Experimental Study on Reservoir Physical Properties and Formation Blockage Risk in Geothermal Water Reinjection in Xining Basin: Taking Well DR2018 as an Example
Abstract
1. Introduction
2. Analysis of Geothermal Water and Reservoir Rocks
2.1. Geothermal Water Properties
2.2. Reservoir Rock Physical Properties
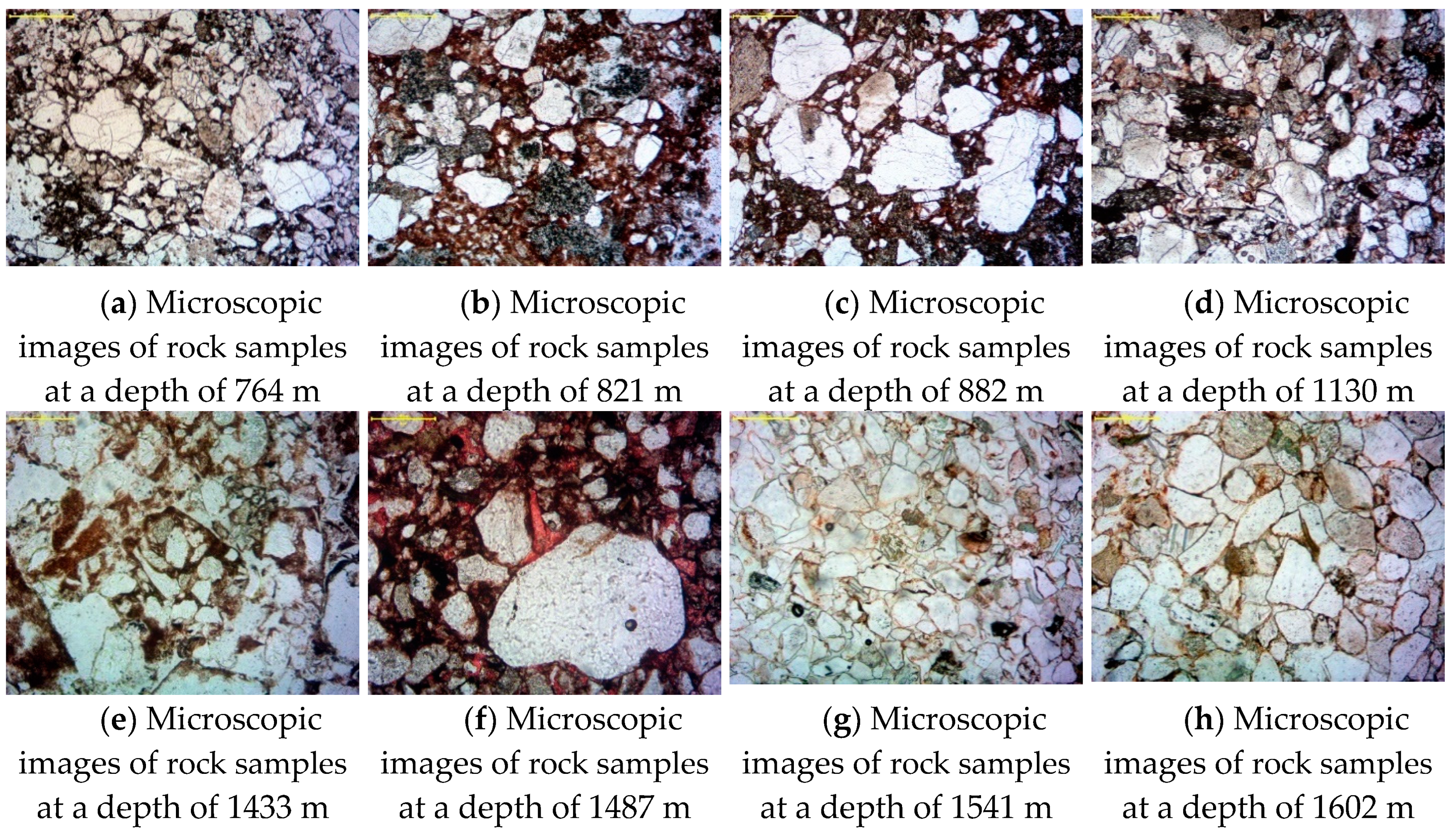
2.2.1. Rock Microscopic Identification
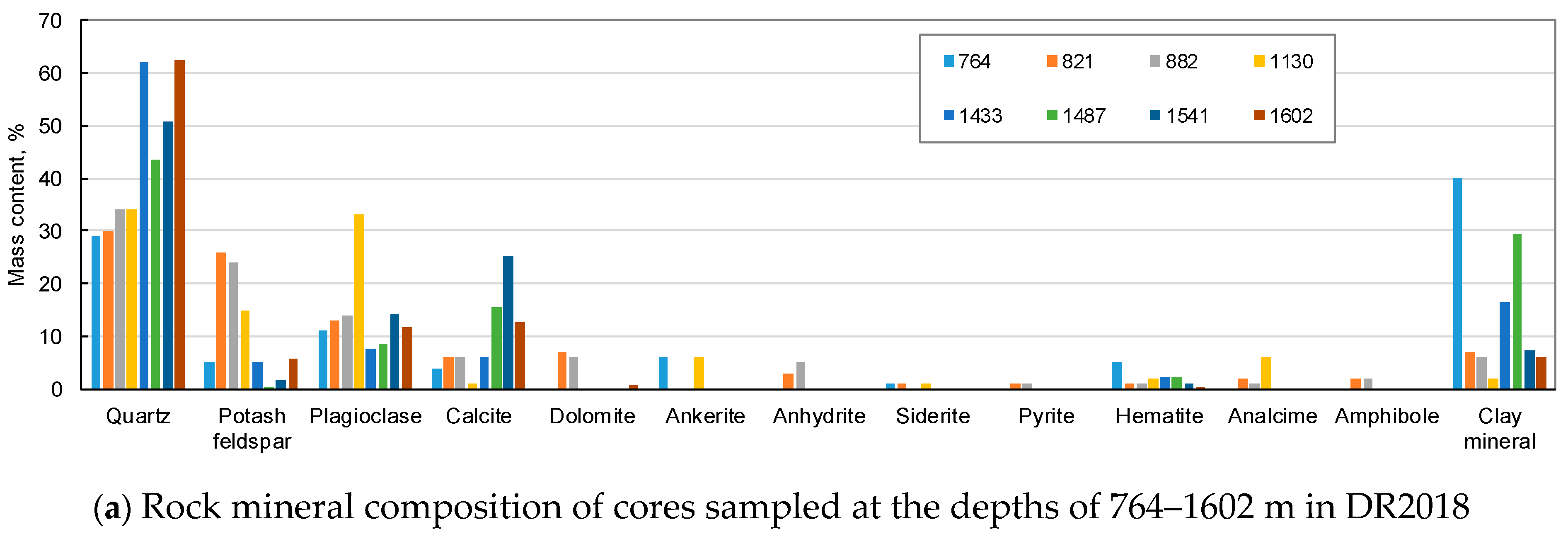
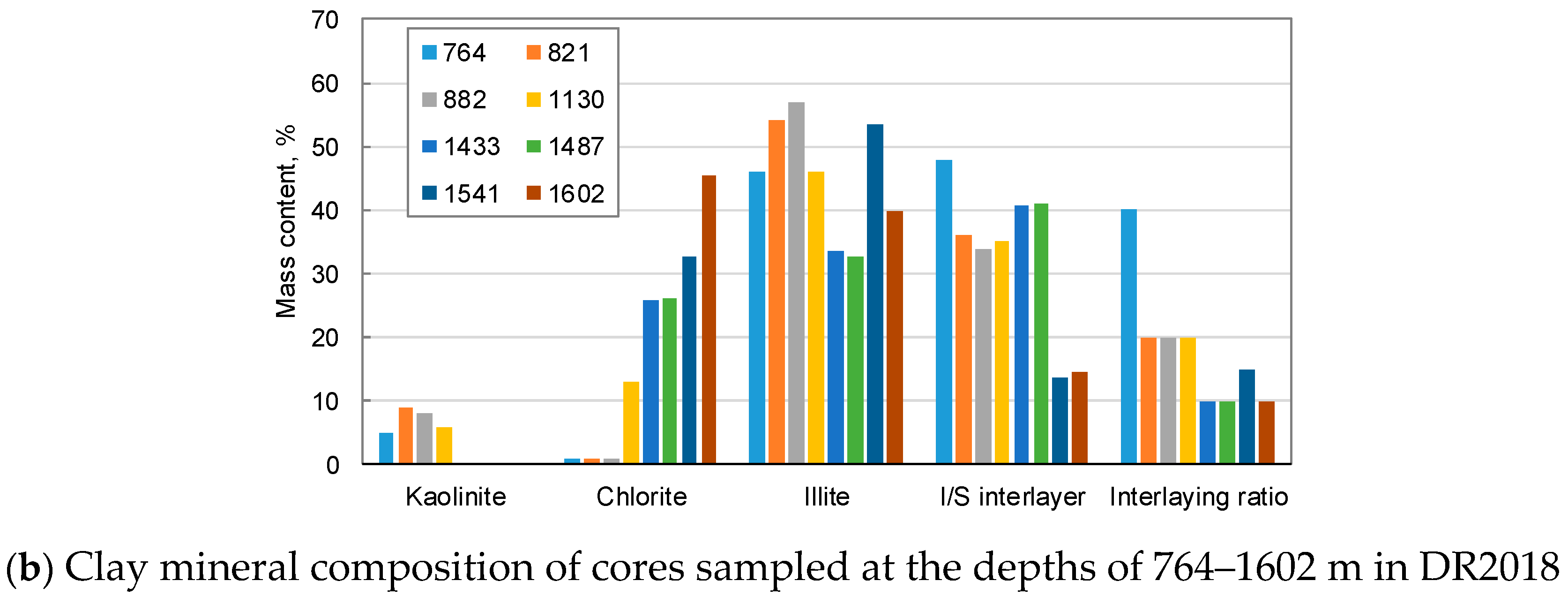
2.2.2. Mineral Composition of Rocks
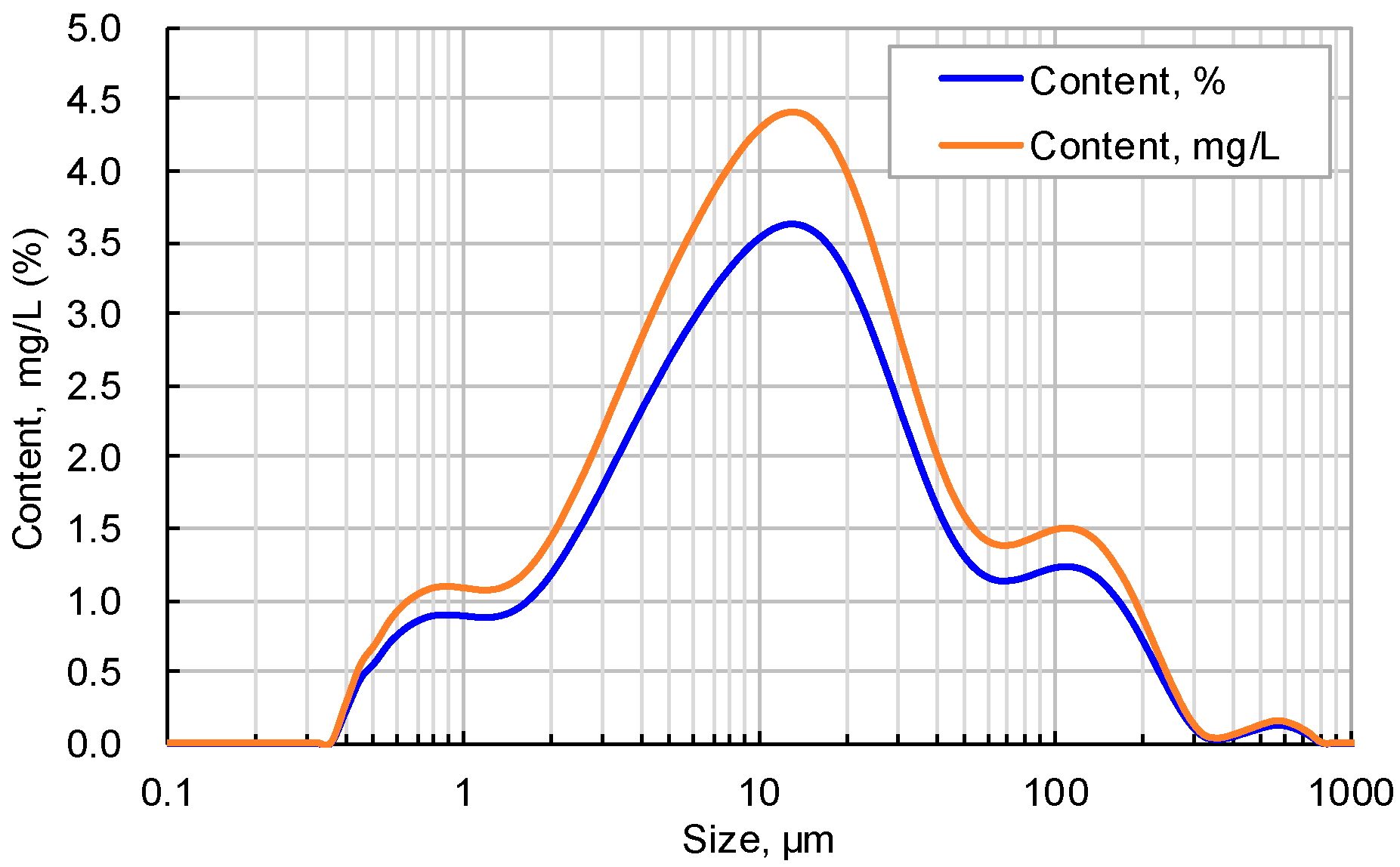
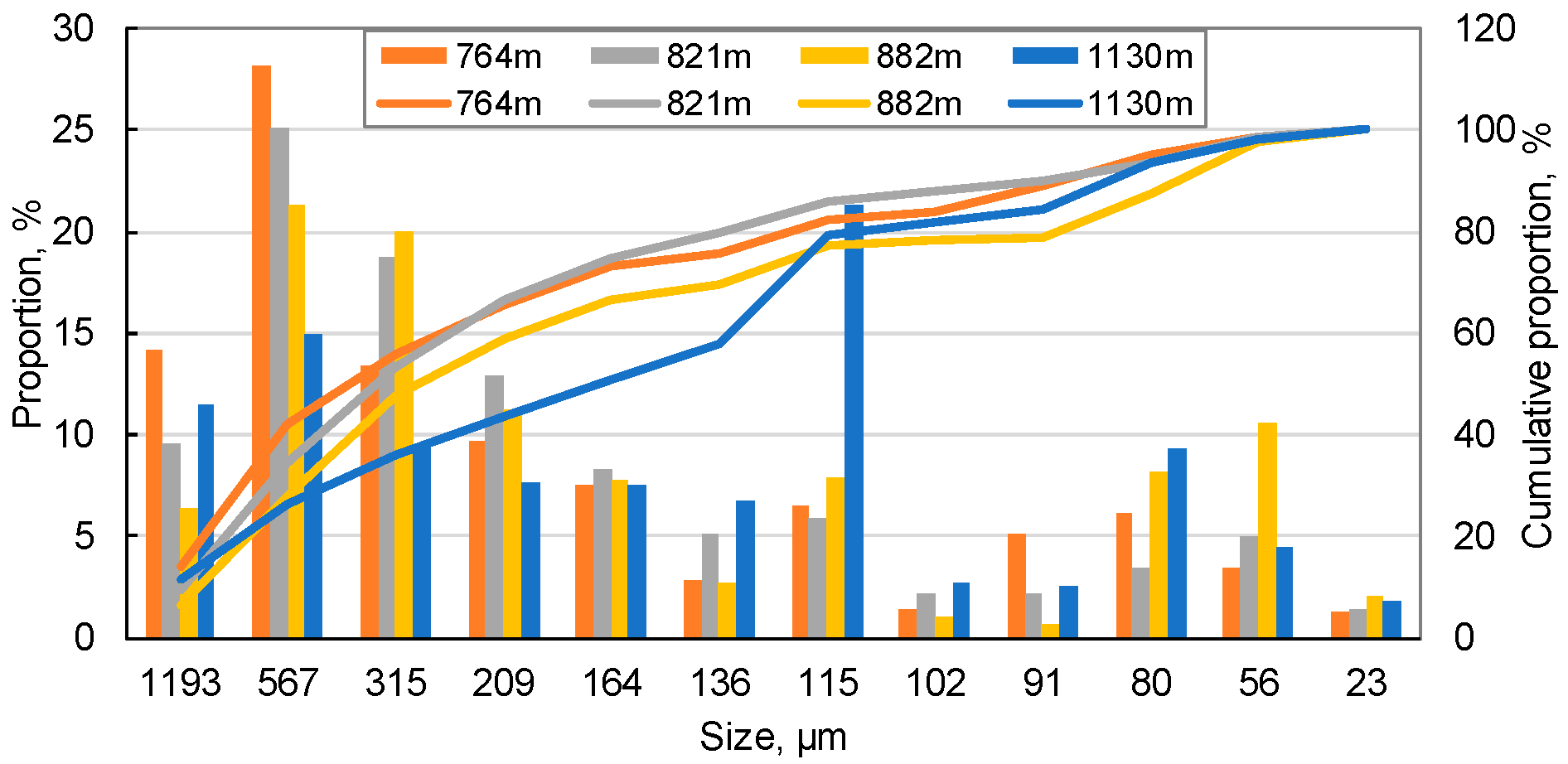
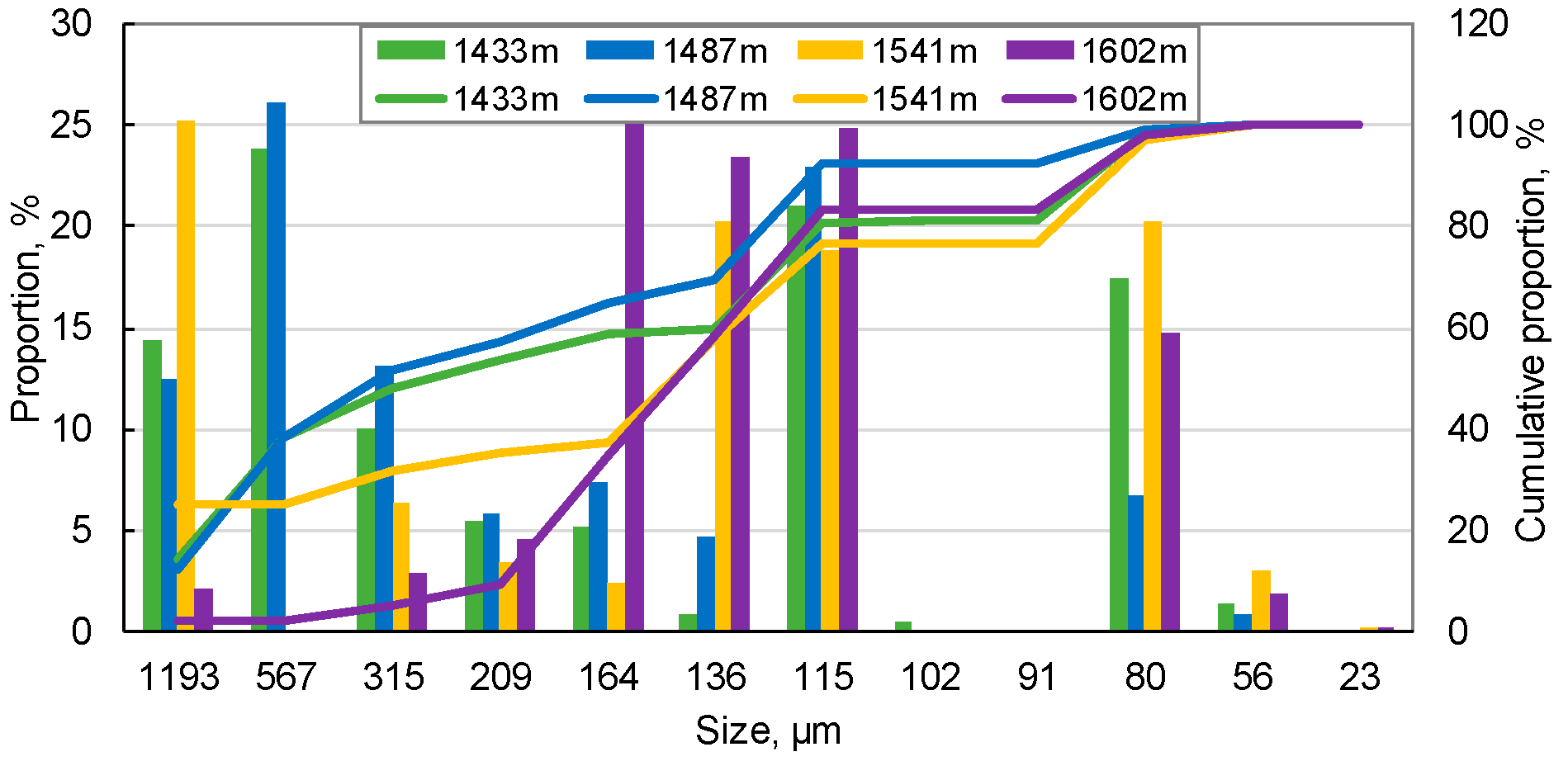
2.2.3. Distribution of Rock Grain Size
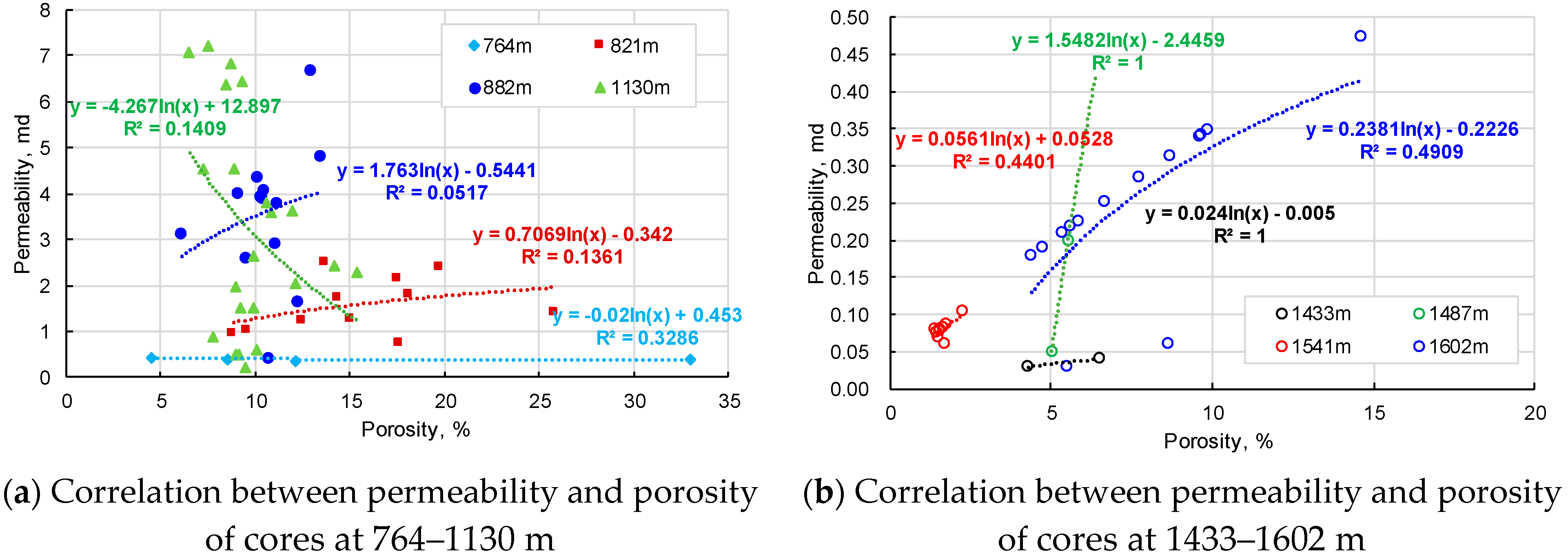
2.2.4. Porosity and Permeability
3. Assessment Experiment of Formation Blockage Risk during Geothermal Reinjection
3.1. Experimental Equipment and Method
3.1.1. Experimental Equipment
3.1.2. Experimental Materials
- (1)
- Water samples: geothermal water of well DR2018 and distilled water.
- (2)
- Rock samples: 20 standard-size cores at 1130 m of well DR2018, which have relatively high permeability and uniform quality with a length of about 4–6 cm and a diameter of 2.5 cm.
- (3)
- Chemicals: CaCl2 and NaHCO3, which are used for generating scaling particles; kaolin clay particles are used to prepare suspended particles in geothermal water.
3.1.3. Experimental Procedures
- (1)
- Put the core into the core holder, add confining pressure and backpressure, and keep the temperature at the designed value using the air bath to simulate the geothermal reservoir condition.
- (2)
- Inject geothermal water, scaling ion solution, or suspended particle solution into the core at a certain flow rate using the constant flow pump to simulate the geothermal reinjection process. Simultaneously, monitor the pressures at the two ends of the core holder in real time and collect the produced water using the measuring cylinder.
- (3)
- After the experiment, open the core holder and observe the blockage on the end face of the core.
- (4)
- Calculate the change of core permeability according to the flooding pressure difference and comprehensively analyze the blockage risk and mechanism in the core during water injection based on the core permeability fluctuation, sand grain size distribution, and phenomena observed.
3.1.4. Experimental Scheme
3.2. Experimental Results and Analysis
3.2.1. Blockage Risk Caused by the Migration of Movable Particles
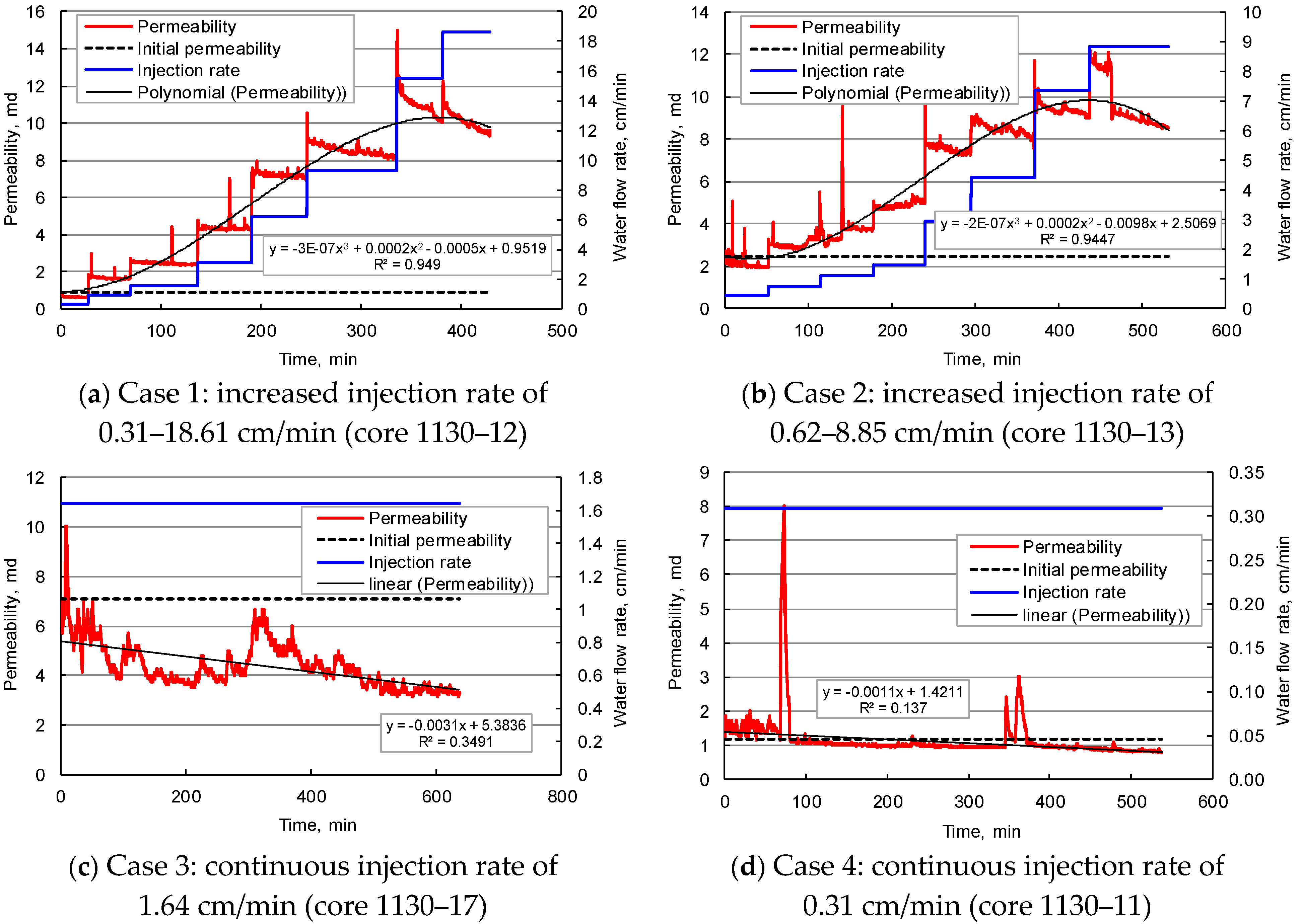
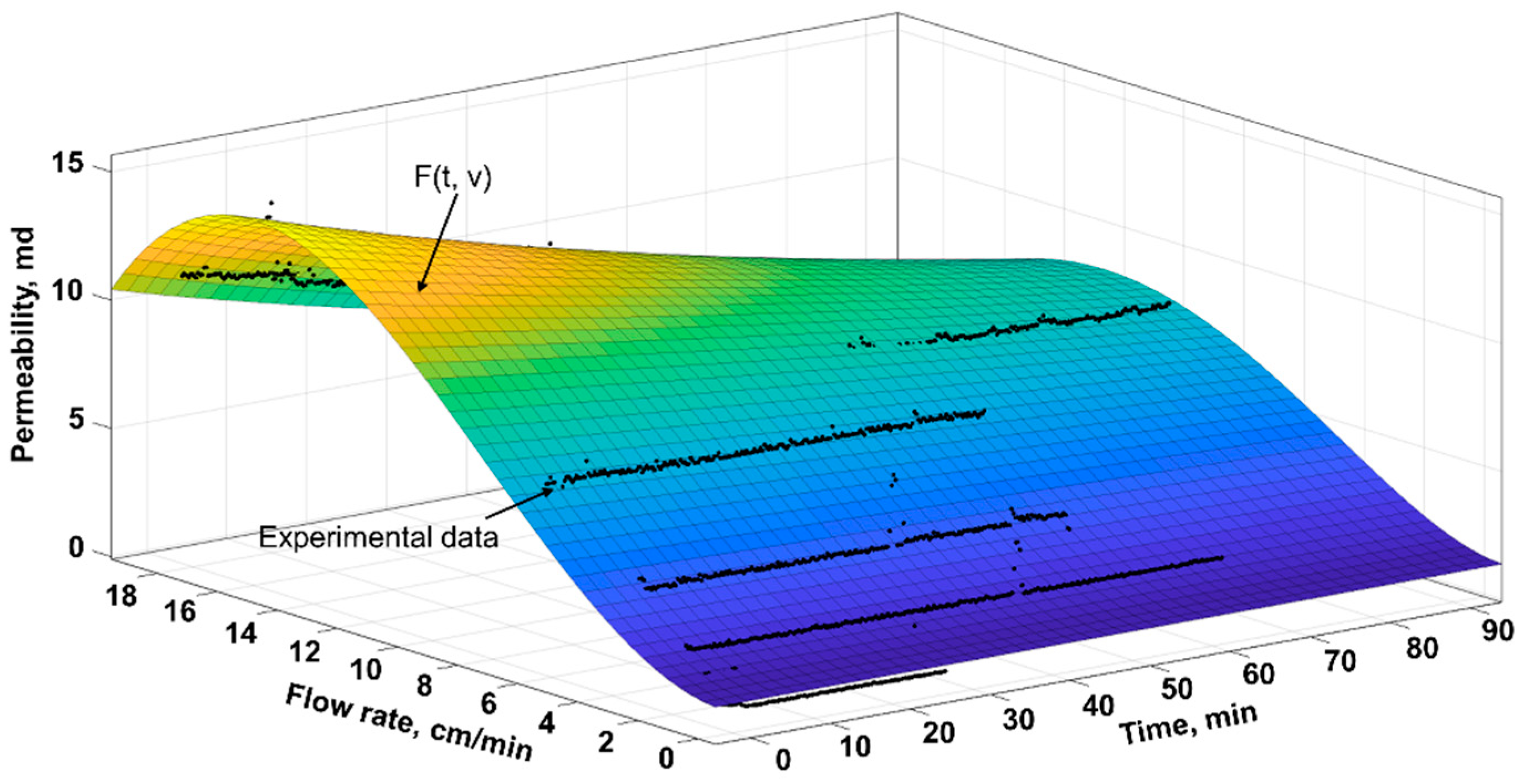
Influence of Water Flow Rate
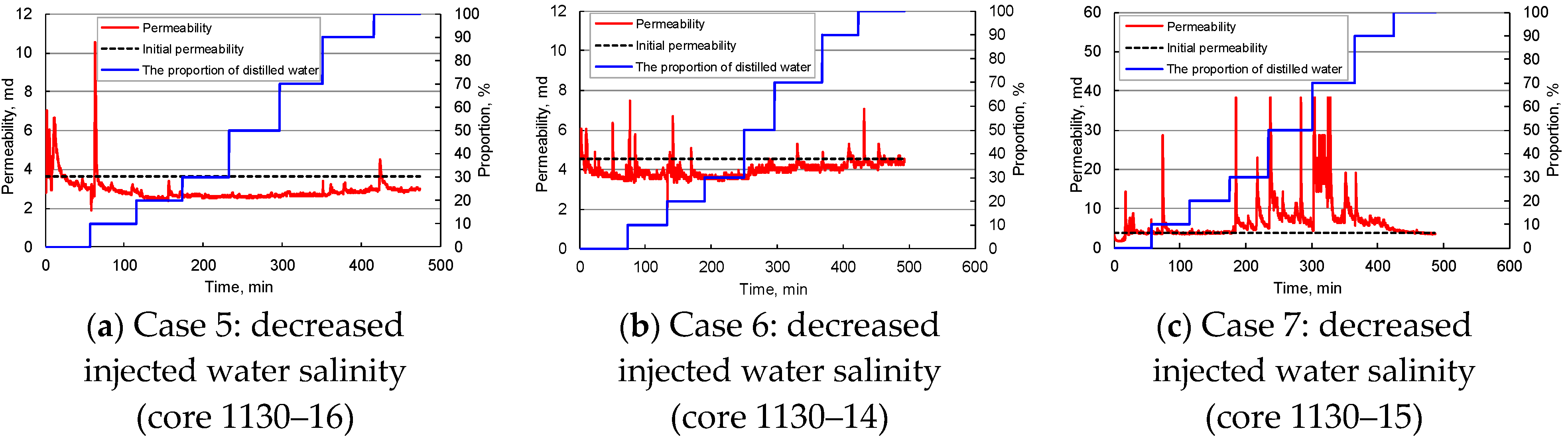
Influence of Injected Water Salinity
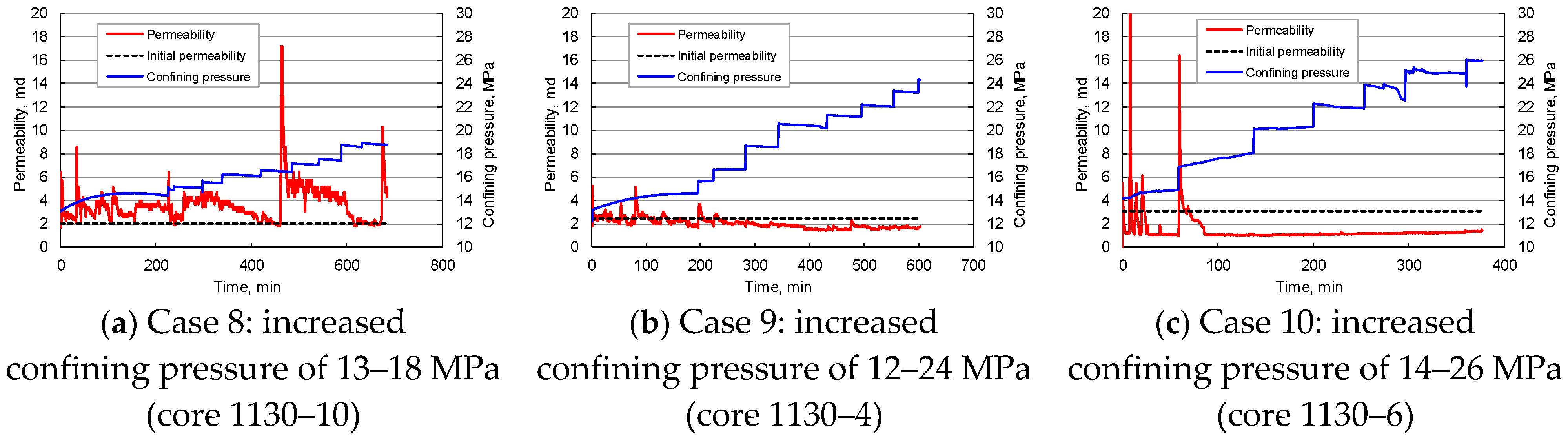
Influence of Confining Pressure
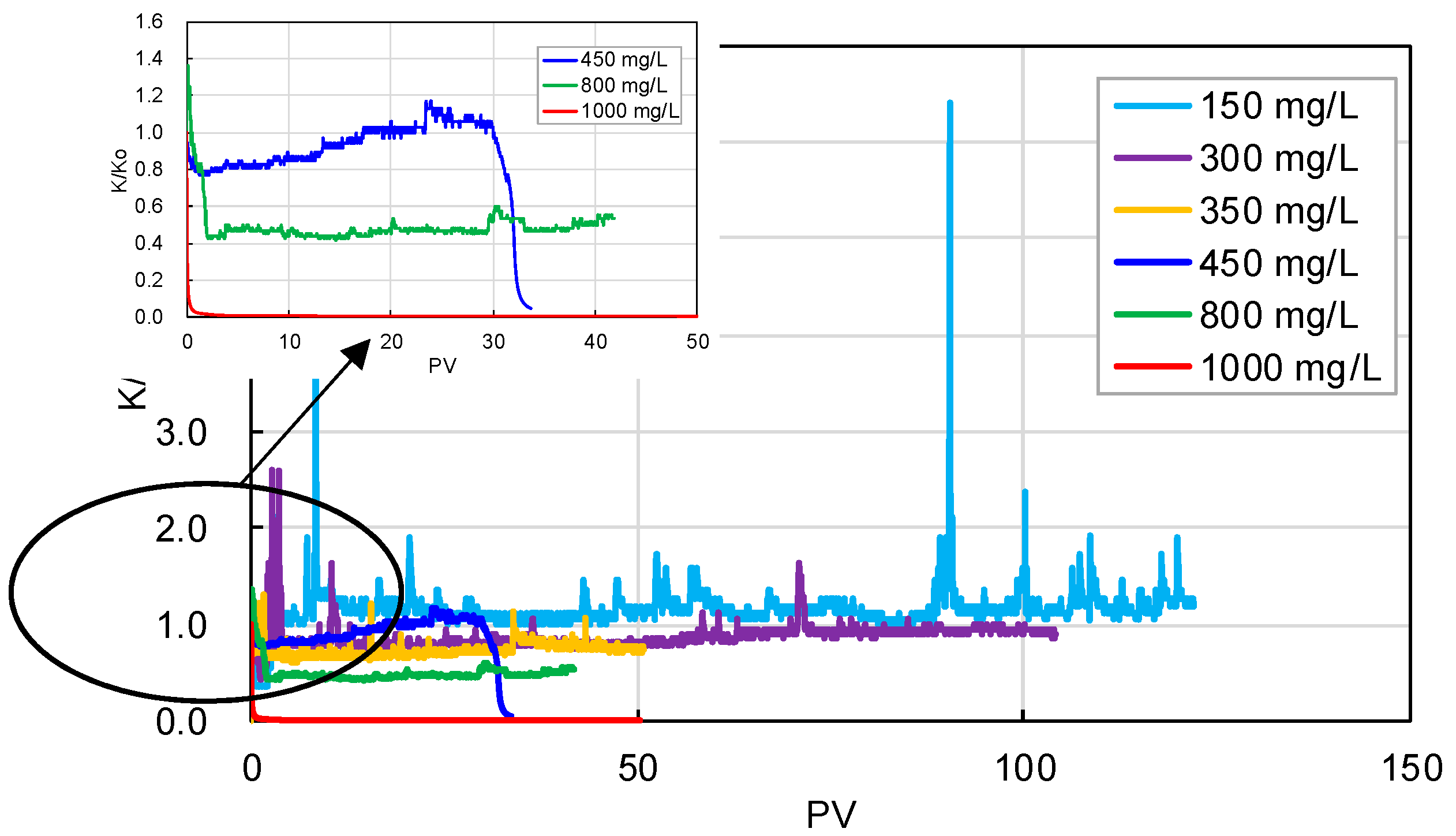
3.2.2. Blockage Risk Caused by the Scaling Particles in Injected Water
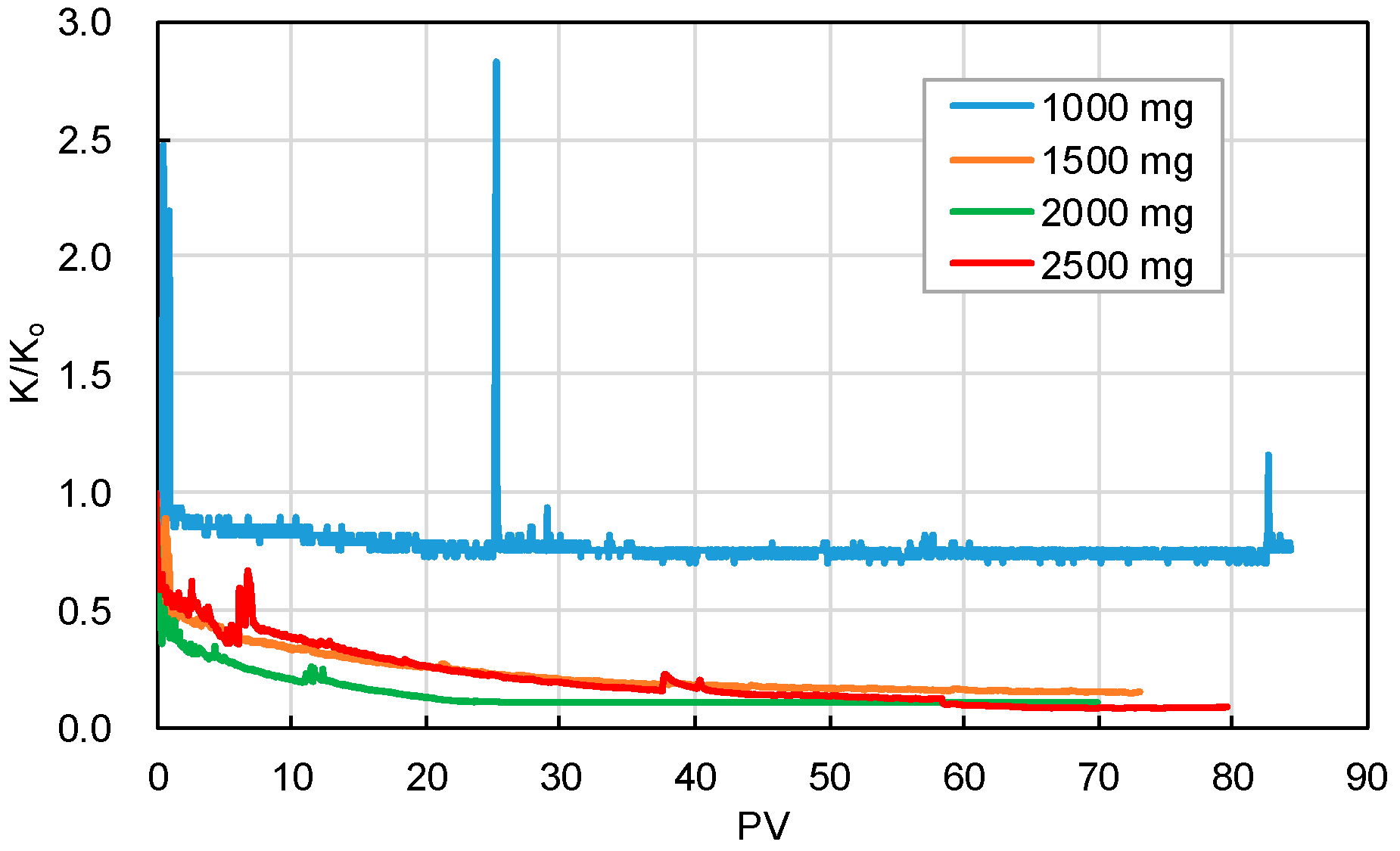
3.2.3. Blockage Risk Caused by the Suspended Particles in Injected Water
4. Discussion
5. Conclusions
- (1)
- The geothermal water in well DR2018 has high salinity and high corrosion and scaling risks. The geothermal reservoir is characterized by a low porosity of 1.64–18.68%, a low permeability of 0.04–7.23 md, and weak rock consolidation of sandstone with clay as the main cement. The sand grain size has a bimodal distribution. The movable clay and sand particles in cores account for 0.18–23.42 wt %, which brings a potential risk of formation blockage for the geothermal water reinjection.
- (2)
- The geothermal reservoir has a significant sensitivity to water flow rate and salinity. The start, migration, deposition, and plugging of clay and sand particles in pores affect the reservoir’s physical properties. Stepped enhancement of water injection rate can increase the core permeability, but when the water flow velocity exceeds 2.95–6.20 cm/min, the core permeability will decline rapidly. Even at low water flow velocity of 0.31–1.64 cm/min, the rock permeability will drop by 35–53% after 25–144 PV injection. With the decrease in the salinity of injected water, the core permeability fluctuates drastically, reflecting the hydration, expansion, and detachment of clay particles in pores and enhancing the reservoir blockage risk. The increase in confining pressure tends to decrease the core permeability, but it may be counteracted by the permeability increase caused by the migration of movable particles.
- (3)
- The intrusive particles in the near-wellbore formation are mainly scaling and suspended clay and sand particles in the injected geothermal water. The higher the content of solid particles in water, the more significant the decrease in core permeability. The blockage risk induced by low-content solid particles in injected water can be covered up by the migration of movable particles in cores. When the content of scaling particles in water is 450–1000 mg/L, the core permeability can decrease by 3–99% after 30 PV injection. In contrast, when the content of suspended particles is 1000–2500 mg/L, the permeability can reduce by 27–92% after 70PV injection. The invasive particles can be easily removed by pretreatment, such as filtration, while the movable particles generated in the reservoir are hard to be eliminated. Hence, the migration and blockage of movable particles in the near-wellbore formation will be the main reason to cause the decline in the well’s geothermal reinjection capacity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature List
| CSP | CaCO3 scaling particles |
| DR | Dire, Chinese abbreviation for “geothermal” |
| GW | Geothermal water |
| md | Millidarcy, equivalent to 10−15 m2 |
| PV | Pore volume |
| SKCP | Suspended kaolin clay particles |
References
- Liu, J. The status of geothermal reinjection. Hydrogeol. Eng. Geol. 2003, 30, 100–104, (In Chinese with English abstract). [Google Scholar]
- Ochi, J.; Vernoux, J.F. Permeability decrease in sandstone reservoirs by fluid injection: Hydrodynamic and chemical effects. J. Hydrol. 1998, 208, 237–248. [Google Scholar] [CrossRef]
- Lun, Z. Study on the blockage characteristics of reservoir pores due to suspension particles of injection water. J. Logist. Eng. Univ. 2006, 3, 30–33, (In Chinese with English abstract). [Google Scholar]
- Ma, Z.; Hou, C.; Xi, L.; Yun, H.; Sun, C. Reinjection clogging mechanism of used geothermal water in a super-deep-porous reservoir. Hydrogeol. Eng. Geol. 2013, 40, 133–139, (In Chinese with English abstract). [Google Scholar]
- Badalyan, A.; Carageorgos, T.; You, Z.; Schacht, U.; Bedrikovetsky, P.; Matthews, C.; Hand, M. A New Experimental Procedure for Formation Damage Assessment in Geothermal Wells. In Thirty-Ninth Workshop on Geothermal Reservoir Engineering; Stanford University: Stanford, CA, USA, 2014. [Google Scholar]
- Oliveira, M.A.; Vaz, A.S.; Siqueira, F.D.; Yang, Y.; You, Z.; Bedrikovetsky, P. Slow migration of mobilised fines during flow in reservoir rocks: Laboratory study. J. Pet. Sci. Eng. 2014, 122, 534–541. [Google Scholar] [CrossRef]
- Rosenbrand, E.; Haugwitz, C.; Jacobsen, P.S.M.; Kjøller, C.; Fabricius, I.L. The effect of hot water injection on sandstone permeability. Geothermics 2014, 50, 155–166. [Google Scholar] [CrossRef]
- You, Z.; Yang, Y.; Badalyan, A.; Bedrikovetsky, P.; Hand, M. Mathematical modelling of fines migration in geothermal reservoirs. Geothermics 2016, 59, 123–133. [Google Scholar] [CrossRef]
- Russell, T.; Pham, D.; Petho, G.; Neishaboor, M.T.; Badalyan, A.; Behr, A.; Bedrikovetsky, P. Kaolinite mobilisation in unconsolidated porous media: Effect of brine salinity and salt type Na and Ca salts. In Proceedings of the SPE-191922-MS SPE Asia Pacific Oil and Gas Conference and Exhibition, Brisbane, Australia, 23–25 October 2018. [Google Scholar]
- You, Z.; Badalyan, A.; Yang, Y.; Bedrikovetsky, P.; Hand, M. Fines migration in geothermal reservoirs: Laboratory and mathematical modelling. Geothermics 2019, 77, 344–367. [Google Scholar] [CrossRef]
- Carpenter, C. Study of Carbonate Reservoirs Examines Fines Migration in CO2-Saturated-Brine Flow. J. Pet. Technol. 2019, 71, 76–77. [Google Scholar] [CrossRef]
- Sun, P.; Song, H.; Qi, J.; Wang, J.; Xu, Z.; Yuan, M. Study on sand control techniques for loose sandstone geothermal wells in Guantao formation of Neogene. Sci. Technol. Innov. 2019, 201906, 12–14, (In Chinese with English abstract). [Google Scholar]
- Niknam, P.H.; Talluri, L.; Fiaschi, D.; Manfrida, G. Improved Solubility Model for Pure Gas and Binary Mixture of CO2-H2S in Water: A Geothermal Case Study with Total Reinjection. Energies 2020, 13, 2883. [Google Scholar] [CrossRef]
- Niknam, P.H.; Talluri, L.; Fiaschi, D.; Manfrida, G. Sensitivity analysis and dynamic modelling of the reinjection process in a binary cycle geothermal power plant of Larderello area. Energy 2021, 214, 118869. [Google Scholar] [CrossRef]
- Steefel, C.; Lasaga, A. A coupled model for transport of multiple chemical species and kinetic precipitation/dissolution reactions with application to reactive flow in single phase hydrothermal systems. Am. J. Sci. 1994, 294, 529–592. [Google Scholar] [CrossRef]
- Jafari, S.; Salmanzadeh, M.; Rahnama, M.; Ahmadi, G. Investigation of particle dispersion and deposition in a channel with a square cylinder obstruction using the lattice Boltzmann method. J. Aerosol Sci. 2010, 41, 198–206. [Google Scholar] [CrossRef]
- Ungemach, P. Reinjection of cooled geothermal brines into sandstone reservoirs. Geothermics 2003, 32, 743–761. [Google Scholar] [CrossRef]
- Rose, P.; Xu, T.; Kovac, K.; Mella, M.; Pruess, K. Chemical Stimulation in Near-Wellbore Geothermal Formations: Silica Dissolution in the Presence of Calcite at High Temperature and High pH. In Proceedings of the Thirty-Second Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 22–24 January 2007. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China. HJ 493-2009 Water Quality Sample—Technical Regulation of the Preservation and Handing of Samples; China Environmental Press: Beijing, China, 2009.
- Ministry of Geology and Mineral Resources of the People’s Republic of China. DZ/T 0064.1-80-1993 Groundwater Quality Inspection Method; Standards Press of China: Beijing, China, 1993.
- State Bureau of Petroleum and Chemical Industry of China. GB/T 5368-2000 Thin Section Examination of Rock; Standards Press of China: Beijing, China, 2000. [Google Scholar]
- Standardization Administration of China. GB/T 14505-2010 Method for Chemical Analysis of Rocks and Ores—General Rules and Regulations; Standards Press of China: Beijing, China, 2010.
- National Energy Administration of China. SY/T 5434-2018 Analysis Method for Particle Size of Clastic Rocks; Standards Press of China: Beijing, China, 2018.
- Yu, L.; Zhang, L.; Zhang, R.; Ren, S.R. Assessment of natural gas production from hydrate-bearing sediments with unconsolidated argillaceous siltstones via a controlled sandout method. Energy 2018, 160, 654–667. [Google Scholar] [CrossRef]
- Zhang, L.; Chao, J.; Geng, S.; Zhen, Z.; Chen, H.; Luo, Y.; Qin, G. Particle migration and blockage in geothermal reservoirs during water reinjection: Laboratory experiment and reaction kinetic model. Energy 2020, 206, 118234. [Google Scholar] [CrossRef]
- Standardization Administration of China. GB/T 29172–2012 Practices for Core Analysis; Standards Press of China: Beijing, China, 2012. [Google Scholar]
- Shi, J.; Gong, W.; Cao, W.; Xu, Y. A research into the damage mechanism of velocity-sensitivity in sandstone of a reservoir. J. Chengdu Univ. Technol. 2003, 30, 501–504, (In Chinese with English abstract). [Google Scholar]
- Miyamoto, M.; Ichikawa, H.; Fukumori, Y.; Akine, Y.; Tokuuye, K. Design and Preparation of Gadolinium-Reservoir Microcapsules for Neutron-Capture Therapy by Means of the Wurster Process. Chem. Pharm. Bull. 1997, 45, 2043–2050. [Google Scholar] [CrossRef][Green Version]
- Larsen, T.; Lioliou, M.; Ostvold, T.; Josang, L.; Randhol, P. Kinetics of CaCO3 scale formation during core flooding. In Proceedings of the SPE-114045-MS SPE International Oilfield Scale Conference, Aberdeen, UK, 28–29 May 2008. [Google Scholar]
- Ma, L.; Li, X.; Tan, J.; Cai, Y.; Ma, Y. Experimental study on dissolution of carbonate and scaling tendency of formation water in CO2 flooding. J. Xi’an Shiyou Univ. 2016, 31, 68–72, (In Chinese with English abstract). [Google Scholar]
- Xu, G.; Ma, Z.; Zhou, X.; Xi, L.; Sun, C. Study on the mechanism of chemical clog for the recharging of geopressured thermal water--taking the recharging well No. 1 in Xianyang as the example. Geotech. Investig. Surv. 2013, 41, 40–44, (In Chinese with English abstract). [Google Scholar]
- Brown, C.A.; Compton, R.G.; Narramore, C.A. The Kinetics of Calcite Dissolution/Precipitation. J. Colloid Interface Sci. 1993, 160, 372–379. [Google Scholar] [CrossRef]
- Moghadasi, J.; Jamialahmadi, M.; Muller-Steinhagen, H.; Sharif, A. Formation damage due to scale formation in porous media resulting from water injection. In Proceedings of the SPE-86524-MS SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 17–28 February 2004. [Google Scholar]
- Lu, Y.; Du, X.; Chi, B.; Yang, X.; Li, S.; Wang, Z. The porous media clogging due to suspended solid during the artificial rechange of groundwater. J. Jilin Univ. 2011, 41, 448–453, (In Chinese with English abstract). [Google Scholar]
- Zhou, X. Reinjection Plugging Mechanism Researching of Sedimentary Basin Type Porous Geothermal Water—As the Reinjection Well in Sanqiao of Xi’an for an Example; Chang’an University: Xi’an China, 2013; (In Chinese with English abstract). [Google Scholar]
- Wang, L. A Study of Geothermal Reinjection in the Guantao Reservoir in Tianjin; China University of Geosciences: Beijing, China, 2014; (In Chinese with English abstract). [Google Scholar]
- Wu, J.; Zhang, J.; Li, X.; Shi, F.; Zhang, P.; Bai, M. Experiment on artificial pressure re-injection of geothermal water in Xi’an suburb. J. Water Resour. Water Eng. 2014, 25, 215–218, (In Chinese with English abstract). [Google Scholar]
- Seibt, P.; Kellner, T. Practical experience in the reinjection of cooled thermal waters back into sandstone reservoirs. Geothermics 2003, 32, 733–741. [Google Scholar] [CrossRef]
- Xu, T.; Pruess, K. Numerical Simulation of Injectivity Effects of Mineral Scaling and Clay Swelling in a Fractured Geothermal Reservoir; Lawrence Berkeley National Lab (LBNL): Berkeley, CA, USA, 2004. [Google Scholar]
- Sandrine, P.; Francois, V.; Patrick, N. Chemical stimulation techniques for geothermal wells: Experiments on the three-well EGS system at Soultz-sous-Forêts, France. Geothermic 2009, 38, 349–359. [Google Scholar]





| Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|
| K+, mg/L | 73.10 | Li, mg/L | 0.56 | Rn, Bq/L | <0.01 |
| Na+, mg/L | 12,328.00 | Sr, mg/L | 15.57 | Metaboric acid, mg/L | 13.68 |
| K+ + Na+, mg/L | 12,401.10 | Zn, mg/L | 0.04 | Salinity, mg/L | 36,005.20 |
| Ca2+, mg/L | 284.10 | Se, mg/L | <0.02 | H2SiO3, mg/L | 43.21 |
| Mg2+, mg/L | 71.00 | As, mg/L | <0.02 | Free CO2, mL/L | 23.3 |
| Cl−, mg/L | 9745.8 | Hg, mg/L | <0.02 | pH | 7.56 |
| SO42−, mg/L | 12,122.94 | Cd, mg/L | <0.02 | Total alkalinity, mg/L | 2262.79 |
| HCO3−, mg/L | 1380.30 | B, mg/L | 1.78 | Total hardness, mg/L | 1001.23 |
| CO32−, mg/L | 0.00 | Ag, mg/L | <0.02 | Suspended particles, mg/L | 122 |
| NH4+, mg/L | 2.36 | Ba, mg/L | 0.1 | Dissolved gas, mL/L | 214 |
| Fe2+, mg/L | <0.02 | Pb, mg/L | <0.02 | CO2 in dissolved gas, mol% | 61.47 |
| Fe3+, mg/L | <0.02 | Mo, mg/L | 0.11 | H2S in dissolved gas, mol% | 10.34 |
| F−, mg/L | 0 | Cu, mg/L | <0.02 | CH4 in dissolved gas, mol% | 14.82 |
| NO3−, mg/L | 25.15 | Mn, mg/L | 0.15 | ||
| Cr6+, mg/L | <0.02 | Al, mg/L | 0.04 |
| Grain Size | Sample Depth, m | 764 | 821 | 882 | 1130 | 1433 | 1487 | 1541 | 1602 |
|---|---|---|---|---|---|---|---|---|---|
| >100 μm (as skeleton) | Average particle diameter, μm | 503.84 | 431.55 | 399.54 | 396.12 | 398.25 | 400.18 | 399.09 | 158.59 |
| Max. pore throat, μm | 208.59 | 178.66 | 165.41 | 163.99 | 164.88 | 165.68 | 165.22 | 65.66 | |
| Min. pore throat, μm | 77.94 | 66.76 | 61.81 | 61.28 | 61.61 | 61.91 | 61.74 | 24.53 | |
| <100 μm (with movable tendency) | Small grain content, wt % | 17.54 | 14.30 | 22.68 | 20.88 | 19.28 | 7.68 | 23.42 | 16.59 |
| <1/2 pore throat particles, wt % | 17.54 | 10.51 | 19.33 | 12.87 | 18.94 | 7.67 | 23.42 | 0.18 | |
| 1/2–1/5 pore throat particles, wt % | 16.31 | 9.34 | 17.77 | 11.51 | 18.87 | 7.60 | 23.22 | 0.11 | |
| <1/5 pore throat particles, wt % | 1.23 | 1.18 | 1.56 | 1.36 | 0.07 | 0.07 | 0.20 | 0.07 |
| Case | Injection Fluid | Injection Rate, mL/min | Confining Pressure, MPa | Core | Initial K, md | Porosity, % | Purpose |
|---|---|---|---|---|---|---|---|
| 1 | DR2018 | 0.1–6 | 14 | 1130–12 | 0.89 | 7.82 | The influence of injection rate |
| 2 | 0.1–6 | 14 | 1130–13 | 2.46 | 14.19 | ||
| 3 | 0.5 | 14 | 1130–17 | 7.07 | 6.53 | ||
| 4 | 0.1 | 14 | 1130–11 | 1.2 | 6.97 | ||
| 5 | DR2018 + distilled water | 0.5 (0–100%) | 14 | 1130–16 | 3.62 | 10.86 | The influence of salinity |
| 6 | 0.5 (0–100%) | 14 | 1130–14 | 4.54 | 7.25 | ||
| 7 | 0.5 (0–100%) | 14 | 1130–15 | 3.83 | 10.64 | ||
| 8 | DR2018 | 0.5 | 14–25 | 1130–10 | 2.06 | 12.15 | The influence of confining pressure |
| 9 | 0.5 | 14–25 | 1130–4 | 2.46 | 9.36 | ||
| 10 | 0.5 | 14–25 | 1130–6 | 3.07 | 9.46 | ||
| 11 | DR2018 + NaHCO3 + CaCl2 | 0.5 (150 mg/L) | 14 | 1130–18 | 7.23 | 7.52 | The influence of scaling particles |
| 12 | 0.5 (300 mg/L) | 14 | 1130–19 | 6.83 | 8.71 | ||
| 13 | 0.5 (350 mg/L) | 14 | 1130–22 | 7.25 | 11.99 | ||
| 14 | 0.5 (450 mg/L) | 14 | 1130–23 | 3.52 | 9.86 | ||
| 15 | 0.5 (800 mg/L) | 14 | 1130–20 | 8.20 | 8.86 | ||
| 16 | 0.5 (1000 mg/L) | 14 | 1130–21 | 2.67 | 9.92 | ||
| 17 | DR2018 + kaolin clay particles | 0.5 (1000 mg/L) | 14 | 1130–7 | 6.56 | 6.55 | The influence of suspended particles |
| 18 | 0.5 (1500 mg/L) | 14 | 1130–3 | 4.81 | 13.4 | ||
| 19 | 0.5 (2000 mg/L) | 14 | 1130–2 | 4.73 | 26.38 | ||
| 20 | 0.5 (2500 mg/L) | 14 | 1130–5 | 3.42 | 9.87 |
| In the Experiments | Calculated by OLIstudio ScaleChem | |||
|---|---|---|---|---|
| Case | CaCl2, mol/L | NaHCO3, mol/L | CaCO3 Scaling Concentration, mg/L | CaCO3 Scaling Concentration, mg/L |
| 11 | 0.0047 | 0.0092 | 150.00 | 166.30 |
| 12 | 0.0066 | 0.0129 | 300.00 | 293.43 |
| 13 | 0.0075 | 0.0147 | 350.00 | 360.25 |
| 14 | 0.0088 | 0.0174 | 450.00 | 460.06 |
| 15 | 0.0137 | 0.0270 | 800.00 | 838.16 |
| 16 | 0.0162 | 0.0319 | 1000.00 | 1036.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Qin, G.; Luo, Y.; Geng, S.; Yang, L.; Wen, R.; Chao, J.; Zhang, L. Experimental Study on Reservoir Physical Properties and Formation Blockage Risk in Geothermal Water Reinjection in Xining Basin: Taking Well DR2018 as an Example. Energies 2021, 14, 2671. https://doi.org/10.3390/en14092671
Zhao Z, Qin G, Luo Y, Geng S, Yang L, Wen R, Chao J, Zhang L. Experimental Study on Reservoir Physical Properties and Formation Blockage Risk in Geothermal Water Reinjection in Xining Basin: Taking Well DR2018 as an Example. Energies. 2021; 14(9):2671. https://doi.org/10.3390/en14092671
Chicago/Turabian StyleZhao, Zhen, Guangxiong Qin, Yinfei Luo, Songhe Geng, Linchao Yang, Ronghua Wen, Jiahao Chao, and Liang Zhang. 2021. "Experimental Study on Reservoir Physical Properties and Formation Blockage Risk in Geothermal Water Reinjection in Xining Basin: Taking Well DR2018 as an Example" Energies 14, no. 9: 2671. https://doi.org/10.3390/en14092671
APA StyleZhao, Z., Qin, G., Luo, Y., Geng, S., Yang, L., Wen, R., Chao, J., & Zhang, L. (2021). Experimental Study on Reservoir Physical Properties and Formation Blockage Risk in Geothermal Water Reinjection in Xining Basin: Taking Well DR2018 as an Example. Energies, 14(9), 2671. https://doi.org/10.3390/en14092671








