureck, U.; Vorndran, E. Artificial inorganic biohybrids: The functional combination of microorganisms and cells with inorganic materials. Acta Biomater. 2018. [Google Scholar] [CrossRef]
- Perullini, M.; Rivero, M.M.; Jobbágy, M.; Mentaberry, A.; Bilmes, S.A. Plant cell proliferation inside an inorganic host. J. Biotechnol. 2007, 127, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N.; Rai, L.C. Removal and assessment of toxicity of Cu and Fe to Anabaena doliolum and Chloreila vulgaris using free and immobilized cells. World J. Microbiol. Biotechnol. 1992, 8, 110–114. [Google Scholar]
- Yashveer, S. Photosynthetic activity, and lipid and hydracarbon production by alginate-immobilized cells of Botryococcus in relation to growth phase. J. Microbiol. Biotechnol. 2003, 13, 687–691. [Google Scholar]
- Pannier, A.; Soltmann, U.; Soltmann, B.; Altenburger, R.; Schmitt-Jansen, M. Alginate/silica hybrid materials for immobilization of green microalgae Chlorella vulgaris for cell-based sensor arrays. J. Mater. Chem. B 2014, 2, 7896–7909. [Google Scholar] [CrossRef] [PubMed]
- Desmet, J.; Meunier, C.F.; Danloy, E.P.; Duprez, M.-E.; Hantson, A.-L.; Thomas, D.; Cambier, P.; Rooke, J.C.; Su, B.-L. Green and sustainable production of high value compounds via a microalgae encapsulation technology that relies on CO2 as a principle reactant. J. Mater. Chem. A 2014, 2, 20560–20569. [Google Scholar] [CrossRef]
- Lode, A.; Krujatz, F.; Brüggemeier, S.; Quade, M.; Schütz, K.; Knaack, S.; Weber, J.; Bley, T.; Gelinsky, M. Green bioprinting: Fabrication of photosynthetic algae-laden hydrogel scaffolds for biotechnological and medical applications. Eng. Life Sci. 2015, 15, 177–183. [Google Scholar] [CrossRef]
- Malik, S.; Hagopian, J.; Mohite, S.; Lintong, C.; Stoffels, L.; Giannakopoulos, S.; Beckett, R.; Leung, C.; Ruiz, J.; Cruz, M.; et al. Robotic extrusion of algae-laden hydrogels for large-scale applications. Glob. Chall. 2020, 4, 1900064. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Smith, S.M.; Raston, C.L. Application of various immobilization techniques for algal bioprocesses. In Biomass and Biofuels from Microalgae. Biofuel and Biorefinery Technologies; Moheimani, N., McHenry, M., de Boer, K., Bahri, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 2, pp. 19–44. [Google Scholar]
- Kuu, W.Y.; Polack, J.A. Improving immobilized biocatalysts by gel phase polymerization. Biotechnol. Bioeng. 1983, 25, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Faafeng, B.A.; van Donk, E.; Källqvist, S.T. In situ measurement of algal growth potential in aquatic ecosystems by immobilized algae. Appl. Phycol. 1994, 6, 301–308. [Google Scholar] [CrossRef]
- Serp, D.; Cantana, E.; Heinzen, C.; Stockar, U.V.; Marison, I.W. Characterization of an encapsulation device for the production of monodisperse alginate beads for cell immobilization. Biotechnol. Bioeng. 2000, 70, 41–53. [Google Scholar] [CrossRef]
- Lau, P.S.; Tam, N.F.Y.; Wong, Y.S. Effect of carrageenan immobilization on the physiological activities of Chlorella vulgaris. Bioresour. Technol. 1998, 63, 115–121. [Google Scholar] [CrossRef]
- Chevalier, P.; de la Noüe, J. Wastewater nutrient removal with microalgae immobilized in carrageenan. Enzym. Microb. Technol. 1985, 7, 621–624. [Google Scholar] [CrossRef]
- McHugh, D.J. A guide to the seaweed industry. In FAO Fisheries Technical Paper; FAO: Rome, Italy, 2003; p. 441. [Google Scholar]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-May, B.; del Pilar Sánchez-Saavedra, M.; Lizardi, J.; Voltolina, D. Growth of Synechococcus sp. immobilized in chitosan with different times of contact with NaOH. J. Appl. Phycol. 2007, 19, 181–183. [Google Scholar] [CrossRef]
- Mallick, N.; Rai, L.C. Removal of inorganic ions from wastewaters by immobilized microalgae. World J. Microbiol. Biotechnol. 1994, 10, 439–443. [Google Scholar] [CrossRef]
- Ekins-Coward, T.; Boodhoo, K.V.K.; Velasquez-Orta, S.; Caldwell, G.; Wallace, A.; Barton, R.; Flickinger, M.C. A microalgae biocomposite-integrated spinning disk bioreactor (SDBR): Toward a scalable engineering approach for bioprocess intensification in light-driven CO2 absorption applications. Ind. Eng. Chem. Res. 2019, 58, 5936–5949. [Google Scholar] [CrossRef]
- Vorlop, K.D.; Klein, J. Entrapment of microbial cells in chitosan. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 135, pp. 259–268. [Google Scholar]
- Castro-Ceseña, A.B.; del Pilar Sánchez-Saavedra, M.; Ruíz-Güereca, D.A. Optimization of entrapment efficiency and evaluation of nutrient removal (N and P) of Synechococcus elongatus in novel core-shell capsules. J. Appl. Phycol. 2016, 28, 2343–2351. [Google Scholar] [CrossRef]
- Cortez, S.; Nicolau, A.; Flickinger, M.C.; Mota, M. Biocoatings: A new challenge for environmental biotechnology. Biochem. Eng. J. 2017, 121, 25–37. [Google Scholar] [CrossRef]
- Guy, A. The science and art of paint formulation. Chem. Phys. Coat. 2004, 317–346. [Google Scholar] [CrossRef]
- Lyngberg, O.K.; Stemke, D.J.; Schottel, J.L.; Flickinger, M.C. A single-use luciferase-based mercury biosensor using Escherichia coli HB101 immobilized in a latex copolymer film. J. Ind. Microbiol. Biotechnol. 1999, 23, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.S.; Flickinger, M.C.; Velev, O.D. Deposition of composite coatings from particle-particle and particle-yeast blends by convective-sedimentation assembly. J. Colloid Interface Sci. 2012, 380, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Price, K.; Wu, W.; McCormick, A.V.; Francis, L.F. Measurements of stress development in latex coatings. In Protective Coatings: Film Formation and Properties; Wen, M., Dušek, K., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 225–240. [Google Scholar]
- Bunning, T.J.; Lawton, C.W.; Klei, H.E.; Sundstrom, D.W. Physical property improvements of a pellicular biocatalyst. Bioprocess. Eng. 1991, 7, 71–75. [Google Scholar] [CrossRef]
- Flickinger, M.C.; Bernal, O.I.; Schulte, M.J.; Broglie, J.J.; Duran, C.J.; Wallace, A.; Mooney, C.B.; Velev, O.D. Biocoatings: Challenges to expanding the functionality of waterborne latex coatings by incorporating concentrated living microorganisms. J. Coat. Technol. Res. 2017, 14, 791–808. [Google Scholar] [CrossRef]
- Martens, N.; Hall, E.A.H. Immobilisation of photosynthetic cells based on film-forming emulsion polymers. Anal. Chim. Acta 1994, 292, 49–63. [Google Scholar] [CrossRef]
- Bernal, O.I.; Pawlak, J.J.; Flickinger Michael, C. Microbial paper: Cellulose fiber-based photo-absorber producing hydrogen gas from acetate using dry-stabilized Rhodopseudomonas palustris. BioResources 2017, 12, 4013–4030. [Google Scholar] [CrossRef][Green Version]
- Bernal, O.I.; Mooney, C.B.; Flickinger, M.C. Specific photosynthetic rate enhancement by cyanobacteria coated onto paper enables engineering of highly reactive cellular biocomposite “leaves”. Biotechnol. Bioeng. 2014, 111, 1993–2008. [Google Scholar] [CrossRef] [PubMed]
- Lyngberg, O.K.; Ng, C.P.; Thiagarajan, V.; Scriven, L.E.; Flickinger, M.C. Engineering the microstructure and permeability of thin multilayer latex biocatalytic coatings containing E. coli. Biotechnol. Prog. 2001, 17, 1169–1179. [Google Scholar] [CrossRef]
- In-na, P.; Umar, A.A.; Wallace, A.D.; Flickinger, M.C.; Caldwell, G.S.; Lee, J.G.M. Loofah-based microalgae and cyanobacteria biocomposites for intensifying carbon dioxide capture. J. Co2 Util. 2020, 42, 101348. [Google Scholar] [CrossRef]
- Wicks, J.Z.W.; Jones, F.; Peppas, S. Organic coatings: Science and technology volume 1: Film formation, components and appearance. Dry. Technol. 1993, 11, 1477. [Google Scholar] [CrossRef]
- Reyes, Y.; Campos-Terán, J.; Vázquez, F.; Duda, Y. Properties of films obtained from aqueous polymer dispersions: Study of drying rate and particle polydispersity effects. Model. Simul. Mater. Sci. Eng. 2007, 15, 355. [Google Scholar] [CrossRef]
- Chen, X.; Fischer, S.; Men, Y. Temperature and relative humidity dependency of film formation of polymeric latex dispersions. Langmuir 2011, 27, 12807–12814. [Google Scholar] [CrossRef]
- Limousin, E.; Ballard, N.; Asua, J.M. The influence of particle morphology on the structure and mechanical properties of films cast from hybrid latexes. Prog. Org. Coat. 2019, 129, 69–76. [Google Scholar] [CrossRef]
- Mesic, B.; Cairns, M.; Järnstrom, L.; Joo Le Guen, M.; Parr, R. Film formation and barrier performance of latex based coating: Impact of drying temperature in a flexographic process. Prog. Org. Coat. 2019, 129, 43–51. [Google Scholar] [CrossRef]
- Schulte, M.J.; Wiltgen, J.; Ritter, J.; Mooney, C.B.; Flickinger, M.C. A high gas fraction, reduced power, syngas bioprocessing method demonstrated with a Clostridium ljungdahlii OTA1 paper biocomposite. Biotechnol. Bioeng. 2016, 113, 1913–1923. [Google Scholar] [CrossRef]
- Gosse, J.L.; Chinn, M.S.; Grunden, A.M.; Bernal, O.I.; Jenkins, J.S.; Yeager, C.; Kosourov, S.; Seibert, M.; Flickinger, M.C. A versatile method for preparation of hydrated microbial–latex biocatalytic coatings for gas absorption and gas evolution. J. Ind. Microbiol. Biotechnol. 2012, 39, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Bernal, O.I.; Bharti, B.; Flickinger, M.C.; Velev, O.D. Fabrication of photoreactive biocomposite coatings via electric field-assisted assembly of cyanobacteria. Langmuir 2017, 33, 5304–5313. [Google Scholar] [CrossRef]
- Chen, Y.; Krings, S.; Booth, J.R.; Bon, S.A.F.; Hingley-Wilson, S.; Keddie, J.L. Introducing porosity in colloidal biocoatings to increase bacterial viability. Biomacromolecules 2020. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; In-na, P.; Kapralov, M.V.; Lee, J.G.M.; Caldwell, G.S. Textile-based cyanobacteria biocomposites for potential environmental remediation applications. J. Appl. Phycol. 2021. [Google Scholar] [CrossRef]
- Koszewska, M. Circular economy—Challenges for the textile and clothing industry. Autex Res. J. 2018, 18, 337–347. [Google Scholar] [CrossRef]
- Melki, S.; Biguenet, F.; Dupuis, D. Hydrophobic properties of textile materials: Robustness of hydrophobicity. J. Text. Inst. 2019, 110, 1221–1228. [Google Scholar] [CrossRef]
- Khosravi, A.; King, J.A.; Jamieson, H.L.; Lind, M.L. Latex barrier thin film formation on porous substrates. Langmuir 2014, 30, 13994–14003. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.K.; He, F.A.; Fan, J.T. Moisture-responsive fabrics based on the hygro deformation of yarns. Text. Res. J. 2009, 80, 1172–1179. [Google Scholar] [CrossRef]
- Dhiman, R.; Chattopadhyay, R. Absorbency of synthetic urine by cotton nonwoven fabric. J. Text. Inst. 2020, 1–8. [Google Scholar] [CrossRef]
- Alassod, A.; Xu, G. Comparative study of polypropylene nonwoven on structure and wetting characteristics. J. Text. Inst. 2020, 1–8. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Peng, W.; Ozbolat, V. Application areas of 3D bioprinting. Drug Discov. Today 2016, 21, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guo, C.; Kumarasena, A.; Omenetto, F.G.; Kaplan, D.L. 3D printing of functional microalgal silk structures for environmental applications. ACS Biomater. Sci. Eng. 2019, 5, 4808–4816. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Kumar, S.; Srivastava, V.; Mishra, T.; Mishra, B.N. 3D bioprinting in plant science: An interdisciplinary approach. Trends Plant Sci. 2020, 25, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Inna, P.; Caldwell, G.S.; Lee, J.G.M. Living textile biocomposites deliver enhanced carbon dioxide capture. J. Ind. Text. 2021. In press. [Google Scholar]
- Watkinson, S.C. The Fungi; Elsevier Science & Technology: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Honegger, R. Lichen-forming fungi and their photobionts. In The Mycota; Springer: Berlin/Heidelberg, Germany, 2009; pp. 307–333. [Google Scholar]
- Chen, Y.; Su, N.; Zhang, K.; Zhu, S.; Zhu, Z.; Qin, W.; Yang, Y.; Shi, Y.; Fan, S.; Wang, Z.; et al. Effect of fiber surface treatment on structure, moisture absorption and mechanical properties of luffa sponge fiber bundles. Ind. Crops Prod. 2018, 123, 341–352. [Google Scholar] [CrossRef]
- Dautzenberg, F.; Mukherjee, M. Process intensification using multifunctional reactors. Chem. Eng. Sci. 2001, 56, 251–267. [Google Scholar] [CrossRef]
- Joshi, S.; Gogate, P. Process intensification of biofuel production from microalgae. In Energy from Microalgae; Springer: Berlin/Heidelberg, Germany, 2018; pp. 59–87. [Google Scholar]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Cho, S.M.; Park, Y.I.; Los, D.A.; Pronina, N.A. CO2-concentrating mechanism in cyanobacterial photosynthesis: Organization, physiological role, and evolutionary origin. Photosynth. Res. 2013, 117, 133–146. [Google Scholar] [CrossRef]
- Bitog, J.P.; Lee, I.-B.; Lee, C.-G.; Kim, K.-S.; Hwang, H.-S.; Hong, S.-W.; Seo, I.-H.; Kwon, K.-S.; Mostafa, E. Application of computational fluid dynamics for modeling and designing photobioreactors for microalgae production: A review. Comput. Electron. Agric. 2011, 76, 131–147. [Google Scholar] [CrossRef]
- Dangi, A.K.; Sharma, B.; Hill, R.T.; Shukla, P. Bioremediation through microbes: Systems biology and metabolic engineering approach. Crit. Rev. Biotechnol. 2019, 39, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-H.; Camsund, D.; Lindblad, P.; Heidorn, T. Design and characterization of molecular tools for a synthetic biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res. 2010, 38, 2577–2593. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Gerotto, C.; Norici, A.; Giordano, M. Toward enhanced fixation of CO2 in aquatic biomass: Focus on microalgae. Front. Energy Res. 2020, 8, 213. [Google Scholar] [CrossRef]
- Dana, G.V.; Kuiken, T.; Rejeski, D.; Snow, A.A. Four steps to avoid a synthetic-biology disaster. Nature 2012, 483, 29. [Google Scholar] [CrossRef]
- Gressel, J.; van der Vlugt, C.J.; Bergmans, H.E. Cultivated microalgae spills: Hard to predict/easier to mitigate risks. Trends Biotechnol. 2014, 32, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. Government regulation of the uses of genetically modified algae and other microorganisms in biofuel and bio-based chemical production. In Algal Biorefineries; Springer: Berlin/Heidelberg, Germany, 2015; pp. 23–60. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldwell, G.S.; In-na, P.; Hart, R.; Sharp, E.; Stefanova, A.; Pickersgill, M.; Walker, M.; Unthank, M.; Perry, J.; Lee, J.G.M. Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing. Energies 2021, 14, 2566. https://doi.org/10.3390/en14092566
Caldwell GS, In-na P, Hart R, Sharp E, Stefanova A, Pickersgill M, Walker M, Unthank M, Perry J, Lee JGM. Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing. Energies. 2021; 14(9):2566. https://doi.org/10.3390/en14092566
Chicago/Turabian StyleCaldwell, Gary S., Pichaya In-na, Rachel Hart, Elliot Sharp, Assia Stefanova, Matthew Pickersgill, Matthew Walker, Matthew Unthank, Justin Perry, and Jonathan G. M. Lee. 2021. "Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing" Energies 14, no. 9: 2566. https://doi.org/10.3390/en14092566
APA StyleCaldwell, G. S., In-na, P., Hart, R., Sharp, E., Stefanova, A., Pickersgill, M., Walker, M., Unthank, M., Perry, J., & Lee, J. G. M. (2021). Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing. Energies, 14(9), 2566. https://doi.org/10.3390/en14092566