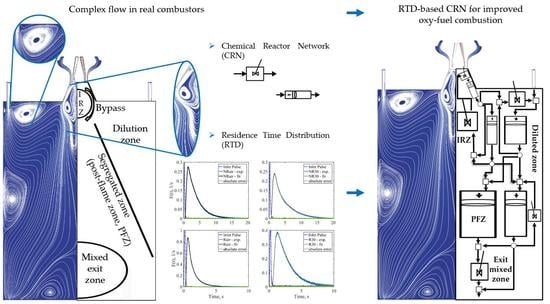
The impact of oxidizers on the mixing and reactive behaviour of the combustor is discussed in the following section. The parameters introduced earlier are employed to comment on the features of systems, varying through different diluents as the system delivers the same output power. For brevity, the isothermal case is discussed first followed by results concerning the reactive cases.
3.1. Non-Reactive Flow: Comparison between the Oxidizers
Figure 6 shows the CRN-fits for NRair and NR30, superimposed to better highlight the differences. The onset of NR30 is delayed with respect to NRair, as can be understood from the convective time delay in
Table 3. Additionally, the oxy-fuel condition causes a broader shape of the RTD. The shape of the RTD is described by the mean residence time,
, and the spread of the distribution,
, and broader RTDs normally exhibit a higher value for these parameters.
Accordingly, in
Table 3, both mentioned parameters are larger for NR30 comparing to NRair, in accordance with experimental findings [
46,
47]. These features agree with the lower flow rate fed to the system for NR30 with respect to NRair, as reported in
Table 1. Therefore, a lower bulk velocity is associated with NR30, justifying the delayed arrival of the tracer to the detection point. This lower velocity expresses a lower impulse of the flow entering the combustion chamber, and is also responsible for a more significant share of the combustor volume associated with the IRZ, consistent with experimental evidence [
52]. In this recirculation zone, more mass flow is entrained for the oxyfuel atmosphere concerning the total mass flow fed to the quarl zone,
in
Table 3, to which the lower bulk velocity is also attributed [
61].
The discussion is extended with the aid of
Table 3, clarifying further phenomena that contribute to the major broadening of the NR30 distribution. Under oxy-fuel conditions, a higher total mass flow is entrained in the lateral dead zones described earlier. In addition, a slightly higher percentage of mass flow from the dilution region is entrained in the main (central) flow. The dead zones in the dilution region of the combustor (see schematization in
Figure 3) present the most prominent residence times in the system under the definition of dead zones [
10]. As a result, the mass flow from these zones experiences a longer mixing time, and its tracer concentration is severely broadened. A higher share of this flow joins the descending bulk flow (see the factor
in
Table 3). These combined factors are most likely the explanation for the different broadening of the RTD curves.
3.2. Reactive Flow: Comparison between the Oxidizers
The RTD-fits obtained after optimizing the parameters of the reactive CRN in different operating conditions are compared in
Figure 7.
Table 4 contains the values of the key parameters chosen to compare these operating conditions. What clearly emerges is the considerable difference between the spread of the two distributions and the strong resemblance between R30 and NR30 (shown in
Figure 6). Referring to
Figure 6, the maximum value of the distribution corresponding to NR30 is around 0.25
, and that corresponding to R30 is around 0.4
(
Figure 7). Instead, the two maxima corresponding to NRair (around 0.28
) and Rair (around 0.9
) are distant from each other, as expected when comparing a cold flow distribution to a reactive flow (higher temperatures). The broadening of the R30 distribution is characterized in
Table 4 by higher
and
. The discrepancy between the convective time delays increases more than twofold for the oxy-fuel combustion.
For the isothermal case (see the previous section), the broader distribution calculated for R30 is partly due to the lower flow rate fed to the system compared to Rair, as explained in
Section 2.1. For this reason, higher residence times are associated with oxy-fuel flows in reactive conditions.
Besides the lower mass fed to the system, higher entrainment in recirculation zones is also calculated for R30. The parameter
in
Table 4 is approximately one order of magnitude higher when feeding the O
2/CO
2 mixture as oxidizer, while the amount of mass flow entrained in the lateral recirculation zones,
, is nearly doubled. Therefore, a significantly larger portion of the flow experiences intense mixing inside the system, broadening the flow distribution. The value of
shows a slightly higher number for R30. According to the modelling section, the flame is represented by a CSTR. Once again, a higher percentage of the flow is entrained in a mixing reactor while investigating oxyfuel combustion as compared to Rair. This phenomenon corroborates the observations about the higher mixing intensity in the oxy-fuel case. The entrainment of colder flow from the dilution region,
, is quite similar for both atmospheres, being slightly lower for R30.
To further discuss the reactive system,
Figure 8 shows two sample profiles inside the combustor. Species concentrations and temperatures from the CRN model are calculated over the profiles and plotted in order to compare the reactive behaviour with different oxidizers.
Figure 9 shows the temperature trend, CO, and CH
4 concentration along the selected profiles in
Figure 8. The comparison starts from the vertical temperature profile, where the first zone from the right side is the IRZ. The temperatures do not significantly differ here, being approximately 1800 K for both conditions. The intense mixing and high reactivity by the IRZ are apparent, as the CO values are below 500 ppm (R30 exhibits the higher value), and no methane is present in this zone (
Figure 9). In the post-flame region, R30 shows a higher temperature along the whole region. The CO concentration shows a sharp rise for Rair due to the combined effect of direct transport of flow from the reaction regions and the side dilution region. The amount of carbon monoxide decreases until the temperatures are high enough to promote the reactions (around 1000 K), and increases again as at the low temperatures diminishes OH formation and CO is no longer oxidized to CO
2 through the reaction CO + OH = CO
2 + H. R30 exhibits a slightly different situation, as the CO content experiences an increase due to transport from CO-rich zones. However, the intensity of this peak is approximately one order of magnitude lower than that calculated for Rair. In other words, despite the higher ϕ that characterizes R30 (
Table 1), the CO mole fraction in the PFZ is significantly lower during oxyfuel combustion.
This behavior is explained by the higher
and
calculated for R30 (
Table 4), showing that a higher share of the inlet flow is entrained in the high-temperature IRZ-CSTR and flame-CSTR. Higher reactant consumption in these reactor zones closer to the burner region also results in less fresh fuel available to react later in the system. The entrainment of lateral dilution gas is to some extent lower for R30 (
), mitigating CO mixing from colder regions and slower reactivity due to mixing cold fluid pockets in the reactive stream. In addition, the higher R30-temperatures in the PFZ also enhance CO reaction with OH, promoting CO
2 formation.
It is also worth mentioning that the availability of unreacted fuel near the exit of the combustor influences the different CO yield in the two cases. The possibility of fuel slip has also been postulated before, based on species measurements [
53]. Among the model variables described in
Section 2, the developed CRN model includes a flow variable that accounts for a fuel slip event which pushes flow from the quarl region to the post-flame region. Therefore, it accounts for possible unreacted methane being transported toward the exit. The value of this fuel slip variable is one order of magnitude lower for R30, meaning that the residence time in the oxy-fuel case is long enough to consume almost all feed.
This higher fuel conversion explains the diverse trend of the CO concentration in the different operating conditions, which is also evident from the horizontal profile. This profile cuts through the flame and bypass zones, and R30 is characterized by a higher temperature in both regions. The bypass shows a higher CO content for Rair, confirming that a higher amount of CO is transported to the post flame region. The in-flame CO content is comparable for the two investigated conditions. No CH
4 is calculated in the bypass region for either case. A lower reactivity in the flame reactor is found for Rair, with a significantly higher fuel content (approximately one order of magnitude higher) with respect to R30. A plausible explanation lies in the higher flame and bypass R30-temperatures, causing this mixture to achieve complete oxidation faster. Following the above discussion, and as evident from
Figure 9, the oxy-fuel condition shows higher operating temperatures. This might at first be unexpected due to the higher specific heat of CO
2 with respect to that of N
2, as also shown in
Table 5.
However, according to
Table 1 and
Section 2.1, the two investigated operating points feature a different total flow rate. This choice is necessary to investigate the impact of the diluent on systems that deliver the same power, in line with previous studies [
63,
64], where burner aerodynamics were exploited to achieve successful flame stabilization. The momentum fluxes were found to play a crucial role in flame shape; thus, they have been kept equal to a reference flame in [
63]. Nevertheless, the fuel flow rate is the same in both cases, determining a higher fuel-to-oxidizer ratio for R30, which also exhibits higher O
2 availability with respect to Rair. These observations describe the higher oxy-fuel temperatures and its lower CO emission; in addition to the more efficient CO conversion at higher temperatures, the loss of in-flame reactivity due to entrainment of cold flow from the dilution region is expected to be lower with increased O
2 availability.
3.4. Comparison between Equilibrium and CRN Species Predictions
A comparison between CRN-values and equilibrium predicted values is given in
Table 7. The equilibrium values were calculated using the hypothesis of plug flow at a constant temperature, set at 1500 K. The results show that equilibrium CO content is more significant for O
2/CO
2 conditions [
4,
24,
65]. The higher CO emission for air combustion compared to oxyfuel combustion is attributed to the different mixing aspects highlighted in previous sections. A more substantial mixing is noticed for R30, ensuring higher residence times in high-temperature mixed areas.
The main specie concentrations do not show a significant deviation from the equilibrium values, whereas CO and CH
4 values deviate from those predicted by the equilibrium calculations. The reason lies mainly in the temperature non-uniformity in the combustor: as seen in
Figure 9, temperatures can range from approximately 500 K (dilution zone) to 2000 K (reaction zone). The mixing of pockets of fluid with different temperatures results in loss of reactivity in the combustor, especially in the dilution zone. The resulting unreacted CO or CH
4 amount is then entrained from the dilution region into the main flow stream. In the PFZ, it is not able to react further due to the lower temperatures. Flame quenching attributable to entrainment from the dilution region into the flame is expected to be lower with increased O
2 availability, as in the case of R30. This explains the lower CO emission for this oxy-fuel atmosphere, as pointed out in the previous section.