Applications of Carbon in Rechargeable Electrochemical Power Sources: A Review
Abstract
1. Introduction
2. Lead–Acid Batteries
3. Lithium-Ion Batteries
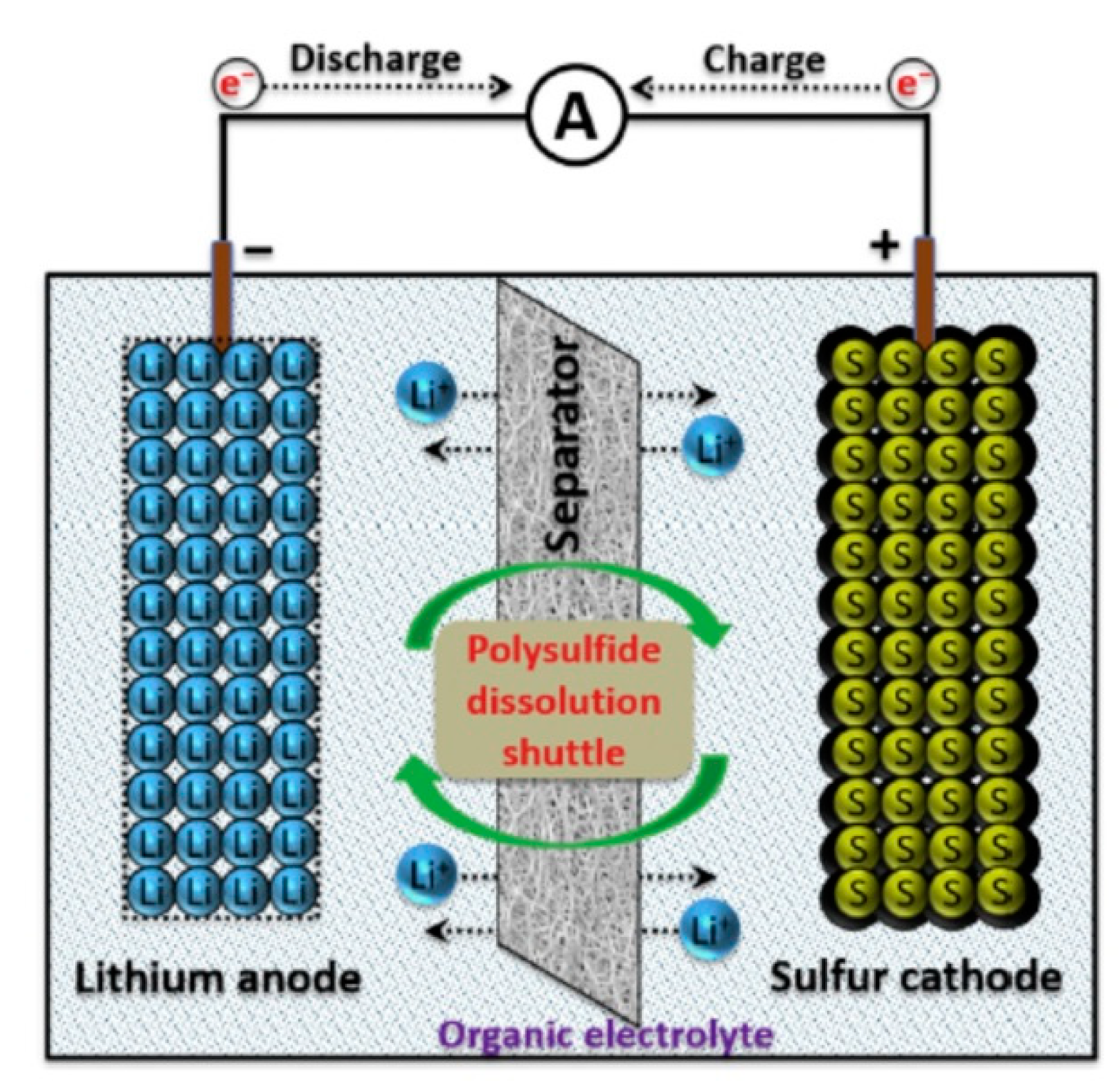
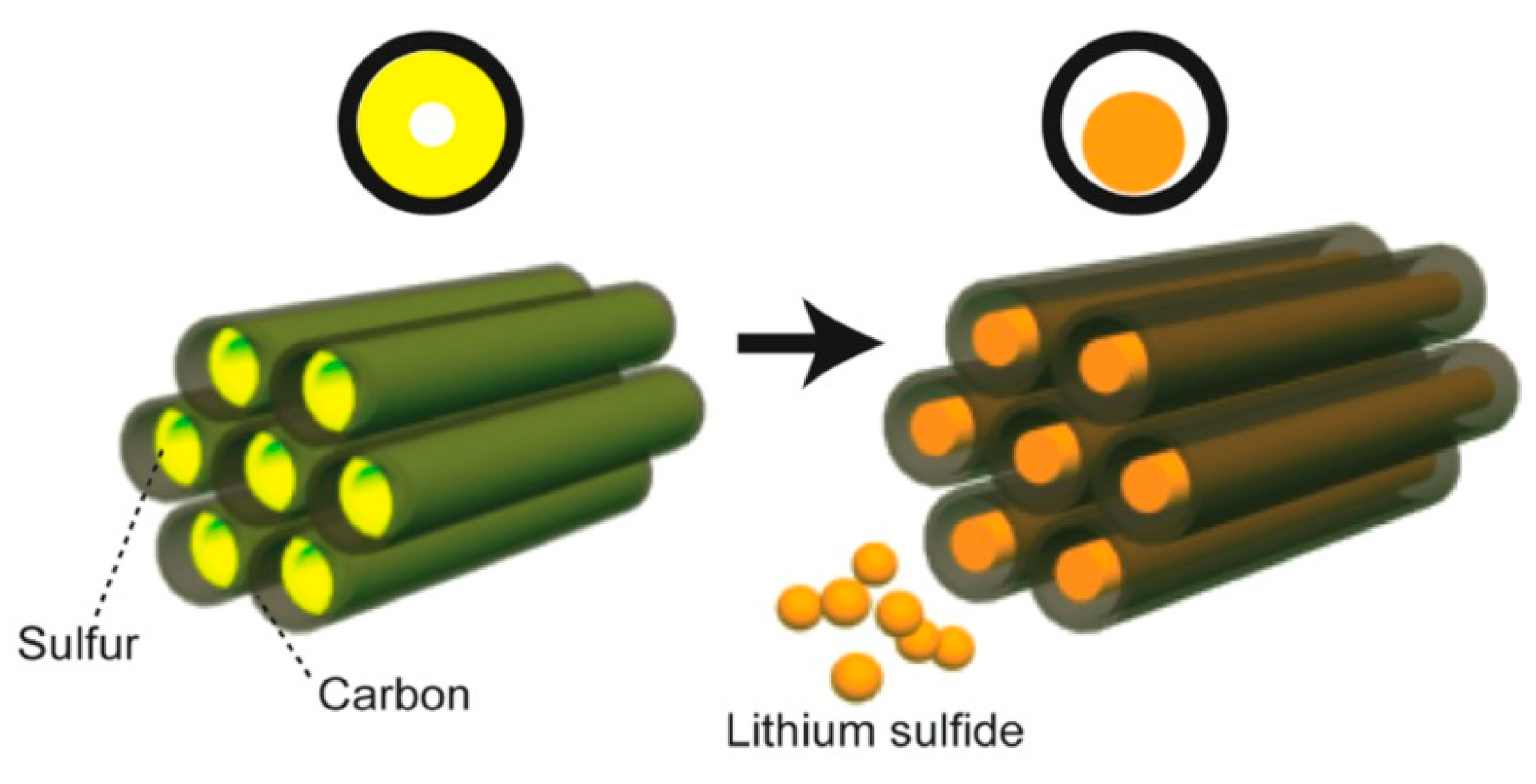
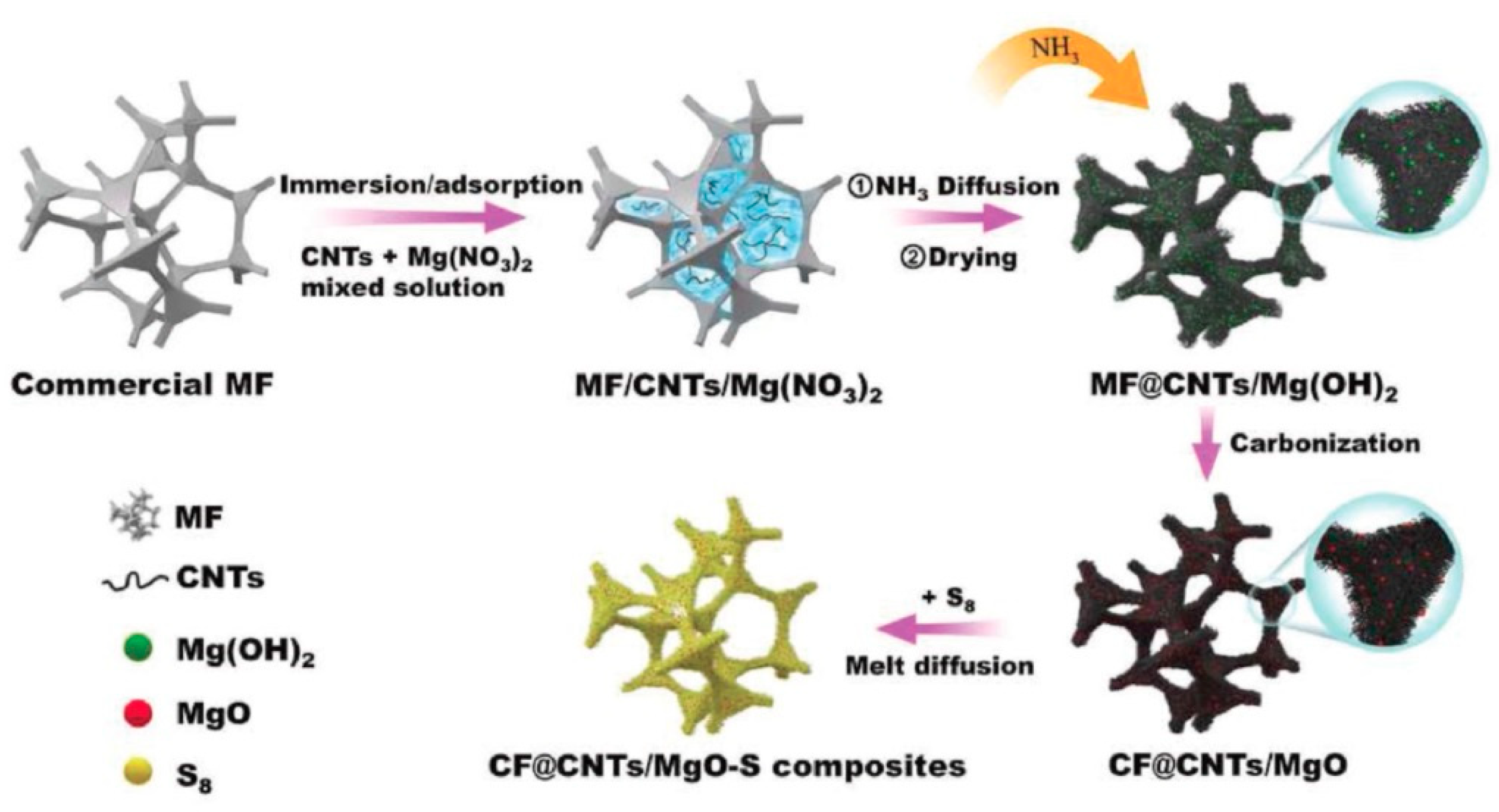
4. Lithium–Sulfur Batteries
5. Sodium-Ion Batteries
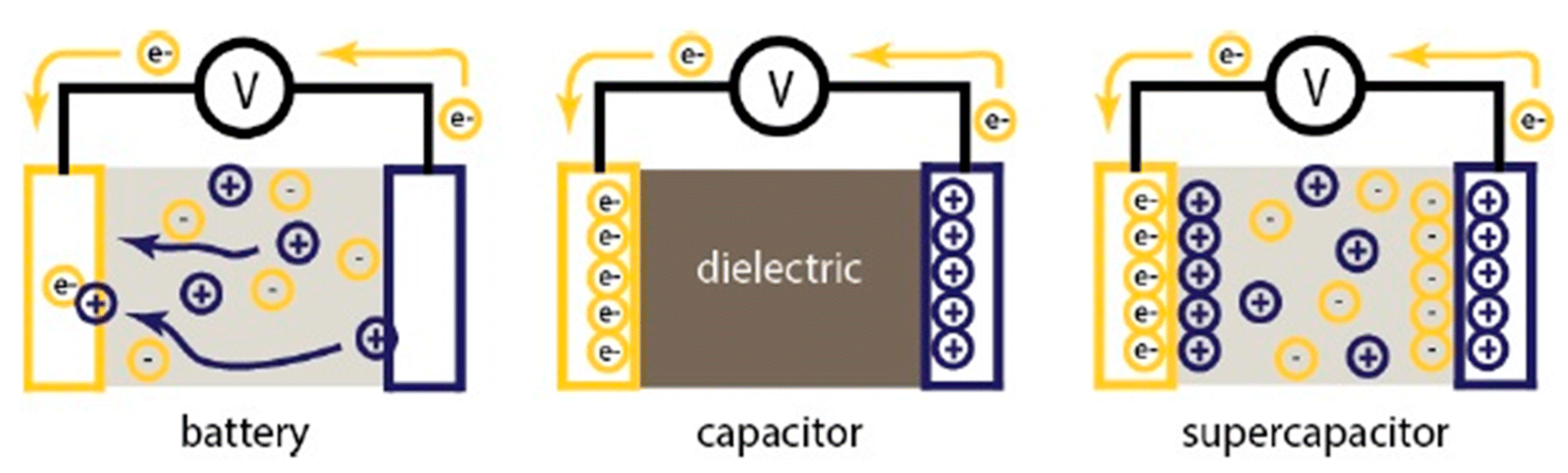
6. Supercapacitors
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs. Available online: https://population.un.org/wpp/Download/Standard/Population/ (accessed on 11 March 2021).
- An Official Website of the European Union. Available online: https://ec.europa.eu/clima/policies/strategies/2050_en (accessed on 11 March 2021).
- May, G.J.; Davidson, A.; Monahov, B. Lead Batteries for Utility Energy Storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, L.; Cheng, H.M. Carbon-Based Fibers for Advanced Electrochemical Energy Storage Devices. Chem. Rev. 2020, 120, 2811–2878. [Google Scholar] [CrossRef] [PubMed]
- Pethaiah, S.S.; Kumar, J.A.; Kalyani, P. Improvement in the Discharge Characteristics of Zinc–Carbon Primary Cells: A Comparative Study with Various Carbon Additives. Ionics 2011, 17, 339–342. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Liu, C.; Wang, S.; Zhou, P.; Zhou, T.; Miao, Z.; Xing, W.; Zhuo, S.; Zhou, J. Fluorinated Multi-Walled Carbon Nanotubes as Cathode Materials of Lithium and Sodium Primary Batteries: Effect of Graphitization of Carbon Nanotubes. J. Mater. Chem. A 2019, 7, 7128–7137. [Google Scholar] [CrossRef]
- Jayakumar, A. A Comprehensive Assessment on the Durability of Gas Diffusion Electrode Materials in PEM fuel cell stack. Front. Energy 2019, 13, 325–338. [Google Scholar] [CrossRef]
- Banerjee, R.; Bevilacqua, N.; Mohseninia, A.; Wiedemann, B.; Wilhelm, F.; Scholta, J.; Zeis, R. Carbon Felt Electrodes for Redox Flow Battery: Impact of Compression on Transport Properties. J. Energy Storage 2019, 26, 100997. [Google Scholar] [CrossRef]
- Mobasser, S.; Firoozi, A.A. Review of Nanotechnology Applications in Science and Engineering. J. Civ. Eng. Urban. 2016, 6, 84–93. [Google Scholar]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey Jr., J.T.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of Carbon Nanomaterials for Advanced Polymernanocomposites: A Comparison Study Between CNT and Graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef]
- Pillot, C. Current Status and Future Trends of the Global Li-ion Battery Market; Avicenne Energy: London, UK, 2018. [Google Scholar]
- Scopus Abstract & Citation Database. Available online: https://www.scopus.com/ (accessed on 20 April 2021).
- Pillot, C. The Rechargeable Battery Market and Main Trends 2016–2025; Avicenne Energy: Fort Lauderdale, FL, USA, 2017. [Google Scholar]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002; pp. 35.6–35.21. [Google Scholar]
- Srinivasan, V. Batteries for Vehicular Applications. Am. Inst. Phys. 2008, 1044, 283–296. [Google Scholar]
- Morris, M.; Tosunoglu, S. Comparison of Rechargeable Battery Technologies. Proceedings of ASME Early Career Technical Conference, Atlanta, GA, USA, 2–3 November 2012. [Google Scholar]
- Winter, M.; Brood, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar]
- Connolly, D. A Review of Energy Storage Technologies, version 3; University of Limerick: Limerick, Ireland, 2009. [Google Scholar]
- Kurzweil, P.; Garche, J. Overview of Batteries for Future Automobiles. In Lead-Acid Batteries for Future Automobiles; Garche, J., Karden, E., Mosley, P.T., Rand, D.A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-Sulfur Batteries: Advances and Trends. Electrochem 2020, 1, 226–259. [Google Scholar] [CrossRef]
- Mandal, S.; Thangarasu, S.; Thong, P.T.; Kim, S.C.; Shim, J.Y.; Jung, H.Y. Positive Electrode Active Material Development Opportunities through Carbon Addition in the Lead-Acid Batteries: A Recent Progress. J. Power Sources 2021, 485, 229336. [Google Scholar] [CrossRef]
- Xia, M.; Nie, J.; Zhang, Z.; Lu, X.; Wang, Z.L. Suppressing Self-Discharge of Supercapacitors via Electrorheological Effect of Liquid Crystals. Nano Energy 2018, 47, 43–50. [Google Scholar] [CrossRef]
- Pavlov, D. Lead-Acid Batteries: Science and Technology; Elsevier B. V: Amsterdam, The Netherlands, 2011; pp. 22–23. [Google Scholar]
- Lach, J.; Wróbel, K.; Wróbel, J.; Podsadni, P.; Czerwiński, A. Applications of Carbon in Lead-Acid Batteries: A Review. J. Solid State Electrochem. 2019, 23, 693–705. [Google Scholar] [CrossRef]
- Hao, Z.; Xu, X.; Wang, H.; Liu, J.; Yan, H. Review on the Roles of Carbon Materials in Lead-Carbon Batteries. Ionics 2018, 24, 951–965. [Google Scholar] [CrossRef]
- Moseley, P.T.; Rand, D.A.J.; Peters, K. Enhancing the Performance of Lead-Acid Batteries with Carbon—In Pursuit of an Understanding. J. Power Sources 2015, 295, 268–274. [Google Scholar] [CrossRef]
- Moseley, P.T.; Rand, D.A.J.; Davidson, A.; Monahov, B. Understanding the Functions of Carbon in the Negative Active-Mass of the Lead–Acid Battery: A Review of Progress. J. Energy Storage 2018, 19, 272–290. [Google Scholar] [CrossRef]
- Ebner, E.; Burow, D.; Panke, J.; Börger, A.; Feldhoff, A.; Atanassova, P.; Valenciano, J.; Wark, M.; Rühl, E. Carbon Blacks for Lead-Acid Batteries in Micro-Hybrid Applications—Studied by Transmission Electron Microscopy and Raman Spectroscopy. J. Power Sources 2013, 222, 554–560. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Zhang, W.; Cao, G.; Zhao, H.; Yang, Y. Enhancing Cycle Performance of Lead-Carbon Battery Anodes by Lead-Doped Porous Carbon Composite and Graphite Additives. Mater. Lett. 2017, 206, 113–116. [Google Scholar] [CrossRef]
- Blecua, M.; Fatas, E.; Ocon, P.; Gonzalo, B.; Merino, C.; de la Fuente, F.; Valenciano, J.; Trinidad, F. Graphitized Carbon Nanofibers: New Additive for the Negative Active Material of Lead Acid Batteries. Electrochim. Acta 2017, 257, 109–117. [Google Scholar] [CrossRef]
- Mahajan, V.; Bharj, R.S.; Bharj, J. Role of Nano-Carbon Additives in Lead-Acid Batteries: A Review. Bull. Mater. Sci. 2019, 42, 21. [Google Scholar] [CrossRef]
- Marom, R.; Ziv, B.; Banerjee, A.; Cahana, B.; Luski, S.; Aurbach, D. Enhanced Performance of Starter Lighting Ignition Type Lead-Acid Batteries with Carbon Nanotubes as an Additive to the Active Mass. J. Power Sources 2015, 296, 78–85. [Google Scholar] [CrossRef]
- Hu, H.Y.; Xie, N.; Wang, C.; Wu, F.; Pan, M.; Li, H.F.; Wu, P.; Wang, X.D.; Zeng, Z.; Deng, S.; et al. Enhancing the Performance of Motive Power Lead-Acid Batteries by High Surface Area Carbon Black Additives. Appl. Sci. 2019, 9, 186. [Google Scholar] [CrossRef]
- Logeshkumar, S.; Manoharan, R. Influence of Some Nanostructured Materials Additives on the Performance of Lead Acid Battery Negative Electrodes. Electrochim. Acta 2014, 144, 147–153. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, H.; Yu, C.; Cao, X.; Cheng, L.; Li, R.; Yang, S.; Dai, C. Effects of Carbon Additives on the HRPSoC Performance of Lead Carbon Batteries and Their Low Temperature Performance. Int. J. Electrochem. Sci. 2017, 12, 10882–10893. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Zhang, W.; Guod, H.; Cao, G.; Zhao, H.; Yang, Y. A New Nano Lead-Doped Mesoporous Carbon Composite as Negative Electrode Additives for Ultralong-Cyclability Lead-Carbon Batteries. Chem. Eng. J. 2018, 337, 201–209. [Google Scholar] [CrossRef]
- Hong, B.; Jiang, L.; Xue, H.; Liu, F.; Jia, M.; Li, J.; Liu, Y. Characterization of Nano-Lead-Doped Active Carbon and its Application in Lead-Acid Battery. J Power Sources 2014, 270, 332–341. [Google Scholar] [CrossRef]
- Wang, F.; Hu, C.; Lian, J.; Zhou, M.; Wang, K.; Yan, J.; Jiang, K. Phosphorus-Doped Activated Carbon as a Promising Additive for High Performance Lead Carbon Batteries. RSC Adv. 2017, 7, 4174–4178. [Google Scholar] [CrossRef]
- Hong, B.; Yu, X.; Jiang, L.; Xue, H.; Liu, F.; Li, Y.; Liu, Y. Hydrogen Evolution Inhibition with Diethylenetriamine Modification of Activated Carbon for a Lead-Acid Battery. RSC Adv. 2014, 4, 33574–33577. [Google Scholar] [CrossRef]
- Ball, R.J.; Evans, R.; Thacker, E.L.; Stevens, R. Effect of Valve Regulated Lead/Acid Battery Positive Paste Carbon Fibre Additive. J. Mater. Sci. 2003, 38, 3013–3017. [Google Scholar] [CrossRef]
- Hao, H.; Chen, K.; Liu, H.; Wang, H.; Liu, J.; Yang, K.; Yan, H. A Review of the Positive Electrode Additives in Lead-Acid Batteries. Int. J. Electrochem. Sci. 2018, 13, 2329–2340. [Google Scholar] [CrossRef]
- Banerjee, A.; Ziv, B.; Shilina, Y.; Levi, E.; Luski, S.; Aurbach, D. Single-Wall Carbon Nanotube Doping in Lead-Acid Batteries: A New Horizon. ACS Appl. Mater. Interfaces 2017, 9, 3634–3643. [Google Scholar] [CrossRef]
- Shen, H.; Jin, Y.; Zhao, Z.; Sun, Y.; Huang, B.; Wang, J. Preparation of Graphite-Based Lead Carbon Cathode and its Performance of Batteries. Micro Nano Lett. 2019, 14, 915–918. [Google Scholar] [CrossRef]
- Shi, J.; Lin, N.; Wang, Y.; Liu, D.; Lin, H. The Application of Rice Husk-Based Porous Carbon in Positive Electrodes of Lead Acid Batteries. J. Energy Storage 2020, 30, 101392. [Google Scholar] [CrossRef]
- Yin, J.; Lin, N.; Lin, Z.; Wang, Y.; Chen, C.; Shi, J.; Bao, J.; Lin, H.; Feng, S.; Zhang, W. Hierarchical Porous Carbon@PbO1-x Composite for High-Performance Lead-Carbon Battery Towards Renewable Energy Storage. Energy 2020, 193, 116675. [Google Scholar] [CrossRef]
- Czerwiński, A. Sposób Galwanicznego Nanoszenia Ołowiu lub Tlenku Ołowiowego na Przewodzące Materiały Węglowe. RP Patent 167796, 12 May 1995. [Google Scholar]
- Czerwiński, A.; Żelazowska, M. Electrochemical Behavior of Lead Deposited on Reticulated Vitreous Carbon. J. Electroanal. Chem. 1996, 410, 55–60. [Google Scholar] [CrossRef]
- Czerwiński, A.; Żelazowska, M. Electrochemical Behavior of Lead Dioxide Deposited on Reticulated Vitreous carbon (RVC). J. Power Sources 1997, 64, 29–34. [Google Scholar] [CrossRef]
- Czerwiński, A.; Obrębowski, S.; Kotowski, J.; Rogulski, Z.; Skowroński, J.; Bajsert, M.; Przystałowski, M.; Buczkowska-Biniecka, M.; Jankowska, E.; Baraniak, M.; et al. Hybrid Lead-Acid Battery with Reticulated Vitreous Carbon as a Carrier- and Current-Collector of Negative Plate. J. Power Sources 2010, 195, 7530–7534. [Google Scholar] [CrossRef]
- Gyenge, E.; Jung, J.; Mahato, B. Electroplated Reticulated Vitreous Carbon Current Collectors for Lead–Acid Batteries: Opportunities and Challenges. J. Power Sources 2003, 113, 388–395. [Google Scholar] [CrossRef]
- Czerwiński, A.; Rogulski, Z.; Obrębowski, S.; Lach, J.; Wróbel, K.; Wróbel, J. Positive Plate for Carbon Lead-Acid Battery. Int. J. Electrochem. Sci. 2014, 9, 4826–4839. [Google Scholar]
- Czerwiński, A.; Wróbel, J.; Lach, J.; Wróbel, K.; Podsadni, P. The Charging-Discharging Behavior of the Lead-Acid Cell with Electrodes Based on Carbon Matrix. J. Solid State Electrochem. 2018, 22, 2703–2714. [Google Scholar] [CrossRef]
- Kirchev, A.; Serra, L.; Dumenil, S.; Brichard, G.; Alias, M.; Jammet, B.; Vinit, L. Carbon Honeycomb Grids for Advanced Lead-Acid Batteries. Part III: Technology Scale-Up. J. Power Sources 2015, 299, 324–333. [Google Scholar] [CrossRef]
- Jang, Y.I.; Dudney, N.J.; Tiegs, T.N.; Klett, J.W. Evaluation of the Electrochemical Stability of Graphite Foams as Current Collectors for Lead Acid Batteries. J. Power Sources 2006, 161, 1392–1399. [Google Scholar] [CrossRef]
- Ma, L.W.; Chen, B.Z.; Chen, Y.; Yuan, Y. Pitch-Based Carbon Foam Electrodeposited with Lead as Positive Current Collectors for Lead Acid Batteries. J. Appl. Electrochem. 2009, 39, 1609–1615. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Shi, X.C.; Xu, H.; Shang, W.; Yuan, Y.; Xiao, L.P. Preparation and Electrochemical Properties of Pitch-Based Carbon Foam as Current Collectors for Lead Acid Batteries. Electrochim. Acta 2008, 53, 2245–2249. [Google Scholar] [CrossRef]
- Lannelongue, J.; Cugnet, M.; Guillet, N.; Kirchev, A. Electrochemistry of Thin-Plate Lead-Carbon Batteries Employing Alternative Current Collectors. J. Power Sources 2017, 352, 194–207. [Google Scholar] [CrossRef]
- Hariprakash, B.; Gaffoor, S.A. Lead-Acid Cells with Lightweight, Corrosion-Protected, Flexible-Graphite Grids. J. Power Sources 2007, 173, 565–569. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Cheng, J.; Zhang, W.; Cao, G.; Zhao, H.; Yang, Y. Novel Polymer-Graphite Composite Grid as a Negative Current Collector for Lead-Acid Batteries. J. Power Sources 2016, 334, 31–38. [Google Scholar] [CrossRef]
- Kirchev, A.; Kircheva, N.; Perrin, M. Carbon Honeycomb Grids for Advanced Lead-Acid Batteries. Part I: Proof of Concept. J. Power Sources 2011, 196, 8773–8788. [Google Scholar] [CrossRef]
- Kirchev, A.; Dumenil, S.; Alias, M.; Christin, R.; de Mascarel, A.; Perrin, M. Carbon Honeycomb Grids for Advanced Lead-Acid Batteries. Part II: Operation of the Negative Plates. J. Power Sources 2015, 279, 809–824. [Google Scholar] [CrossRef]
- Christie, S.; Wong, Y.S.; Titelman, G.; Abrahamson, J. Lead-Acid Battery Construction. US Patent 9543589, 10 January 2017. [Google Scholar]
- Lam, L.T.; Louey, R. Development of Ultra-Battery for Hybrid-Electric Vehicle Applications. J. Power Sources 2006, 158, 1140–1148. [Google Scholar] [CrossRef]
- Cooper, A.; Furakawa, J.; Lam, L.; Kellaway, M. The UltraBattery—A New Battery Design for a New Beginning in Hybrid Electric Vehicle Energy Storage. J. Power Sources 2009, 188, 642–649. [Google Scholar] [CrossRef]
- Monahov, B. The beneficial Role of Carbon in the Negative Plate of Advanced Lead-Carbon Batteries. Ecs Trans. 2012, 41, 45–69. [Google Scholar] [CrossRef]
- Broussely, M.; Pistoia, G. Industrial Applications of Batteries, 1st ed.; Elsevier: London, UK, 2007; pp. 2–28. [Google Scholar]
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capigila, C. Review on Recent Progress of Nanostructured Anode Materials for Li-ion Batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Ratynski, M.; Hamankiewicz, B.; Krajewski, M.; Boczar, M.; Ziolkowska, D.; Czerwinski, A. Impact of Natural and Synthetic Graphite Milling Energy on Lithium-Ion Electrode Capacity and Cycle Life. Carbon 2019, 145, 82–89. [Google Scholar] [CrossRef]
- Park, C.M.; Choi, W.; Hwa, Y.; Kim, J.H.; Jeong, G.; Sohn, H.J. Characterizations and Electrochemical Behaviors of Disproportionated SiO and its Composite for Rechargeable Li-ion Batteries. J. Mater. Chem. 2010, 20, 4854–4860. [Google Scholar] [CrossRef]
- Pistoia, G. Lithium Batteries—New Materials, Developments and Perspectives; Elsevier: New York, NY, USA, 1994; pp. 1–97. [Google Scholar]
- Chen, Z.; Wang, Q.; Amine, K. Improving the Performance of Soft Carbon for Lithium-Ion Batteries. Electrochim. Acta 2006, 51, 3890–3894. [Google Scholar] [CrossRef]
- Sato, K.; Noguchi, M.; Demachi, A.; Oki, N.; Endo, M. A Mechanism of Lithium Storage in Disordered Carbons. Science 1994, 264, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for Lithium Insertion in Carbonaceous Materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Papanek, P.; Radosavljevic, M.; Fischer, J.E. Lithium Insertion in Disordered Carbon-Hydrogen Alloys: Intercalation vs Covalent Binding. Chem. Mater. 1996, 8, 1519–1526. [Google Scholar] [CrossRef]
- Fujimoto, H.; Tokumitsu, K.; Mubuchi, A.; Chinnasamy, N.; Kasuh, T. The Anode Performance of the Hard Carbon for the Lithium Ion Battery Derived from the Oxygen-Containing Aromatic Precursors. J. Power Sources 2010, 195, 7452–7456. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.L.; Wang, Y.G.; Wang, C.X.; Xia, Y.Y. Pitch Modified Hard Carbons as Negative Materials for Lithium-Ion Batteries. Electrochim. Acta 2012, 74, 1–7. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; Huang, X. Influence of Micropore Structure on Li-Storage Capacity in Hard Carbon Spherules. Solid State Ion. 2005, 176, 1151–1159. [Google Scholar] [CrossRef]
- Rao, X.; Lou, Y.; Chen, J.; Lu, H.; Cheng, B.; Wang, W.; Fang, H.; Li, H.; Zhong, S. Polyacrylonitrile Hard Carbon as Anode of High Rate Capability for Lithium Ion Batteries. Front. Energy Res. 2020, 8, 3. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Wang, C. Spherical Hard Carbon Prepared from Potato Starch Using as Anode Material for Li-ion Batteries. Mater. Lett. 2011, 65, 3368–3370. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.Y.; Li, J.; Zou, Y.L.; Tang, J.J. Study of Nano-Porous Hard Carbons as Anode Materials for Lithium Ion Batteries. Mater. Chem. Phys. 2012, 135, 445–450. [Google Scholar] [CrossRef]
- Zhao, J.; Buldum, A.; Han, J.; Ping, J. First-Principles Study of Li-Intercalated Carbon Nanotube Ropes. Phys. Rev. Lett. 2000, 85, 1706–1709. [Google Scholar] [CrossRef]
- Meunier, V.; Kephart, J.; Roland, C.; Bernholc, J. Ab Initio Investigations of Lithium Diffusion in Carbon Nanotube Systems. Phys. Rev. Lett. 2002, 88, 075506–01. [Google Scholar] [CrossRef] [PubMed]
- Schauermann, C.M.; Ganter, M.J.; Gaustad, G.; Babbitt, C.W.; Raffaelle, R.P.; Landi, B.J. Recycling Single-Wall Carbon Nanotube Anodes from Lithium Ion Batteries. J. Mater. Chem. 2012, 22, 12008–12015. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, C.; Qian, W.; Xie, Q.; Zheng, C.; Kong, C.; Wei, F. Carbon Nanotube Production and Application in Energy Storage. Asia-Pac. J. Chem. Eng. 2013, 8, 234–245. [Google Scholar] [CrossRef]
- Lv, R.; Zou, L.; Gui, X.; Kang, F.; Zhu, Y.; Zhu, H.; Wei, J.; Gu, J.; Wang, K.; Wu, D. High-Yield Bamboo-Shaped Carbon Nanotubes from Cresol for Electrochemical Application. Chem. Commun. 2008, 2046–2048. [Google Scholar] [CrossRef]
- Zhou, J.; Song, H.; Fu, B.; Wu, B.; Chen, X. Synthesis and High-Rate Capability of Quadrangular Carbon Nanotubes with One Open end as Anode Materials for Lithium-Ion Batteries. J. Mater. Chem. 2010, 20, 2794–2800. [Google Scholar] [CrossRef]
- Oktaviano, H.S.; Yamada, K.; Waki, K. Nano-Drilled Multiwalled Carbon Nanotubes: Characterizations and Application for LIB Anode Materials. J. Mater. Chem. 2012, 22, 25167. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon Nanotubes for Lithium Ion Batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- DiLeo, R.A.; Castiglia, A.; Ganter, M.J.; Rogers, R.E.; Cress, C.D.; Raffaelle, R.P.; Landi, B.J. Enhanced Capacity and Rate Capability of Carbon Nanotube Based Anodes with Titanium Contacts for Lithium Ion Batteries. ACS Nano 2010, 4, 6121–6131. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Zhang, X.; Luo, B.; Jin, M.; Liang, M.; Dayeh, S.A.; Picraux, S.T.; Zhi, L. Adaptable Silicon-Carbon Nanocables Sandwiched between Reduced Graphene Oxide Sheets as Lithium Ion Battery Anodes. ACS Nano 2013, 7, 1437–1445. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, L.; Toprakci, O.; Liang, Y.; Alcoutlabi, M. Electrospun Nanofiber-Based Anodes, Cathodes, and Separators for Advanced Lithium-Ion Batteries. Polym. Rev. 2011, 51, 239–264. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, Y.; Wang, J.; Jiang, K.; Fan, S. Conformal Fe3O4 Sheath on Aligned Carbon Nanotube Scaffolds as High-Performance Anodes for Lithium Ion Batteries. Nano Lett. 2013, 13, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, P.; Julien, C.; Islam, S.S. Carbon Nanotubes in Li-ion Batteries: A Review. Mater. Sci. Eng. B 2016, 213, 12–40. [Google Scholar] [CrossRef]
- Chen, S.; Shen, L.; van Aken, P.A.; Maier, J.; Yu, Y. Dual-Functionalized Double Carbon Shells Coated Silicon Nanoparticles for High Performance Lithium-Ion Batteries. Adv. Mater. 2017, 1605650. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. Rational Design of Three-Dimensional Graphene Encapsulated with Hollow FeP@Carbon Nanocomposite as Outstanding Anode Material for Lithium Ion and Sodium Ion Batteries. ACS Nano 2017, 11, 11602–11616. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Hu, L.; Cheng, C.; Sun, K.; Guo, X.; Shao, Q.; Li, J.; Wang, N.; Guo, Z. Nano-TiNb2O7/Carbon Nanotubes Composite Anode for Enhanced Lithium-Ion Storage. Electrochim. Acta 2018, 260, 65–72. [Google Scholar] [CrossRef]
- Pan, D.; Wang, S.; Zhao, B.; Wu, M.; Zhang, H.; Wang, Y.; Jiao, Z. Li Storage Properties of Disordered Graphene Nanosheets. Chem. Mater. 2009, 21, 3136–3142. [Google Scholar] [CrossRef]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-Based Electrochemical Energy Conversion and Storage: Fuel Cells, Supercapacitors and Lithium Ion Batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef]
- Hwang, H.J.; Koo, J.; Park, M.; Park, N.; Kwon, Y.; Lee, H. Multilayer Graphynes for Lithium Ion Battery Anode. J. Phys. Chem. C 2013, 117, 6919–6923. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. Large Reversible Capacity of High Quality Graphene Sheets as an Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2010, 55, 3909–3914. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Wang, H.G.; Wu, Z.; Zhang, X.B. In Situ Fabrication of Porous Graphene Electrodes for High-Performance Energy Storage. ACS Nano 2013, 7, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Vinayan, B.P.; Ramaprabhu, S. Facile Synthesis of SnO2 Nanoparticles Dispersed Nitrogen Doped Graphene Anode Material for Ultrahigh Capacity Lithium Ion Battery Applications. J. Mater. Chem. A 2013, 1, 3865–3871. [Google Scholar] [CrossRef]
- Hu, A.; Chen, X.; Tang, Y.; Tang, Q.; Yang, L.; Zhang, S. Self-assembly of Fe3O4 Nanorods on Graphene for Lithium Ion Batteries with High Rate Capacity and Cycle Stability. Electrochem. Commun. 2013, 28, 139–142. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Liu, M.; Shao, Z. A Comprehensive Review of Li4Ti5O12-based Electrodes for Lithium-Ion Batteries: The latest Advancements and Future Perspectives. Mater. Sci. Eng. R 2015, 98, 1–71. [Google Scholar] [CrossRef]
- Fu, R.; Zhou, X.; Fan, H.; Blaisdell, D.; Jagadale, A.; Zhang, X.; Xiong, R. Comparison of Lithium-Ion Anode Materials Using an Experimentally Verified Physics-Based Electrochemical Model. Energies 2017, 10, 2174. [Google Scholar] [CrossRef]
- Liu, J.; Wei, A.X.; Chen, M.; Xia, X. Rational synthesis of Li4Ti5O12/N-C Nanotube Arrays as Advanced High-Rate Electrodes for Lithium-Ion Batteries. J. Mater. Chem. A 2018, 6, 3857–3863. [Google Scholar] [CrossRef]
- Wei, A.; Li, W.; Bai, X.; Zhang, L.; Liu, Z.; Wang, Y. A Facile One-Step Solid-State Synthesis of a Li4Ti5O12/Graphene Composite as an Anode Material for High-Power Lithium-Ion Batteries. Solid State Ion. 2019, 329, 110–118. [Google Scholar] [CrossRef]
- Wei, A.; Mu, J.; He, R.; Bai, X.; Liu, Z.; Zhang, L.; Liu, Z.; Wang, Y. Preparation of Li4Ti5O12/Carbon Nanotubes Composites and LiCoO2/ Li4Ti5O12 Full-Cell with Enhanced Electrochemical Performance for High-Power Lithium-Ion Batteries. J. Phys. Chem. Solids 2020, 138, 109303. [Google Scholar] [CrossRef]
- Lan, C.K.; Bao, Q.; Huang, Y.H.; Duh, J.G. Embedding nano-Li4Ti5O12 in Hierarchical Porous Carbon Matrixes Derived from Water Soluble Polymers for Ultra-Fast Lithium Ion Batteries Anodic Materials. J. Alloys Compd. 2016, 673, 336–348. [Google Scholar] [CrossRef]
- Yao, N.Y.; Liu, H.K.; Liang, X.; Sun, Y.; Feng, X.Y.; Chen, C.H.; Xinag, H.F. Li4Ti5O12 Nanosheets Embedded in Three-Dimensional Amorphous Carbon for Superior-Rate Battery Applications. J. Alloys Compd. 2019, 771, 755–761. [Google Scholar] [CrossRef]
- Stenina, I.; Shaydullin, R.; Kulova, T.; Kuzmina, A.; Tabachkova, N.; Yoroslavtsev, A. Effect of Carbon Additives on the Electrochemical Performance of Li4Ti5O12/C Anodes. Energies 2020, 13, 3941. [Google Scholar] [CrossRef]
- Shen, L.; Lv, H.; Chen, S.; Kopold, P.; van Aken, P.A.; Wu, X.; Maier, J.; Yu, Y. Peapod-like Li3VO4/N-Doped Carbon Nanowires with Pseudocapacitive Properties as Advanced Materials for High-Energy Lithium-Ion Capacitors. Adv. Mater. 2017, 29, 1700142. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Liu, W.; Wang, K.; Li, C.; Li, Z.; Ma, Y. Electrochemical Performances and Capacity Fading Behaviors of Activated Carbon/Hard Carbon Lithium Ion Capacitor. Electrochim. Acta 2017, 235, 158–166. [Google Scholar] [CrossRef]
- Decaux, C.; Lota, G.; Raymoundo-Piñero, E.; Frąckowiak, E.; Béguin, F. Electrochemical Performance of a Hybrid Lithium-Ion Capacitor with a Graphite Anode Preloaded from Lithium Bis(Trifluoromethane)Sulfonimide-Based Electrolyte. Electrochim. Acta 2012, 86, 282–286. [Google Scholar] [CrossRef]
- Brückner, J.; Thieme, S.; Böttger-Hiller, F.; Bauer, I.; Grossmann, H.T.; Strubel, P.; Althues, H.; Spange, S.; Kaskel, S. Carbon-Based Anodes for Lithium Sulfur Full Cells with High Cycle Stability. Adv. Funct. Mater. 2014, 24, 1284–1289. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y. Carbon-Metal Oxide Nanocomposites as Lithium-Sulfur Battery Cathodes. Funct. Mater. Lett. 2018, 11, 1830007. [Google Scholar] [CrossRef]
- Donoro, A.; Cintora-Juarez, D.; Etacheri, V. Carbon Nanomaterials for Rechargeable Lithium-Sulfur Batteries. In Carbon Based Nanomaterials for Advanced Thermal and Electrochemical Energy Storage and Conversion; Paul, R., Etacheri, V., Wang, Y., Lin, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 279–309. [Google Scholar]
- Zhang, X.Q.; Liu, C.; Gao, Y.; Zhang, J.M.; Wang, Y.Q. Research Progress of Sulfur/Carbon Composite Cathode Materials and the Corresponding Safe Electrolytes for Advanced Li-S Batteries. Nano 2020, 15, 2030002. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H.; Park, M.J. Sulfur-Rich Polymers with Functional Linkers for High-Capacity and Fast-Charging Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1802423. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.M. The Regulating Role of Carbon Nanotubes and Graphene in Lithium–Ion and Lithium–Sulfur Batteries. Adv. Mater. 2019, 31, 1800863. [Google Scholar] [CrossRef]
- Sun, J.; Ma, J.; Fan, J.; Pyun, J.; Geng, J. Rational Design of Sulfur-Containing Composites for High-Performance Lithium–Sulfur Batteries. Apl Mater. 2019, 7, 020904. [Google Scholar] [CrossRef]
- Thangavel, R.; Kannan, A.G.; Ponraj, R.; Kaliyappan, K.; Yoon, W.S.; Kim, D.W.; Lee, Y.S. Cinnamon-Derived Hierarchically Porous Carbon as an Effective Lithium Polysulfide Reservoir in Lithium–Sulfur Batteries. Nanomaterials 2020, 10, 1220. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, Y.; Jiang, B.; Lv, Y. Encapsulation of Cathode in Lithium-Sulfur Batteries with a Novel Two-Dimensional Carbon Allotrope: DHP-Graphene. Sci. Rep. 2017, 7, 14948. [Google Scholar] [CrossRef]
- Zhang, B.; Xiao, M.; Wang, S.; Han, D.; Song, S.; Chen, G.; Meng, Y. Novel Hierarchically Porous Carbon Materials Obtained from Natural Biopolymer as Host Matrixes for Lithium−Sulfur Battery Applications. ACS Appl. Mater. Interfaces 2014, 6, 13174–13182. [Google Scholar] [CrossRef]
- Fang, X.; Peng, H. A Revolution in Electrodes: Recent Progress in Rechargeable Lithium–Sulfur Batteries. Small 2015, 11, 1488–1511. [Google Scholar] [CrossRef]
- Li, S.; Jin, B.; Zhai, X.; Li, H.; Jiang, Q. Review of Carbon Materials for Lithium-Sulfur Batteries. ChemistrySelect 2018, 3, 2245–2260. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Wang, Y.; Li, J.; Li, H.; Zeng, J.; Wang, J.; Hwang, B.J.; Zhao, J. High Sulfur-Containing Carbon Polysulfide Polymer as a Novel Cathode Material for Lithium-Sulfur Battery. Sci. Rep. 2017, 7, 11386. [Google Scholar] [CrossRef] [PubMed]
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li-Sulfur Battery Systems: An Integral Perspective. Adv. Energy Mater. 2015, 5, 1500212. [Google Scholar] [CrossRef]
- Mao, Y.; Li, G.; Guo, Y.; Li, Z.; Liang, C.; Peng, X.; Lin, Z. Foldable Interpenetrated Metal-Organic Frameworks/Carbon Nanotubes Thin Film for Lithium–Sulfur Batteries. Nat. Commun. 2017, 8, 14628. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wang, S.; Tao, D.; Yang, Z. Acetylene Black/Sulfur Composites Synthesized by a Solution Evaporation Concentration Crystallization Method and Their Electrochemical Properties for Li/S Batteries. Energies 2013, 6, 3466–3480. [Google Scholar] [CrossRef]
- Fu, A.; Wang, C.; Pei, F.; Cui, J.; Fang, X.; Zhen, N. Recent Advances in Hollow Porous Carbon Materials for Lithium–Sulfur Batteries. Small 2019, 15, 1804786. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Sun, Y.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.; Huang, Y. Insight into the Electrode Mechanism in Lithium-Sulfur Batteries with Ordered Microporous Carbon Confined Sulfur as the Cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, H.; Xia, Y.; Liang, C.; Zhang, W.; Luo, J.; Gan, Y.; Zhang, J.; Tao, X.; Fan, H.J. High-Content of Sulfur Uniformly Embedded in Mesoporous Carbon: A New Electrodeposition Synthesis and an Outstanding Lithium–Sulfur Battery Cathode. J. Mater. Chem. A 2017, 5, 5905–5911. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Zhang, F.; Huang, Y.; Chen, Y. Sulfur-Infiltrated Graphene-Based Layered Porous Carbon Cathodes for High-Performance Lithium Sulfur Batteries. ACS Nano 2014, 8, 5208–5215. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Pei, S.; Qian, X.; Hou, P.X.; Cheng, H.M.; Liu, C.; Li, F. Toward More Reliable Lithium−Sulfur Batteries: An All-Graphene Cathode Structure. ACS Nano 2016, 10, 8676–8682. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Song, H.; Jeong, Y. Flexible catholyte@carbon Nanotube Film Electrode for High-Performance Lithium Sulfur Battery. Carbon 2017, 113, 371–378. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, H.; Fu, C.; Chen, Z.; Xu, C.; Kuang, Y. Chemical Modification of Pristine Carbon Nanotubes and their Exploitation as the Carbon Hosts for Lithium-Sulfur Batteries. Int. J. Hydrog. Energy 2016, 41, 21850–21860. [Google Scholar] [CrossRef]
- Razzaq, A.A.; Yao, Y.; Shah, R.; Qi, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-Performance Lithium Sulfur Batteries Enabled by a Synergy Between Sulfur and Carbon Nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Gueon, D.; Hwang, J.T.; Yang, S.B.; Cho, E.; Sohn, K.; Yang, D.K.; Moon, J.H. Spherical Macroporous Carbon Nanotube Particles with Ultrahigh Sulfur Loading for Lithium–Sulfur Battery Cathodes. ACS Nano 2018, 12, 226–233. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Niu, Z.; Chen, J. Advanced Nanostructured Carbon-Based Materials for Rechargeable Lithium-Sulfur Batteries. Carbon 2019, 141, 400–416. [Google Scholar] [CrossRef]
- Deng, W.; Hu, A.; Chen, X.; Zhang, S.; Tang, Q.; Liu, Z.; Fan, B.; Xiao, K. Sulfur-Impregnated 3D Hierarchical Porous Nitrogen-Doped Aligned Carbon Nanotubes as High-Performance Cathode for Lithium-Sulfur Batteries. J. Power Sources 2016, 322, 138–146. [Google Scholar] [CrossRef]
- Ponraj, R.; Kannan, A.G.; Ahn, J.H.; Lee, J.H.; Kang, J.; Han, B.; Kim, D.W. Effective Trapping of Lithium Polysulfides Using a Functionalized Carbon Nanotube-Coated Separator for Lithium−Sulfur Cells with Enhanced Cycling Stability. ACS Appl. Mater. Interfaces 2017, 9, 38445–38454. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Yan, N.; Zhang, H.; Li, X.; Zhang, H. 1-D Oriented Cross-Linking Hierarchical Porous Carbon Fibers as a Sulfur Immobilizer for High Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2016, 4, 5965–5972. [Google Scholar] [CrossRef]
- Yun, J.H.; Kim, J.H.; Kim, D.K.; Lee, H.W. Suppressing Polysulfide Dissolution via Cohesive Forces by Interwoven Carbon Nanofibers for High-Areal-Capacity Lithium-Sulfur Batteries. Nano Lett. 2018, 18, 475–481. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, W.; Jang, J.; Manthiram, A. Sulfur-Embedded Activated Multichannel Carbon Nanofiber Composites for Long-Life, High-Rate Lithium–Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1601943. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, Q.; Cha, J.J.; Yang, Y.; Li, W.; Seh, Z.W.; Cui, Y. Amphiphilic Surface Modification of Hollow Carbon Nanofibers for Improved Cycle Life of Lithium Sulfur Batteries. Nano Lett. 2013, 13, 1265–1270. [Google Scholar] [CrossRef]
- Gu, X.; Lai, C.; Liu, F.; Yang, W.; Hou, Y.; Zhang, S. A Conductive Interwoven Bamboo Carbon Fiber Membrane for Li–S batteries. J. Mater. Chem. A 2015, 3, 9502–9509. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Zhao, Y.; Tan, T.; Zhang, Y.; Chen, Z. Preparation of Hierarchical Porous Carbon from Waterweed and Its Application in Lithium/Sulfur Batteries. Energies 2018, 11, 1535. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Y.; Zhong, Q.; Lai, D.; Yao, J. Porous Nitrogen-Doped Carbon Derived from Silk Fibroin Protein Encapsulating Sulfur as a Superior Cathode Material for High-Performance Lithium–Sulfur Batteries. Nanoscale 2015, 7, 17791–17797. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, W.; Chen, Y.; Fan, H.; Su, D.; Wang, G. MOF-Derived Porous N–Co3O4@N–C Nanododecahedra Wrapped with Reduced Graphene Oxide as a High Capacity Cathode for Lithium–Sulfur Batteries. J. Mater. Chem. A 2018, 6, 2797–2807. [Google Scholar] [CrossRef]
- Campbell, B.; Bell, J.; Bay, H.H.; Favors, Z.; Ionescu, R.; Ozkan, C.S.; Ozkan, M. SiO2-Coated Sulfur Particles with Mildly Reduced Graphene Oxide as a Cathode Material for Lithium–Sulfur Batteries. Nanoscale 2015, 7, 7051–7055. [Google Scholar] [CrossRef]
- Yu, M.; Ma, J.; Song, H.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R. Atomic Layer Deposited TiO2 on a Nitrogen-Doped Graphene/Sulfur Electrode for High Performance Lithium–Sulfur Batteries. Energy Environ. Sci. 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Su, Z.; Tong, C.J.; He, D.Q.; Lai, C.; Liu, L.M.; Wang, C.; Xi, K. Ultra-small B2O3 Nanocrystals Grown in Situ on Highly Porous Carbon Microtubes for Lithium–Iodine and Lithium–Sulfur Batteries. J. Mater. Chem. A 2016, 4, 8541–8547. [Google Scholar] [CrossRef]
- Tao, Y.; Wei, Y.; Liu, Y.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Kinetically-Enhanced Polysulfide Redox Reactions by Nb2O5 Nanocrystals for High-Rate Lithium–Sulfur Batter. Energy Environ. Sci. 2016, 9, 3230–3239. [Google Scholar] [CrossRef]
- Xie, J.; Li, B.Q.; Peng, H.J.; Song, Y.W.; Zhao, M.; Chen, X.; Zhang, Q.; Huang, J.Q. Implanting Atomic Cobalt within Mesoporous Carbon toward Highly Stable Lithium–Sulfur Batteries. Adv. Mater. 2019, 31, 1903813. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Yan, X.; Su, Q.; Lu, Y.; Qiu, J.; Wang, Z.; Lin, X.; Huang, J.; Liu, R.; et al. Superhierarchical Cobalt-Embedded Nitrogen-Doped Porous Carbon Nanosheets as Two-in-One Hosts for High-Performance Lithium–Sulfur Batteries. Adv. Mater. 2018, 30, 1706895. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Wu, H.; Liu, H.; Huang, J.; Zheng, Y.; Yang, L.; Jing, P.; Zhang, Y.; Dou, S.; Liu, H. A Flexible 3D Multifunctional MgO-Decorated Carbon Foam@CNTs Hybrid as Self-Supported Cathode for High-Performance Lithium-Sulfur Batteries. Adv. Funct. Mater. 2017, 27, 1702573. [Google Scholar] [CrossRef]
- Kim, A.Y.; Kim, M.K.; Kim, J.Y.; Wen, Y.; Gu, L.; Dao, V.D.; Choi, H.S.; Byun, D.; Lee, J.K. Ordered SnO Nanoparticles in MWCNT as A Functional Host Material for High-Rate Lithium-Sulfur Battery Cathode. Nano Res. 2017, 10, 2083–2095. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Z.; Cheng, B.; Chen, R.; Hu, Y.; Ma, L.; Zhu, G.; Liu, J.; Jin, Z. Self-Templated Formation of Interlaced Carbon Nanotubes Threaded Hollow Co3S4 Nanoboxes for High-Rate and Heat-Resistant Lithium−Sulfur Batteries. J. Am. Chem. Soc. 2017, 139, 12710–12715. [Google Scholar] [CrossRef]
- He, J.; Luo, L.; Chen, Y.; Manthiram, A. Yolk–Shelled C@Fe3O4 Nanoboxes as Efficient Sulfur Hosts for High-Performance Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1702707. [Google Scholar] [CrossRef]
- Chen, M.; Lu, Q.; Jiang, S.; Huang, C.; Wang, X.; Wu, B.; Xiang, X.; Wu, Y. MnO2 Nanosheets Grown on the Internal/External Surface of N-Doped Hollow Porous Carbon Nanospheres as the Sulfur Host of Advanced Lithium-Sulfur Batteries. Chem. Eng. J. 2018, 335, 831–842. [Google Scholar] [CrossRef]
- Fang, M.; Chen, Z.; Liu, Y.; Quan, J.; Yang, C.; Zhu, L.; Xu, Q.; Xu, Q. Design and Synthesis of Novel Sandwich-Type C@TiO2@C Hollow Microspheres as Efficient Sulfur Hosts for Advanced Lithium–Sulfur Batteries. J. Mater. Chem. A 2018, 6, 1630–1638. [Google Scholar] [CrossRef]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.B.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen-Doped Mesoporous Carbon Promoted Chemical Adsorption of Sulfur and Fabrication of High-Areal-Capacity Sulfur Cathode with Exceptional Cycling Stability for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, F.; Wang, M.; Zhang, Y.; Ma, Q.; Lin, J.; Feng, Z.; Wang, H. Metal Organic Framework Derivative Improving Lithium Metal Anode Cycling. Adv. Funct. Mater. 2020, 30, 1907579. [Google Scholar] [CrossRef]
- Yao, S.; Guo, R.; Xie, F.; Wu, Z.; Gao, K.; Zhang, C.; Shen, X.; Li, T.; Qin, S. Electrospun Three-Dimensional Cobalt Decorated Nitrogen Doped Carbon Nanofibers Network as Freestanding Electrode for Lithium/Sulfur Batteries. Electrochim. Acta 2020, 337, 135765. [Google Scholar] [CrossRef]
- Chen, K.; Sun, Z.; Fang, R.; Shi, Y.; Cheng, H.M.; Li, F. Metal–Organic Frameworks (MOFs)-Derived Nitrogen-Doped Porous Carbon Anchored on Graphene with Multifunctional Effects for Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707592. [Google Scholar] [CrossRef]
- Yim, T.; Park, M.S.; Yu, J.S.; Kim, K.J.; Im, K.Y.; Kim, J.H.; Jeong, G.; Jo, Y.N.; Woo, S.G.; Kang, K.S.; et al. Effect of chemical reactivity of polysulfide toward carbonate-basedelectrolyte on the electrochemical performance of Li–S batteries. Electrochim. Acta 2013, 107, 454–460. [Google Scholar] [CrossRef]
- Mohan, E.H.; Sarada, B.V.; Naidu, R.V.R.; Salian, G.; Haridasa, A.K.; Rao, B.V.A.; Rao, T.N. Graphene-Modified Electrodeposited Dendritic Porous Tin Structures as Binder Free Anode for High Performance Lithium-Sulfur Batteries. Electrochim. Acta 2016, 219, 701–710. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Dahn, J.R. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803–A811. [Google Scholar] [CrossRef]
- Qiu, S.; Xiao, L.; Sushko, M.L.; Han, K.S.; Shao, Y.; Yan, M.; Liang, X.; Mai, L.; Feng, J.; Cao, Y.; et al. Manipulating Adsorption–Insertion Mechanisms in Nanostructured Carbon Materials for High-Efficiency Sodium Ion Storage. Adv. Energy Mater. 2017, 7, 1700403. [Google Scholar] [CrossRef]
- Balogun, M.S.; Luo, Y.; Qiu, W.; Liu, P.; Tong, Y. A review of carbon materials and their composites with alloy metals for sodium ion battery anodes. Carbon 2016, 98, 162–178. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Ponrouch, A.; Goni, A.R.; Palacin, M.R. High Capacity Hard Carbon Anodes for Sodium Ion Batteries in Additive Free Electrolyte. Electrochem. Commun. 2013, 27, 85–88. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-Density Sodium and Lithium Ion Battery Anodes from Banana Peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Qian, T.; Wang, M.; Shen, X.; Hu, N.; Sun, Z.; Yan, C. A Sustainable Route from Biomass Byproduct Okara to High Content Nitrogen-Doped Carbon Sheets for Efficient Sodium Ion Batteries. Adv. Mater. 2016, 28, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Paranthaman, M.P.; Akato, K.; Naskar, A.K.; Levine, A.M.; Lee, R.J.; Kim, S.O.; Zhang, J.; Dai, S.; Manthiram, A. Tire-Derived Carbon Composite Anodes for Sodium-Ion Batteries. J. Power Sources 2016, 316, 232–238. [Google Scholar] [CrossRef]
- Wang, H.; Yu, W.; Shi, J.; Mao, N.; Chen, S.; Liu, W. Biomass Derived Hierarchical Porous Carbons as High-Performance Anodes for Sodium-Ion Batteries. Electrochim. Acta 2016, 188, 103–110. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Jiang, E.; Amiralian, N.; Annamalai, P.K.; Martin, D.J.; Kumar, N.A.; Zhao, X.S. Spinifex Nanocellulose Derived Hard Carbon Anodes for High-Performance Sodium-Ion Batteries. Sustain. Energy Fuels 2017, 1, 1090–1097. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Jain, Z.; Jiang, H.; Razink, J.J.; Stickle, W.F.; Neuefeind, J.C.; Ji, X. Defective Hard Carbon Anode for Na-Ion Batteries. Chem. Mater 2018, 30, 4536–4542. [Google Scholar] [CrossRef]
- Luo, W.; Schardt, J.; Bommier, C.; Wang, B.; Razink, J.; Simonsen, J.; Ji, X. Carbon Nanofibers Derived from Cellulose Nanofibers as a Long-Life Anode Material for Rechargeable Sodium-Ion Batteries. J. Mater. Chem. A 2013, 1, 10662–10666. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Li, Y.; Qiao, M.; Hu, Y.S.; Titirici, M.M.; Lieder, M. Hard Carbon Derived from Rice Husk as Low Cost Negative Electrodes in Na-ion Batteries. J. Energy Chem. 2019, 29, 17–22. [Google Scholar] [CrossRef]
- Darjazi, H.; Staffolani, A.; Sbrascini, L.; Bottoni, L.; Tossici, R.; Nobili, F. Sustainable Anodes for Lithium- and Sodium-Ion Batteries Based on Coffee Ground-Derived Hard Carbon and Green Binders. Energies 2020, 13, 6216. [Google Scholar] [CrossRef]
- Rios, C.M.S.; Simonin, L.; Geyer, A.; Ghimbeu, C.M.; Dupont, C. Unraveling the Properties of Biomass-Derived Hard Carbons upon Thermal Treatment for a Practical Application in Na-Ion Batteries. Energies 2020, 13, 3513. [Google Scholar] [CrossRef]
- Zou, Y.; Li, H.; Qin, K.; Xia, Y.; Lin, L.; Qi, Y.; Jian, Z.; Chen, W. Low-Cost Lignite-Derived Hard Carbon for High-Performance Sodium-Ion Storage. J. Mater. Sci. 2020, 55, 5994–6004. [Google Scholar] [CrossRef]
- Luo, W.; Jian, Z.; Xing, Z.; Wang, W.; Bommier, C.; Lerner, M.M.; Ji, X. Electrochemically Expandable Soft Carbon as Anodes for Na-Ion Batteries. ACS Cent. Sci. 2015, 1, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xiao, J.; Wang, W.; Engelhard, M.; Chen, X.; Nie, Z.; Gu, M.; Saraf, L.V.; Exarhos, G.; Zhang, J.G.; et al. Surface-Driven Sodium Ion Energy Storage in Nanocellular Carbon Foams. Nano Lett. 2013, 13, 3909–3914. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zeng, L.; Yang, Z.; Gu, L.; Wang, J.; Liu, X.; Cheng, J.; Yu, Y. Free-Standing and Binder-Free Sodium-Ion Electrodes with Ultralong Cycle Life and High Rate Performance Based on Porous Carbon Nanofibers. Nanoscale 2014, 6, 693–698. [Google Scholar] [CrossRef]
- Lu, P.; Sun, Y.; Xiang, H.; Liang, X.; Yu, Y. 3D Amorphous Carbon with Controlled Porous and Disordered Structures as a High-Rate Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2017, 1702434. [Google Scholar] [CrossRef]
- Wang, H.G.; Wu, Z.; Meng, F.I.; Ma, D.L.; Huang, X.L.; Wang, L.M.; Zhang, X.B. Nitrogen-Doped Porous Carbon Nanosheets as Low-Cost, High-Performance Anode Material for Sodium-Ion Batteries. ChemSusChem 2013, 6, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, N.; Cui, H.; Wang, C. Enhanced Storage Capability and Kinetic Processes by Pores- and Hetero-Atoms- Riched Carbon Nanobubbles for Lithium-Ion and Sodium-Ion Batteries Anodes. Nano Energy 2014, 4, 81–87. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Park, K.; Lu, W.; Wang, C.; Xue, W.; Yang, F.; Zhou, J.; Suo, L.; Lin, T.; et al. Nitrogen-Doped Carbon for Sodium-Ion Battery Anode by Self-Etching and Graphitization of Bimetallic MOF-Based Composite. Chem. 2017, 3, 152–163. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Liu, S.; Yang, H. Nitrogen-Doped Three-Dimensional Porous Carbon Anode Derived from Hard Halloysite Template for Sodium-Ion Batteries. Appl. Clay. Sci. 2021, 200, 105916. [Google Scholar] [CrossRef]
- Wang, Y.X.; Chou, S.L.; Liu, H.K.; Dou, S.X. Reduced Graphene Oxide with Superior Cycling Stability and Rate Capability for Sodium Storage. Carbon 2013, 57, 202–208. [Google Scholar] [CrossRef]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Hu, Y.; Masuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded Graphite as Superior Anode for Sodium-Ion Batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, K.K.; Benayad, A.; Yoon, S.M.; Park, H.K.; Jung, I.S.; Jin, M.H.; Jeong, H.K.; Kim, J.M.; Choi, J.Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a Green Graphene Oxide Reduction with Reusable Potassium Carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Kumar, N.A.; Gambarelli, S.; Duclairoir, F.; Bidan, G.; Dubois, L. Synthesis of High Quality Reduced Graphene Oxide Nanosheets Free of Paramagnetic Metallic Impurities. J. Mater. Chem. A 2013, 1, 2789–2794. [Google Scholar] [CrossRef]
- Kumar, N.A.; Togami, M.; Oishi, Y.; Tominaga, M.; Takafuji, M.; Ihara, H. Iron Metal Induced Deoxygenation of Graphite Oxide Nanosheets-Insights on the Capacitive Properties of Binder-Free Electrodes. RSC Adv. 2015, 5, 23367–23373. [Google Scholar] [CrossRef]
- Kumar, N.A.; Gaddam, R.R.; Varanasi, S.R.; Yang, D.; Bhatia, S.K.; Zhao, X.S. Sodium Ion Storage in Reduced Graphene Oxide. Electrochim. Acta 2016, 214, 319–325. [Google Scholar] [CrossRef]
- Aslam, M.K.; Xu, M. A Mini-Review: MXene Composites for Sodium/Potassium-Ion Batteries. Nanoscle 2020, 12, 15993–16007. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, A.; Marino, C.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better Cycling Performances of Bulk Sb in Na-Ion Batteries Compared to Li-Ion Systems: An Unexpected Electrochemical Mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Z.; Chen, Y.; Weadock, N.; Wan, J.; Vaaland, O.; Han, X.; Li, T.; Hu, L. Tin Anode for Sodium-Ion Batteries Using Natural Wood Fiber as a Mechanical Buffer and Electrolyte Reservoir. Nano Lett. 2013, 13, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xu, Y.; Zhu, Y.; Liu, Y.; Zheng, S.; Liu, Y.; Langrock, A.; Wang, C. Selenium@Mesoporous Carbon Composite with Superior Lithium and Sodium Storage Capacity. ACS Nano 2013, 7, 8003–8010. [Google Scholar] [CrossRef]
- Mei, Y.; Huang, Y.; Hu, X. Nanostructured Ti-based Anode Materials for Na-ion Batteries. J. Mater. Chem. A 2016, 4, 12001–12013. [Google Scholar] [CrossRef]
- Wang, N.; Chu, C.; Xu, X.; Du, Y.; Yang, J.; Bai, Z.; Dou, S. Comprehensive New Insights and Perspectives into Ti-Based Anodes for Next-Generation Alkaline Metal (Na+, K+) Ion Batteries. Adv. Energy Mater. 2018, 1800888. [Google Scholar] [CrossRef]
- Lee, Y.H.; Luu, T.H.T.; Duong, D.L.; Lee, T.H.; Pham, D.T.; Shaoo, R.; Han, G.; Kim, Y.M.; Lee, Y.H. Monodispersed SnS Nanoparticles Anchored on Carbon Nanotubes for High-Retention Sodium-Ion Batteries. J. Mater. Chem. A 2020, 8, 7861–7869. [Google Scholar] [CrossRef]
- Chen, B.; Qin, H.; Li, K.; Zhang, B.; Liu, E.; Zhao, N.; Shi, C.; He, C. Yolk-shelled Sb@C Nanoconfined Nitrogen/Sulfur Co-Doped 3D Porous Carbon Microspheres for Sodium-Ion Battery Anode with Ultralong High-Rate Cycling. Nano Energy 2019, 66, 104133. [Google Scholar] [CrossRef]
- Schulze, M.C.; Belson, R.M.; Kraynak, L.A.; Prieto, A.L. Electrodeposition of Sb/CNT Composite Films as Anodes for Li- and Na-Ion Batteries. Energy Stor. Mater. 2020, 25, 572–584. [Google Scholar] [CrossRef]
- Peng, B.; Xu, Y.; Wang, X.; Mulder, F.M. A High Performance and Low Cost Sodium Ion Anode Based on A Facile Black Phosphorus—Carbon Nanocomposite. ChemElectroChem 2017, 4, 2140–2144. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, P.; Li, J.; Wang, S.; Li, L. Recent Progress of Phosphorus Composite Anodes for Sodium/Potassium Ion Batteries. Energy Stor. Mater. 2021, 34, 436–460. [Google Scholar] [CrossRef]
- Lu, Y.C.; Dimov, N.; Okada, S.; Bui, T.H. SnSb Alloy Blended with Hard Carbon as Anode for Na-Ion. Batteries Energies 2018, 11, 1614. [Google Scholar] [CrossRef]
- Cha, H.A.; Jeong, H.M.; Kang, J.K. Nitrogen-Doped Open Pore Channeled Graphene Facilitating Electrochemical Performance of TiO2 Nanoparticles as an Anode Material for Sodium Ion Batteries. J. Mater. Chem. A 2014, 2, 5182–5186. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, T.; Chuang, C.; Lu, Y.R.; Chan, T.S.; Du, Z.; Ji, H.; Wan, L.J. Synergy of Black Phosphorus−Graphite−Polyaniline-Based Ternary Composites for Stable High Reversible Capacity Na-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2019, 11, 16656–16661. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, F.; Song, Y.; Li, Y. Revitalizing Carbon Supercapacitor Electrodes with Hierarchical Porous Structures. J. Mater. Chem. A 2017, 5, 17705–17733. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Hubkowska, K.; Koss, U.; Czerwiński, A. Characteristic of Hydrogen-Saturated Pd-Based Alloys for the Application in Electrochemical Capacitors. J. Solid State Electrochem. 2012, 16, 2533–2539. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathia, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Ho, M.Y.; Khiew, P.S.; Isa, D.; Tan, T.K.; Chiu, W.S.; Chia, C.H. A review of Metal Oxide Composite Electrode Materials for Electrochemical Capacitors. Nano 2014, 9, 1430002. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z. Carbon Materials for High Volumetric Performance Supercapacitors: Design, Progress, Challenges and Opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Dubey, R.; Guruviah, V. Review of Carbon-Based Electrode Materials for Supercapacitor Energy Storage. Ionics 2019, 25, 1419–1445. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Lota, G.; Centeno, T.A.; Frackowiak, E. Templated Mesoporous Carbons for Supercapacitor Application. Electrochim. Acta 2005, 50, 2799–2805. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Zhong, H.; Xu, F.; Li, Z.; Fu, R.; Wu, D. High-Energy Supercapacitors Based on Hierarchical Porous Carbon with an Ultrahigh Ion-Accessible Surface Area in Ionic Liquid Electrolytes. Nanoscale 2013, 5, 4678–4682. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.M. Biomass-Derived Porous Carbon Materials with Different Dimensions for Supercapacitor Electrodes: A Review. J. Mater. Chem. A 2019, 7, 16028. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon Nanotube—and Graphene-Based Nanomaterials and Applications in High-Voltage Supercapacitor: A Review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-Based Composite Materials for Supercapacitor Electrodes: A Review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Frackowiak, E.; Abbas, Q.; Béguin, F. Carbon/Carbon Supercapacitors. J. Energy Chem. 2013, 22, 226–240. [Google Scholar] [CrossRef]
- Chien, H.C.; Cheng, W.Y.; Wang, Y.H.; Lu, S.Y. Ultrahigh Specific Capacitances for Supercapacitors Achieved by Nickel Cobaltite/Carbon Aerogel Composites. Adv. Funct. Mater. 2012, 22, 5038–5043. [Google Scholar] [CrossRef]
- Levitt, A.S.; Alhabeb, M.; Hatter, C.B.; Sarycheva, A.; Dion, G.; Gogotsi, Y. Electrospun MXene/Carbon Nanofibers as Supercapacitor Electrodes. J. Mater. Chem. A 2019, 7, 269. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Feng, Y.P. Carbon Nanotubes for Supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. [Google Scholar] [CrossRef]
- Xu, B.; Yue, S.; Sui, Z.; Zhang, X.; Hou, S.; Cao, G.; Yang, Y. What is the Choice for Supercapacitors: Graphene or Graphene Oxide? Energy Environ. Sci. 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Z.; Chao, L.; Wu, X.; Lei, X.; Chang, Z.; Sun, X. Synthesis of Hierarchical Porous N-doped Sandwich-Type Carbon Composites as High-Performance Supercapacitor Electrodes. J. Mater. Chem. A 2015, 3, 3667–3675. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.L.; Simon, P. Ultrahigh-Power Micrometre-Sized Supercapacitors based on Onion-Like Carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Lv, L.; Fan, Y.; Chen, Q.; Zhao, Y.; Hu, Y.; Zhang, Z.; Chen, N.; Qu, L. Three-Dimensional Multichannel Aerogel of Carbon Quantum Dots for High-Performance Supercapacitors. Nanotechnology 2014, 25, 235401. [Google Scholar] [CrossRef] [PubMed]
- Strauss, V.; Marsh, H.; Kowal, M.D.; El-Kady, M.; Kaner, R.B. A Simple Route to Porous Graphene from Carbon Nanodots for Supercapacitor Applications. Adv. Mater. 2018, 30, 1704449. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.; Borenstein, A.; Markovsky, B.; Aurbach, D.; Gedanken, A.; Talianker, M.; Porat, Z. Activated Carbon Modified with Carbon Nanodots as Novel Electrode Material for Supercapacitors. J. Phys. Chem. C 2016, 120, 13406–13413. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Y.; Lu, G.; Chen, N.; Zhang, Z.; Li, H.; Shao, H.; Qu, L. Graphene Quantum Dots–Carbon Nanotube Hybrid Arrays for Supercapacitors. Nanotechnology 2013, 24, 195401. [Google Scholar] [CrossRef]
- ] Niu, Y.; Wang, J.; Zhang, J.; . Shi, Z. Graphene Quantum Dots as a Novel Conductive Additive to Improve the Capacitive Performance for Supercapacitors. J. Electroanal. Chem. 2018, 828, 1–10. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, G.; Hou, Y.; Pan, Z.; Li, H.; Li, W.; Liu, M.; Ye, F.; Yang, X.; Zhang, Y. Vertically Aligned Carbon Nanotubes on Carbon Nanofibers: A Hierarchical Three-Dimensional Carbon Nanostructure for High-Energy Flexible Supercapacitors. Chem. Mater. 2015, 27, 1194–1200. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Zhang, H.; Sun, H.; Zhang, D.; Ma, Y. High-Performance Supercapacitors Based on a Graphene–Activated Carbon Composite Prepared by Chemical Activation. RSC Adv. 2012, 2, 7747–7753. [Google Scholar] [CrossRef]
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated Graphene-Based Carbons as Supercapacitor Electrodes with Macro- and Mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Song, J.; Yang, W.; Wang, M.; Zhu, C.; Zhao, W.; Zheng, J.; Lin, Y. Self-Supporting Activated Carbon/Carbon Nanotube/Reduced Graphene Oxide Flexible Electrode for High Performance Supercapacitor. Carbon 2018, 129, 236–244. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.G.; Neff, D.; Fulvio, P.; Mayes, R.; Zhamu, A.; Fang, Q.; Chen, G.; Meyer III, H.; Jang, B.; et al. Nitrogen-enriched Ordered Mesoporous Carbons Through Direct Pyrolysis in Ammonia with Enhanced Capacitive Performance. J. Mater. Chem. A 2013, 1, 7920–7926. [Google Scholar] [CrossRef]
- Chen, H.; Sun, F.; Wang, J.; Li, W.; Qiao, W.; Ling, L.; Long, D. Nitrogen Doping Effects on the Physical and Chemical Properties of Mesoporous Carbons. J. Phys. Chem. C 2013, 117, 8318–8328. [Google Scholar] [CrossRef]
- Ramirez-Castro, C.; Crosnier, O.; Athouel, L.; Retoux, R.; Belanger, D.; Brousse, T. Electrochemical Performance of Carbon/MnO2 Nanocomposites Prepared via Molecular Bridging as Supercapacitor Electrode Materials. J. Electrochem. Soc. 2015, 162, A5179–A5184. [Google Scholar] [CrossRef]
- Lv, H.; Yuan, Y.; Xu, Q.; Liu, H.; Wang, Y.G.; Xia, Y. Carbon Quantum Dots Anchoring MnO2/Graphene Aerogel Exhibits Excellent Performance as Electrode Materials for Supercapacitor. J. Power Sources 2018, 398, 167–174. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.A.; An, N.; Yang, Y.Y.; Li, Z.M.; Wu, H.Y. Facile Synthesis of MnO2/CNTs Composite for Supercapacitor Electrodes with Long Cycle Stability. J. Phys. Chem. C 2014, 118, 22865–22872. [Google Scholar] [CrossRef]
- Noh, J.; Yoon, C.M.; Kim, Y.K.; Jang, J. High Performance Asymmetric Supercapacitor Twisted from Carbon Fiber/MnO2 and Carbon Fiber/MoO3. Carbon 2017, 116, 470–478. [Google Scholar] [CrossRef]
- Warren, R.; Sammoura, F.; Tounsi, F.; Sanghadasa, M.; Lin, L. Highly Active Ruthenium Oxide Coating via ALD and Electrochemical Activation in Supercapacitor Applications. J. Mater. Chem. A 2015, 3, 15568–15575. [Google Scholar] [CrossRef]
- He, G.; Li, J.; Chen, H.; Shi, J.; Sun, X.; Chen, S.; Wang, X. Hydrothermal Preparation of Co3O4@graphene Nanocomposite for Supercapacitor with Enhanced Capacitive Performance. Mater. Lett. 2012, 82, 61–63. [Google Scholar] [CrossRef]
- Fleker, O.; Borenstein, A.; Lavi, R.; Benisvy, L.; Ruthstein, S.; Aurbach, D. Preparation and Properties of Metal Organic Framework/Activated Carbon Composite Materials. Langmuir 2016, 32, 4935–4944. [Google Scholar] [CrossRef]
- Panda, B.; Dwivedi, I.; Priya, K.; Karandikar, P.B.; Mandake, P.S. Analysis of Aqueous Supercapacitor with Various Current Collectors, Binders and Adhesives. In Proceedings of the 2016 Biennial International Conference on Power and Energy Systems: Towards Sustainable Energy (PESTSE 2016), Bengaluru, India, 21–23 January 2016. [Google Scholar]
- Talapatra, S.; Kar, S.; Pal, S.K.; Vajtai, R.; Ci, L.; Victor, P.; Shaijumon, M.M.; Kaur, S.; Nalamasu, O.; Ajayan, P.M. Direct growth of aligned carbon nanotubes on bulk metals. Nat. Nanotechnol. 2006, 1, 112–116. [Google Scholar] [CrossRef]
- Fischer, A.E.; Pettigrew, K.A.; Rolison, D.R.; Stroud, R.M.; Long, J.W. Incorporation of Homogeneous, Nanoscale MnO2 within Ultraporous Carbon Structures via Self-Limiting Electroless Deposition: Implications for Electrochemical Capacitors. Nano Lett. 2007, 7, 281–286. [Google Scholar] [CrossRef]
- Futaba, D.N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-Engineerable and Highly Densely Packed Single-Walled Carbon Nanotubes and their Application as Super-Capacitor Electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, H.; Qiu, J.; Zhou, C. Inkjet Printing of Single-Walled Carbon Nanotube/RuO2 Nanowire Supercapacitors on Cloth Fabrics and Flexible Substrates. Nano Res. 2010, 3, 594–603. [Google Scholar] [CrossRef]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of Materials and Fabrication Methods. J. Energy Eng. 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Hu, L.; Wu, H.; Xie, X.; Cui, Y. Paper Supercapacitors by a Solvent-Free Drawing Method. Energy Environ. Sci. 2011, 4, 3368–3373. [Google Scholar] [CrossRef]
- Hoffmann, V.; Jung, D.; Alhnidi, M.J.; Mackle, L.; Kruse, A. Bio-Based Carbon Materials from Potato Waste as Electrode Materials in Supercapacitors. Energies 2020, 13, 2406. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, W.; Lin, H.; Li, Y.; Lu, H.; Wang, Y. Hierarchical Porous Carbon Based on the Self-Templating Structure of Rice Husk for High-Performance Supercapacitors. RSC Adv. 2015, 5, 19294–19300. [Google Scholar] [CrossRef]
- Yeleuov, M.; Seidl, C.; Temirgaliyeva, T.; Taurbekov, A.; Prikhodko, N.; Lesbayev, B.; Sultanov, F.; Daulbayev, C.; Kumekov, S. Modified Activated Graphene-Based Carbon Electrodes from Rice Husk for Supercapacitor Applications. Energies 2020, 13, 4943. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Wang, X.; Ok, Y.S.; Elliott, J.A.W.; Chang, S.X.; Chung, H.J. Flexible and Self-Healing Aqueous Supercapacitors for Low Temperature Applications: Polyampholyte Gel Electrolytes with Biochar Electrodes. Sci. Rep. 2017, 7, 1685. [Google Scholar] [CrossRef] [PubMed]
- Su, X.L.; Chen, J.R.; Zheng, G.P.; Yang, J.H.; Guan, X.X.; Liua, P.; Zheng, X.C. Three-Dimensional Porous Activated Carbon Derived from Loofah Sponge Biomass for Supercapacitor Applications. Appl. Surf. Sci. 2018, 436, 327–336. [Google Scholar] [CrossRef]
- Jia, H.; Sun, J.; Xie, X.; Yin, K.; Sun, L. Cicada Slough-Derived Heteroatom Incorporated Porous Carbon for Supercapacitor: Ultra-High Gravimetric Capacitance. Carbon 2019, 143, 309–317. [Google Scholar] [CrossRef]

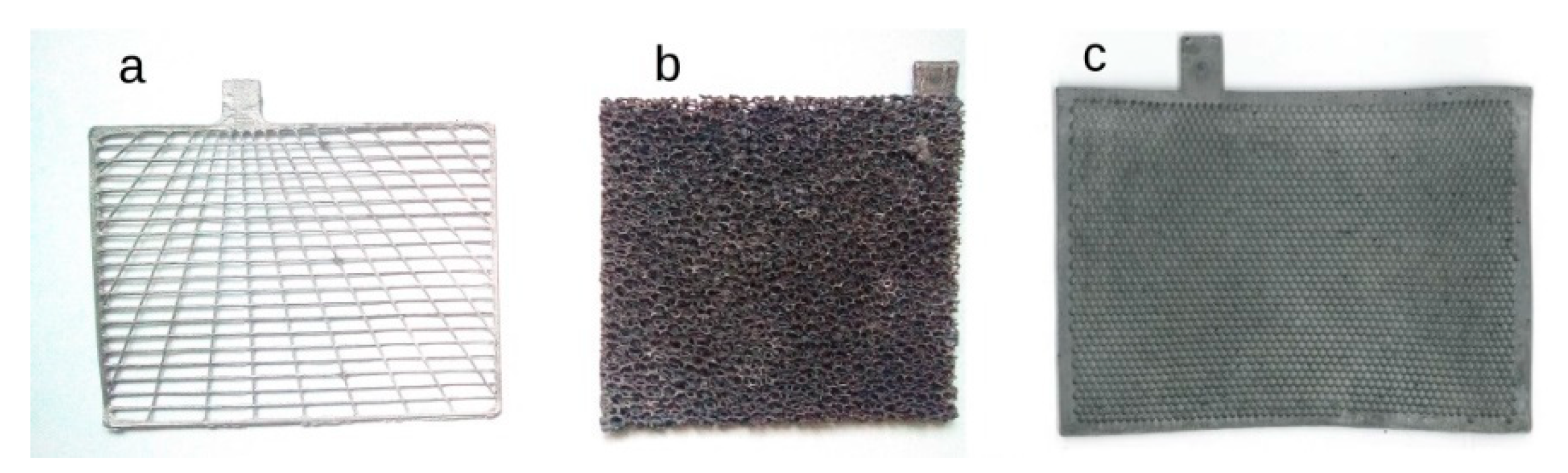
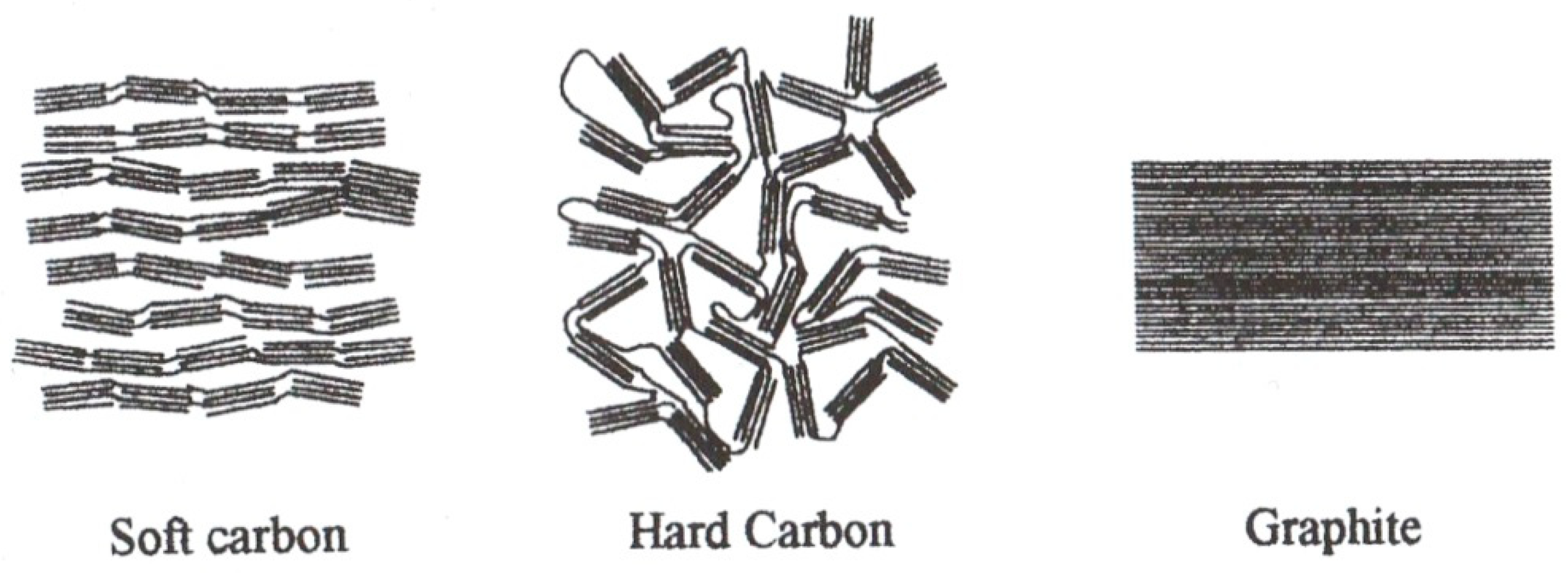

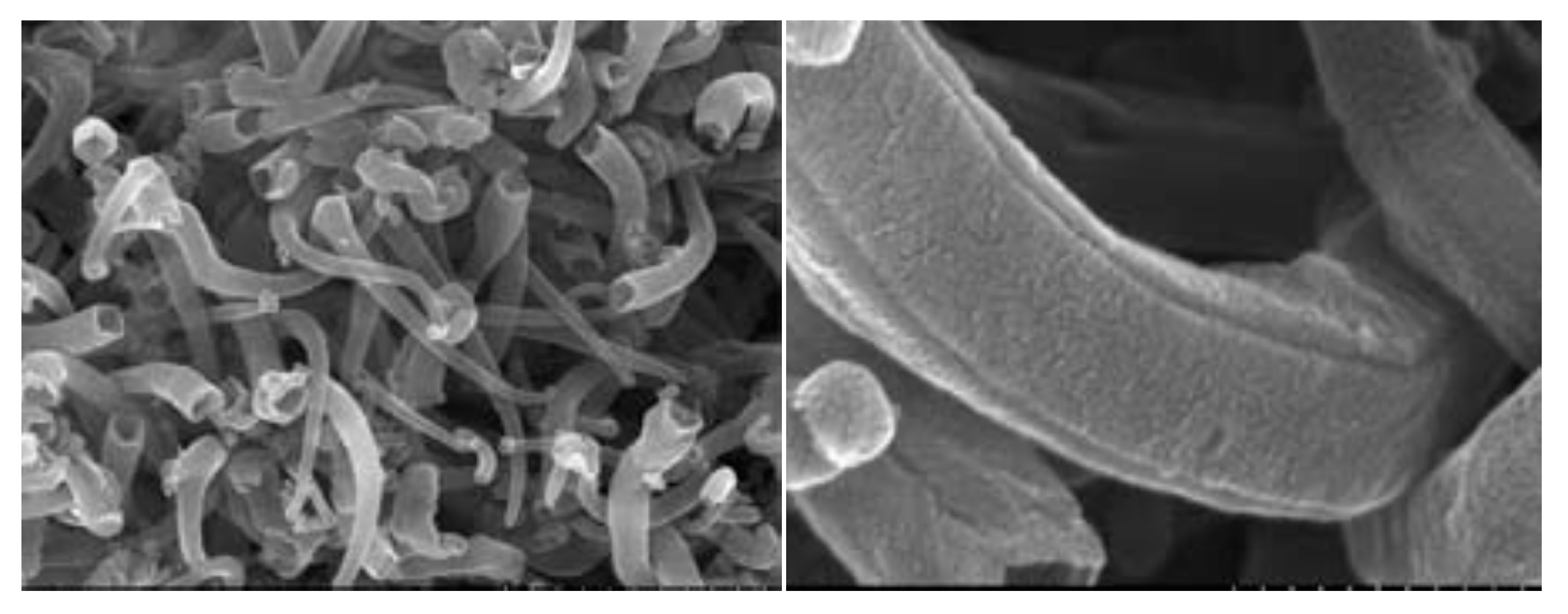
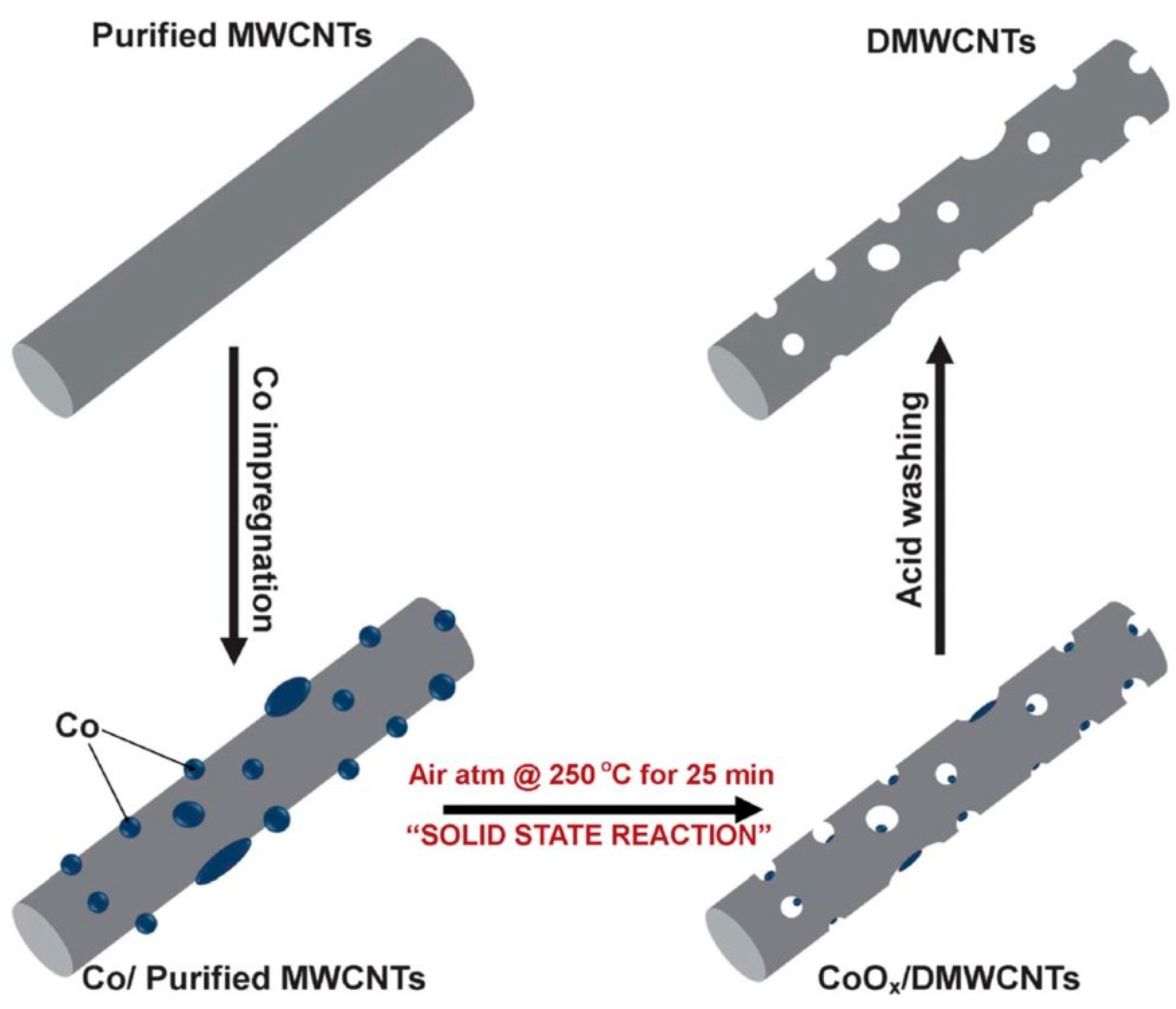





| Rechargeable Electrochemical Power Source | |||||
|---|---|---|---|---|---|
| Lead–Acid Batteries | Lithium-Ion Batteries | Lithium–Sulfur Batteries | Sodium-Ion Batteries | Supercapacitors | |
| Specific energy (Wh/kg) | 35–50 | 80–180 | 150–350 | 75–150 | 0.05–10 |
| Specific power (W/kg) | 150–400 | 200–1000 | 100–300 | up to 1500 | 2000–10,000 |
| Cycle life | Moderate (up to 1000 cycles, 2000 cycles for special designs) | High (up to 3000 cycles) | Low (up to a few hundred cycles) | Moderate (up to 1000 cycles) | Very high (over 10,000 cycles) |
| Self-discharge rate | Low (2–8% per month) | Low (2–10% per month) | Moderate (over 15% per month) | Low (small % per month) | High (over 10% per day) |
| Approximated cost per unit energy (USD/kWh) | 80–200 | 170–350 | ~200 (estimated to be lower than lithium-ion batteries) | Estimated as lower than lithium-ion batteries | Over 10,000 |
| Typical applications | SLI batteries; energy storage systems (power walls, UPS systems); vehicular applications (micro- and mild-hybrid electric vehicles) | Portable electronics; vehicular applications (hybrid-electric, electric, and plug-in-hybrid electric vehicles); energy storage systems | Potentially electric vehicles; portable electronics | Potentially electric vehicles; power tools; small energy storage systems (home storage) | Portable electronics; voltage stabilizers; potentially vehicular applications (start–stop and micro-hybrid electric vehicles) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lach, J.; Wróbel, K.; Wróbel, J.; Czerwiński, A. Applications of Carbon in Rechargeable Electrochemical Power Sources: A Review. Energies 2021, 14, 2649. https://doi.org/10.3390/en14092649
Lach J, Wróbel K, Wróbel J, Czerwiński A. Applications of Carbon in Rechargeable Electrochemical Power Sources: A Review. Energies. 2021; 14(9):2649. https://doi.org/10.3390/en14092649
Chicago/Turabian StyleLach, Jakub, Kamil Wróbel, Justyna Wróbel, and Andrzej Czerwiński. 2021. "Applications of Carbon in Rechargeable Electrochemical Power Sources: A Review" Energies 14, no. 9: 2649. https://doi.org/10.3390/en14092649
APA StyleLach, J., Wróbel, K., Wróbel, J., & Czerwiński, A. (2021). Applications of Carbon in Rechargeable Electrochemical Power Sources: A Review. Energies, 14(9), 2649. https://doi.org/10.3390/en14092649






