1. Introduction
Refractory materials are widely used in high-temperature industrial environments, such as the aerospace, steel and construction industries. Magnesia oxide plays an irreplaceable role in refractory material production because of its resistance to oxidation, deoxidation and thermal decomposition. An electric arc furnace (EAF) was first used to produce fused magnesia in 1991 to facilitate high-quality magnesia oxide production in China [
1]. In the industrial process of MgO production, the main equipment used to produce fused magnesia oxide is a fused magnesia furnace, and the heat resource of the furnace consists of arc heat generation (40%) and resistance heat generation (60%) [
2]. With a rapid increase in MgO requirement in several industries, over 90% of electrically fused magnesia oxide has been produced in a low energy efficiency EAF, which is a serious waste of electrical energy [
3,
4,
5,
6]. There are two common electric fused systems that are used to produce electrically fused magnesia oxide: the two-electrode direct current magnesia furnace (DFMF) and three-electrode alternating current fused magnesia furnace (AFMF). The researchers’ research focuses mainly on power supply systems and automation control. Wang et al. estimated the electrical system losses of electric arc furnaces, and Fu et al. studied the common blow-out phenomenon in electric magnesia furnaces and gave a predictive model that can predict the occurrence of the phenomenon of blasting furnaces in magnesia fusion furnaces [
7,
8]. Compared with the DFMF system, the AFMF system is a widely used method because of its mature technology and good production ability; however, the DFMF system is also used in some plants due to its higher energy efficiency and low equipment cost. Qin improved the basic structural parameters and operation methods of the two-electrode DC electric arc furnace and described the energy-saving characteristics of the two-electrode DC electric arc furnace. In addition, Kong et al. improved the control method of a three-electrode AC electric arc furnace and summarised the advantages of a three-phase electric arc furnace [
9,
10]. Furthermore, it is also clear that substantial energy consumption and large numbers of byproducts exist in both the AFMF system and DFMF system. As the requirement of high-quality MgO increases, AFMF systems play an important role in high-quality MgO production, and the improvement in the energy efficiency of AFMF systems has received much attention in the past decade. Li et al. conducted a series of studies on the cleaner production of magnesium oxide in Liaoning Province, analysed the production process from the perspective of the urban environment and production environment and gave the best production process for refractory materials using magnesium oxide [
5,
11]. Based on the metallurgical system, Chai et al. carried out a comparative modelling of the fused magnesia production process, applied the CPS system to the fused magnesia production process and gave the basic realisation route of the fused magnesia CPS system [
12]. The AFMF system can also be divided into two processes, namely, the magnesite ore smelting process (MOP) and light-burned magnesia process (LMP), due to different raw material preparation methods.
The raw materials of the MOP process are produced in an ore-crushing plant, and magnesite ores are mined from the magnesite mines and are transported to be crushed. The main raw material of the LMP process is light calcinated magnesia. Light calcinated magnesia powder is generally produced in a light-burning plant, where the raw material ore is sent to the light-calcinating equipment for calcination at a temperature of 900–1000 K [
13]. Inside, the ore decomposes and generates light calcinated magnesia. During the light calcination process, the main heat source is the combustion of the gas by the gas producer. After the light calcinated magnesia powder is produced, it is placed for cooling to room temperature.
After cooling, light-calcined magnesia powder can be pressed to pellets and sent to an electric-fused plant. In addition to the equipment used in light-calcined plants, the basic equipment of MOPs and LMPs are almost the same in electric fused plants.
The light-calcined system is the main equipment in the LMP. Unlike in the MOP, a light-calcined system is used to pretreat the raw materials by heating them at a temperature between 800 K and 1000 K. In the temperature range from 800–1000 K, the MgCO
3 in magnesite ores decompose into CO
2 and MgO. Longo et al. provided several chemical reaction equations for MgCO
3 decomposition production at different temperature ranges, and studies have shown that the production of MgO presents different states at different temperature ranges [
14]. Jie Y. et al. summarised the research directions in the field of fused magnesia as optimisation, control, modelling and experimental constraints and also summarised the main problems in the field of fused magnesia and the intelligent technologies that can be applied [
15].
The three-electrode alternating current fused magnesia furnace (AFMF) system plays the most important role in both MOPs and LMPs, and the system in electric fused plants mainly consists of a high-voltage transformer (HVT), material transport equipment (MTE) and an AFMF. High-voltage electrical power is translated into standard electricity with a regime of 2800 kVA through a multistage coil in the HVT. A mathematical model used to calculate the electric loss of HVTs was developed by Gutiérrez et al. considering multistage coil loss (copper loss), wire loss (iron loss) and reactive power [
16]. The electrical loss assessment of an HVT was discussed by Zhou’s group with respect to source-load power uncertainty and electricity price fluctuations [
17]. An MTE system consists of material feeders and material setters; a complex control system was designed by Chai based on a cyber-physical system (CPS) system, and an integrated optimal operation control algorithm was proposed [
18]. Liu designed and modelled all of the equipment to lay magnesite ores, and the structural strength and stability were studied based on ANSYS workbench 17.0. At the same time, a control program compiled by a ladder diagram was also developed [
19]. The AFMF is the most important equipment in both MOPs and LMPs; raw materials are preheated at the upper part of the furnace, and a smelting process occurs in the lower part of the furnace [
7]. Shan explained the mechanism of ore preheating and surface heat transfer and discussed the influencing factors of the resultant heat transfer. The equipment used to heat water, which is needed for use in industrial parks, was designed and applied in Don-xing refractory company to avoid wasting heat [
20]. Fu. et al. performed some research on the prediction of eruptions occurring in the AFMF, and a software and hardware set that can accurately identify the vibration frequency of raw material eruptions was developed together [
21]. Li established a mathematical model to describe phenomena that occurs in furnaces, i.e., the magnetic field, fluid flow field and heat transfer; at the same time, the combustion enthalpy change was also considered in the model [
22]. Although researchers have performed many studies on the control system of AFMF systems and affiliated equipment to achieve better energy efficiency, few studies have provided a clear comparison of MOPs and LMPs with respect to energy performance.
Exergy analysis has been widely used to evaluate the energy efficiency of industrial processes, and the efficiency and rationality of the exergy concept has been proven in metallurgical systems. Rong et al. applied exergy analysis to the rotary kiln-electric furnace (RKEF) system, investigated the potential for energy savings, and used waste heat to preheat the raw materials [
23]. Yu et al. presented comprehensive exergy and energy analyses to compare the energy performance of RKEF systems and sintering-preheating-submerge arc furnace (SPSF) systems and concluded that SPSF systems are more environmentally friendly than RKEF systems and that waste gas could be recycled and show a better energy saving effect [
24]. Zhang et al. studied the exergy loss of each component of electric vehicles, and an exergy analysis was carried out on the heat pump air conditioning system (HPACS) of electric vehicles with a battery thermal management system. The results show that under all operating conditions, the compressor is the main source of system exergy losses [
25,
26,
27,
28,
29]. Harmed K. also carried out corresponding research in the field of seawater desalination. This article focuses on the recovery of sewage as the research object and uses electric energy as the energy supply for evaporation treatment to provide good environmental protection. Energy-exergy loss was evaluated. In addition, the researchers also conducted corresponding evaluations and studies on the reverse osmosis seawater desalination process based on geothermal energy [
30,
31,
32]. Mahmoudan A. proposed a new type of integrated energy system based on a geothermal heat source and LNG radiator and carried out energy, exergy and exergy economic evaluations of the system. At the same time, parameter research was carried out on different decision variables, and finally, the TOPSIS method specified the optimal control scheme [
31]. Although many exergy analyses have been applied in several industrial systems, exergy analyses of MOP and LMP in AFMF systems have not been discussed in previous studies. The preparation process of MgO requires large amounts of electricity, and the energy saving potential urgently needs to be evaluated.
In the present work, energy, exergy, and environmental analyses of a real-scale magnesite smelting process were applied to evaluate the energy efficiency, exergy efficiency and performance with respect to environmental protection of MOP and LMP. In the circumstances of a reduction in carbon emissions, the importance of evaluating the energy and exergy efficiency and eco-friendliness of energy-intensive enterprises is obvious. This study provides a series of key parameter analyses in magnesite smelting process systems and shows the tremendous potential of environmental protection, energy savings and exergy savings
2. System Description
Figure 1 shows the main equipment of the MOP and LMP systems. The two parts in the picture were taken on the top and side of the mould, showing the electrode, the mould and the finished product of fused magnesia waiting to be broken after the production process. The three-phase magnesia smelting furnace is located at No. 1 Magnesite Plant of Hai-cheng Magnesite Group Corporation. The furnace provides experimental data used in the text. The equipment mainly consists of three carbon electrodes, servo motors and crystallisers.
Figure 2 shows the total process of MOP and LMP. The arrows in the map show the translation of materials and directions of energy and mass flow. LMP and MOP utilise different pretreatments of materials of different purities. Ores were first mined from a magnesite mine and transported to a sorting centre in which the mass fraction of different batch ores can be tested and divided into three ranges. The raw materials with mass fraction of MgCO
3 between 0.7 and 0.8 are referred to as inferior ores; these raw materials must be floated in a flotation kiln first to filter out the materials with a mass fraction lower than 0.75 based on the standards summarised from production experience. Materials with a mass fraction lower than 0.75 were used to treat pollution gas. Other materials were sent to a light-calcined warehouse and were mixed with raw materials with a mass fraction between 0.8 and 0.9 (named premium ores). The materials were sent to a light-calcined bed, and after the light-calcined process, the pre-production materials were transported to the electric fused furnace. This total process is referred to as LMP. In addition to the materials mentioned above, ores with mass fractions higher than 0.9 are named excellent ores. These ores are only crushed in crushing plants first and then are transported to electric fused furnaces. This process is named MOP.
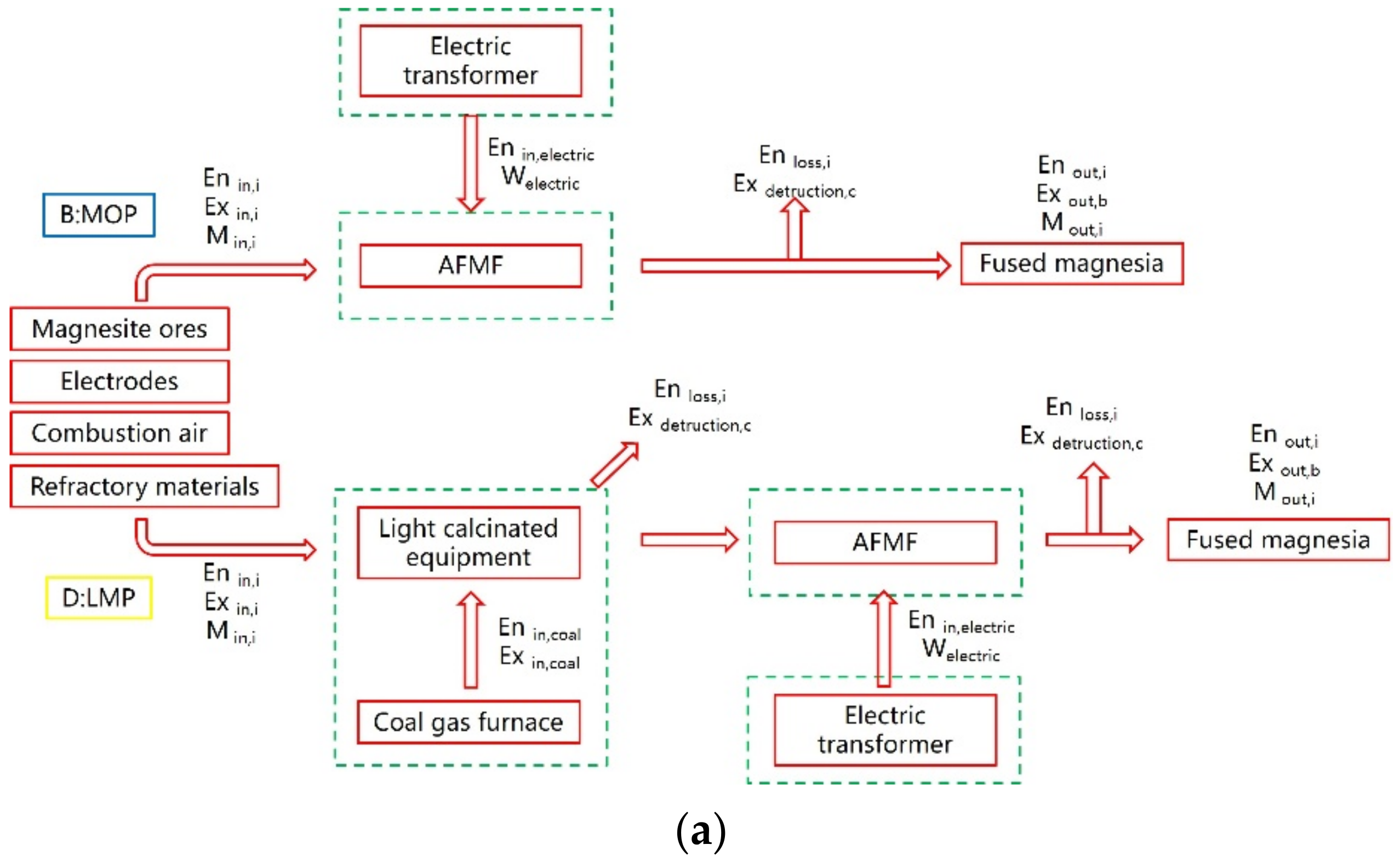
Figure 3a shows the specific production process under the two different processes of LMP and MOP, as well as the boundary division when calculating the mass–energy–exergy balance. First, regarding the mass balance, in the MOP route, ore, electrodes, combustion air and refractory materials are considered input items of the process flow. After the smelting is completed, the fused magnesia, flue gas, remaining ore and byproducts are considered outputs. In the LMP route, the ore is processed in the light calciner and then sent to AFMF to complete smelting. The decomposition process of the ore is completed in the light calciner. The mass input and output items are the same as in the MOP route. In the light calciner, less attention has been given to the flue gas emission of the calcination process because the preparation process of the raw materials is not the focus of this article. The article only calculates the mass output of its light calcinated magnesia powder. Second, regarding the energy balance, in the MOP route,
Figure 3a cannot explain the preheating of the ore or the incomplete solidification of molten magnesium oxide. As a supplement, the detailed flow of internal energy is given in
Figure 3b. Combining the two figures, the information on the energy balance, input items, output items, and loss items of the energy balance are all marked. Unlike the MOP process, the LMP process involves the light calcination process. To make the calculation more detailed, the electrical system, the light calcinating system and the AFMF are divided into several calculation domains. The sum of the results of each calculation domain can obtain the energy balance of the overall LMP process, and the production unit consumption of the two process routes are calculated. Finally, regarding the analysis of exergy balance, the exergy input of the MOP process mainly includes ore, electrodes, reaction air, etc., and the output items mainly include fused magnesia, auxiliary products, etc., unlike MOP, exergy loss also exists in the light calcination process, and the exergy loss is also calculated separately.
Figure 3c shows the distribution of refractory materials, byproducts and fused magnesia after smelting and cooling. The main component of the byproducts is heavy calcinated magnesia. Although its market value is lower than that of fused magnesia, it is also a useful product, so some attention should be given to the output of byproducts.