1. Introduction
The transition from the fossil fuel-based to a sustainable energy foundation society is one of the greatest challenges for the upcoming few decades. To limit the global temperature increase to 1.5–2 °C in the year 2100 compared to the pre-industrial level, anthropogenic greenhouse gas (GHG) emissions must be eliminated in all sectors, including agriculture, households, transport and industry [
1]. To realize this ambitious goal, most countries have committed to the Kyoto Protocol and/or Paris Agreement. Zero emission discharge can be difficult to achieve with current technologies and, thus, new technologies are constantly developed, or renewable substitutes are intended to replace the fossil fuel-based feedstock. This transition can result in a larger demand for special materials and depleting easily accessible sources will further increase the energy demand in the future, e.g., mining [
2]. Thus, feedstock supply may require additional governance to ensure sustainability and economic feasibility [
3].
An increasing demand for metal alloys from primary metallurgy and its sustainable production are the key challenges to minimize greenhouse gas emissions from metallurgy. Currently, ≈5–10% of anthropogenic CO
emissions are emitted by the metallurgical industry [
4,
5,
6]. In steel industry, ≈98% of greenhouse gas emissions are related to CO
and methane emissions from smelting and coke-making [
7], whereas the total emissions from alloying elements production in submerged arc furnaces are highly affected by the indirect emissions from electricity production. Short-term opportunities to decrease greenhouse gas emissions from metallurgy are based on the replacement of fossil fuels, e.g., carbon neutral reductants or blue hydrogen, carbon neutral electricity and carbon capture and storage [
8]. Studies have shown that more than 31% of the CO
emissions can be removed in the production of green steel [
9], reducing the GWP to 1.6 kg CO
-eq. kg
of steel. In addition, the use of charcoal can reduce the SO
emissions by more than 75% compared to fossil fuels [
10]. Combinations of new processes, bio-reductants and carbon capture and storage have shown that the CO
mitigation potentials can be in the range of 1.4–2.7 t CO
t
steel, corresponding to ≈75–150% of the current emissions [
11].
Norway is a main producer for silico-manganese (SiMn), ferro-manganese (FeMn), silicon (Si) and further alloying elements, which resulted in a total release of ≈9 million tonnes CO
equivalent [
12]. Despite the already low specific greenhouse gas emissions from Norwegian’s metallurgical industry, a net zero policy is intended to become a reality by 2050 [
13]. A similar roadmap was released for the decarbonization of the steel industry in Europe [
14]. Renewable reductants in combination with carbon capture and storage (CCS) shall cover ≈75% of the current emissions and ≈45% of the future emissions [
12]. The application of CCS in combination with new technologies, such as sustainable hydrogen, are intended to further reduce and potentially leading to net negative CO
emissions. Biomass and its derivatives are considered as renewable reductants in the metallurgical industry [
15,
16] and can reduce greenhouse gas emissions by ≥ 50% [
17]. However, the use of reductants with insufficient properties can result in a lower efficiency of the process [
18] and consequently higher greenhouse gas emissions.
Classical charcoal provides inferior properties compared to fossil fuel-based reductants used by ferro-alloy production [
19,
20], possibly resulting in a different process behavior in operation [
21]. While a high mechanical stability and low gas reactivity are desirable in conventional large blast furnaces [
22,
23], mechanical properties are of less importance for small scale blast furnaces, electric arc furnaces (EAF) and submerged arc furnaces (SAF) [
24,
25,
26]. Closed hearth SAF require a low volatile matter content based on their off-gas system, and specific electrical properties to provide a homogeneous temperature profile in the furnace. Open hearth furnaces, on the other hand, are more flexible to volatile matter content but require properties to ensure a good gas transport in the burden. Thus, multiple parameters must be improved in order to increase the substitution rate of renewable reductants, such as particle size, mechanical and abrasion strengths, CO
reactivity and chemical composition at similar economics [
18]. In addition, the economics of renewable substitutes are often inferior, e.g., a lower bulk or energy density, resulting in higher costs in handling, transport and storage to a price increase of ≈2 [
27,
28]. Moreover, published data still lack information about the influence of feedstock, production process conditions and upgrading strategies on certain applications on industrial scale [
29]. It is assumed that charcoal can replace fuel-based reductants by more than 40% in SAF, whereas larger to fully replacement require additional processing of the charcoal. Full replacement of fossil fuel-based reductants has been carried out in open hearth furnaces in Brazil at the cost of furnace efficiency [
30].
A great deal of effort has been undertaken in the past few years to optimize specific properties of biomass and its derivatives for the application in metallurgy, the energy sector and other industries, such as construction, biochemistry or medicine. A multifaceted use of biomass can optimize the sustainable usage biomass by developing plans for a diverse usage [
31]. The production of thermochemical and biochemical products have significantly increased in the last few years with a further increase forecasted to ≈25% in 2024 [
32]. Thus, industrial processes may compete for the available potential of renewable feedstock. Therefore, conditioning routes for the biomass and its application should be considered regarding the availability of biomass and alternatives. For example, the potential of biomass is not large enough to be considered as a sustainable replacement of fossil fuel in the power production in addition to other sectors [
31]. Renewable energy from solar, wind or hydropower can replace fossil fuels in this sector, whereas no alternatives currently exist to replace carbon in the reduction of manganese(II) oxide to its metallic form.
To reduce metals to its metallic form by renewables, different process routes have been investigated or developed to produce charcoal with specific properties [
9,
21,
29]. New thermochemical conversion processes, such as hydrothermal carbonization (also called wet torrefaction), seem superior to classical dry pyrolysis in producing binderless pellets [
33,
34], whereas the organic binder can increase the mechanical stability of biomass and charcoal pellets and briquettes [
9,
26,
27,
35,
36,
37]. The combination of different biomass feedstocks showed an improved mechanical stability compared to their individual counterparts [
38]. On the other hand, biomass or charcoal addition to coal blends in metallurgical coke production resulted in inferior mechanical properties at a mass ratios larger than 5–15% [
39,
40,
41]. Thus, the quality of renewable reductants can partially be designed by the different feedstock materials and processes. However, additional upgrading processes, as well as a concomitant mass loss, will decrease the economic feasibility and usable biomass potential of renewable reductants and reducing its sustainability.
To decrease CO
emissions from metallurgy, renewable reductants must remain CO
neutral, to a great extent lower than used fossil fuel-based reductants. The higher reactivity of charcoal compared to fossil fuels leads to an average higher CO and CO
amount in the off-gas [
21]. A higher CO
concentration may be favorable for its utilization in consecutive processes, such as carbon capture and storage or carbon capture and utilization [
42]. The application of such mainstream technologies can reduce the CO
emissions from the iron and steel industry by more than 40% [
43].
The aim of this work was to perform a life cycle assessment (LCA) to evaluate the impact of upgraded charcoal manufacturing on the environment in Norway. The objective of this study was to develop a simplified approach based on ISO 14044 aiming to reduce the CO emissions through the replacement of fossil-based metallurgical coke with the bio-based reductants. The GHG emissions from the manufacturing of charcoal were calculated using different scenarios including novel industrial ways to utilize the liquid pyrolysis by-products and various feedstock pre-treatment processes. For the first time, the life cycle analysis of the charcoal manufacturing was performed through inventory of the energy and materials that are required across the metallurgical industry value chain in Norway.
2. Methodology
The methodology of the present study suggests the execution of the LCA according to the framework of the ISO 14044. This will allow us to analyze the environmental burdens of the metal reduction process. The goal and scope will be identified using literature data followed by Life Cycle Inventory (LCI), assignment to categories and is finished by the brainstorming and interpretation of the category endpoint.
Figure 1 shows the first step with the definition of the aim, objectives, functional unit and system boundaries.
The next step consists of collection, description, verification of inventory regarding process steps, input parameters of water, material, and energy and emissions of the whole charcoal life cycle. The third step is used to quantify parameters based on the inventory analysis that includes selection of impact categories depending on the parameters of goal and scope and assignment of life cycle inventory results to various categories. Normalization and weighting were not performed in a frame of this study. The last step is the interpretation of the results to calculate environmental impacts to describe how environmentally relevant flows will change in response to possible decisions. This impact category for the charcoal manufacturing was divided according to CO emissions released into the air, water and landfill. Each category formed from the life cycle inventory analysis represent a sequence of effects that can cause a certain level of damage to the environment based on the used pre-treatment method, e.g., alkali leaching, heat treatment or briquetting. This type of method generates a simplified and narrowed picture of the ecological impacts of charcoal integration into metallurgical industry. The results of an LCA are not absolute values and therefore cannot serve as a certification on itself. Calculations were carried out with SimaPro software (Version 9, PRé Sustainability B.V.) for each individual process step. Compared to a full life cycle analysis, where also toxicity, eutrophication and further impacts are considered, the scope of this study was limited to the evaluation of the CO emissions.
The input parameter of 1000 kg of charcoal was used in all LCA calculation. Tables 1 and 2 provide the data on the raw feedstock characteristics such as water, volatiles, inorganics contents and composition, etc. Tables 3–9 show the results of estimated input and output parameters based on the literature and LCA calculation to determine the CO emissions for the entire charcoal life cycle. The CO calculations of the charcoal manufacturing will be compared with the emissions from the life cycle assessment of fossil-based metallurgical coke. The results will not guarantee the sustainability of a product, but these are valuable for the comparison of different products and process steps. The local impacts on the charcoal manufacturing in Norway are of importance to link these results with environmental damage on global scale.
4. Results and Discussion
4.1. Life Cycle Inventory Analysis
The summary of the unit processes and the life cycle inventory analysis are summarized in
Table 8 and
Table 9 based on the input parameter from ash removal, briquetting, secondary heat treatment and combinations thereof.
The latter contains possible emissions by inappropriate handling, insufficient cleaning or post-treatment of the products, such as the release of volatile matter, the dissolution of condensed tars and oils by leaching and washing, and the evaporation of bio-oil binder. Extracted ash and neutralized solvents are accounted as landfill but may be upgraded to mineral fertilizer or filling material [
122]. In addition, dissolved bio-oil species and dissoluble hydrocarbons from charcoal necessitate a wastewater treatment before discharging the water and before water recirculation to the ash removal. Recovery of hydrocarbons by liquid–liquid extraction or thermal separation may provide an additional chemical side stream for further applications. However, the complexity of organic species and their application are currently not considered as a chemical feedstock and no market has been established to the knowledge of the authors.
A large uncertainty occurs by the energy demand of the different processes, especially for the secondary heat treatment at elevated temperatures. An effective energy recovery is assumed for the optimal case, in which losses to the environment are minimized. Biomass or charcoal as energy carrier can substitute the power supply for these processes, but the heat supply by charcoal or biomass will further reduce the available biomass potential, which may be the limiting factor in replacing fossil fuels in the future. In addition, energy losses for high temperature processes increase for processes which are heated by the oxidation of fuel due to the larger off-gas volume flow. Direct heating with combustion gases may increase carbon losses by Boudouard reaction, which occur at temperatures larger than 700 °C. Volatile matter release by bio-oil binder and secondary heat treatment is assumed to be an energy source to partially provide heat for the individual processes, as shown in
Table 9.
However, hydrocarbons with a low boiling point may also be used as renewable energy carrier or as chemical feedstock. Moreover, the removal of volatile species of bio-oil prior to the compaction may improve the mechanical properties of the renewable coke due to the decreasing void fraction by volatile release. Bio-pitch binder would require an increased production of bio-oil, which would result in an increased global warming potential of the process chain under the current conditions.
4.2. Upgraded Charcoal
Upgrading of industrial charcoal by ash removal, briquetting and renewable coke production increases the potential to replace large amounts of fossil fuel-based reductants in SAF. The inferior properties of charcoal can be upgraded individually by existing technologies to fulfill requirements of ferro-alloy production in SAF. Washing and leaching can reduce the ash content of bio-reductants, whereas charcoal fines can be compacted by briquetting to decrease the losses by transport and handling. Organic binders, e.g., bio-oil binder, require a heat treatment to increase the mechanical strength of the briquettes, which can be executed at elevated temperature to further reduce the volatile matter content to the requirements of closed hearth SAF. However, the additional processes to improve charcoal’s properties increase the CO
emissions per metric tonne of ash free charcoal, briquettes and renewable coke by the additional energy demand, chemicals (e.g., solvents and binder), carbon losses, and by additional by-product streams, as summarized in
Figure 6 for a renewable power supply.
The centralized upgrading (e.g., at harbor or on-site the SAF) was used to disregard additional transport or handling. Treatment of wastewater to a zero liquid discharge and possible off-gas cleaning were further neglected, which increase the energy demand of the upgrading processes. Ash removal at atmospheric temperature has only a minor effect on additional CO emissions, in which main emissions occur from solvent regeneration and landfill. Thermal drying can further increase the indirect emissions for the ash removed charcoal from fossil power supply. Briquetting showed the largest increase in CO-eq. emissions based on the global warming potential for the bio-oil production. The high global warming potential of bio-oil production nearly doubles the of briquettes and renewable coke, whereas organic losses occur mainly for the secondary heat treatment. The global warming potential increase by organic losses are correlated to the production process of primary charcoal and can increase by charcoal with lower FC content and classical charcoal. However, volatile species contain often oxygenates and permanent gases, e.g., CO and CO, resulting in a lower carbon loss compared to its mass loss. The uncertainty of a lower fixed carbon content between classical and industrial charcoal is negligible compared to the different global warming potential of the production processes. The low conversion efficiency and risk of deforestation by classical charcoal production can increase the global warming potential to a level larger than metallurgical coke.
4.3. Acid and Alkali Leaching
Acid leaching can reduce critical alkali and alkali–earth metals by more than 90%. Most minerals can be removed by a combination of acid and alkali leaching. Single acid leaching of charcoal reduces K and Ca content by 85% and 50% [
55]. A significant removal of potassium and calcium prior to the application in SAF is required due to the recirculation and accumulation in the burden [
123], and the catalytic effect of these elements on the Boudouard reaction [
124]. The low energy demand at low temperature leaching increased the specific CO
emissions of renewable fixed carbon (charcoal) by ≈1.9 kg CO
t
, mainly for the production and regeneration of the used chemicals. A partial recovery of the solvents by gypsum and lime can reduce the emissions per metric tonne of fixed carbon (charcoal) by ≈10% to 1.7 kg t
, but negatively affects the economy of the process. Leaching at elevated temperatures is favorable to remove mineral compounds, but further increase the energy demand for the process. No further thermal decomposition of charcoal is expected at temperatures less than 300 °C. In contrast to coal, charcoal’s density is less than that of most solvents and may float in open vessels, increasing the technical challenge of a homogeneous transport for continuous processes or closed batch systems.
Leaching of charcoal may dissolve condensed bio-oil compounds, concomitantly decreasing the solid yield on ash free basis. This mass loss is mainly assumed for charcoal produced in pressurized pyrolysis, whereas only a minor loss of organics is expected for industrial charcoal. An organic mass loss of 1% is assumed by leaching, which decreases the biomass potential by 2–3%. Washing of classical charcoal can result in an organic loss of up to 1.5% [
125]. A larger weight loss of classical charcoal can be related to the lower pyrolysis temperature and the loss of nonpolar functional groups by the release of volatiles [
47]. Leaching of raw biomass can result in the removal of extractives and the modification of organic structure, resulting in an organic mass loss of 2–30% [
126,
127], concomitantly increasing the energy density by up to 25%. Organic losses occurring by leaching stages will increase the need for wastewater treatment and, thus, increase the indirect global warming potential, which are not considered in this study. In addition, untreated solvent discharge or leakages can harm local water reservoirs or pollute soil by acidification and light hydrocarbons dissolved by the solvent [
128].
Ash removal at elevated temperature and thermal drying can increase the energy demand by ≈900 kW·h t FC. Based on the CO-eq. emissions from the country-specific power supply, the global warming potential increases by up to 810 kg CO-eq. t FC. The increased ash removal at elevated temperature goes along with increased indirect emissions, which may balance the benefit of the additional removed mineral matter. A major uncertainty is the level of alkali metal removal required to ensure a stable operation of the SAF, due to the possible accumulation of alkali metals and feeding by manganese ores.
4.4. Briquetting
Briquetting and pelleting enable the utilization of charcoal fines and the production of renewable reductants with specific size and shape. Bulk charcoal can be crushed and milled prior to the compaction process to provide a wide particle size range in large quantity. The lower energy demand for charcoal crushing compared to biomass milling decreases the required energy demand by ≥50% to ≈25 kW·h. Partial crushing of charcoal prior to briquetting can provide a broader particle size distribution for briquetting, which can improve the mechanical strength of untreated (green) and heat treated (hot) briquettes. The crushing, blending and compaction result in an additional global warming potential per tonne of renewable fixed carbon (charcoal) of 2.6 kg CO-eq. for renewable power supply. However, weak binding forces between charcoal particles necessitate the usage of additional binder to ensure a high mechanical abrasion strength of pellets and briquettes, and to withstand the compression force induced by the load.
Organic binder such as starch, lignin, bio-oil or bio-pitch are superior to inorganic binder on basis of ash content and critical elements [
129]. Bio-oil binder in combination with a secondary heat-treatment provide a renewable reductant with adequate mechanical properties for metallurgical application in SAF [
111]. However, minerals and clay binder can improve the secondary char formation by bio-oil cracking [
130], decreasing direct emissions by volatile release and by its mass loss. The limited availability of bio-oil and the additional emission of ≈575 kg CO
-eq. t
for its current production [
107,
131] increase the overall emissions of charcoal briquettes by ≈170 kg CO
-eq. t
FC. These emissions can be reduced by the recovery of bio-oil as a by-product from primary pyrolysis at the cost of additional power supply.
Charcoal milling and handling prior to the briquetting can result in an additional global warming potential of ≈2.5 kg CO
-eq. t
FC, whereas the utilization of fines and can decrease the global warming potential by up to 30 kg CO
-eq. t
FC. Moreover, the bio-oil conditioning of primary pyrolysis can decrease the additional bio-oil demand, replacing the greenhouse gas emissions from bio-oil production by indirect emissions to produce power. The pyrolysis of beech wood results in the formation of ≈12.5% tar [
132], which is sufficient to form briquettes from about 50% of the produced charcoal. Thus, on-site briquetting at the pyrolysis plant can minimize the global warming potential from bio-oil demand, which is especially beneficial for renewable coke production, decreasing the GWP to ≈350 kg CO
-eq. t
FC. For comparison, the GWP of Brazilian charcoal briquettes was reported to 2100 kg CO
-eq. t
FC [
133], and ≈1.1 kg CO
-eq. t
FC for charcoal pellets [
9].
4.5. Secondary Heat Treatment
High temperature treatment of charcoal and charcoal briquettes can reduce the volatile matter content and CO
reactivity, approaching those of metallurgical coke [
53,
56]. In addition, a secondary heat treatment is required for charcoal briquettes produced with bio-oil binder to enhance the mechanical stability [
60,
68]. Moreover, multiple bio-oil compounds are removed which can be dissolved by rainwater in storage, minimizing the potential risks of groundwater and soil pollution [
128]. The high temperature treatment results in a mass loss of 15–20% [
56], increasing the global warming potential by 15–30 kg CO
t
FC for an additional charcoal production. The minor global warming potential increase is related to the greater FC content of the heat treated charcoal and renewable coke, which increases by ≈10%-points to 95% for wood charcoal.
The combination of acid leaching and secondary heat treatment to reduce the CO
reactivity were similar effective as heat treatment temperatures above 2000 °C [
54,
55]. A secondary heat treatment temperature of 1300 °C reduces the volatile matter content to ≈1.5%, approaching that of metallurgical coke and fulfilling the requirements of closed hearth SAF. While process heat in classical charcoal production can be covered by the combustion of the volatile matter, less than 33% can be covered by the volatile matter in the secondary heat treatment. This energy demand can be provided by electrical power or the combustion of additional feedstock. In the current study, required heat was provided by electrical power, resulting in additional global warming potential of 2 kg CO
-eq. t
FC for renewable power supply, and 100 kg CO
-eq. t
FC based on European energy mix. An additional heat recovery or by-product utilization can further decrease the global warming potential for the secondary heat treatment. Best case scenario would be that the hot charcoal is directly fed into the SAF to minimize heat losses and reduce power supply in the metallurgical process.
4.6. Process Chains
The combination of the processes improves the final product quality of the renewable reductant. The removal of alkali and alkali–earth metals by leaching in combination with a secondary heat treatment can decrease the CO
reactivity and volatile matter content to a level similar of metallurgical coke [
55]. Both properties are critical in closed hearth SAF for FeMn and SiMn production. Accumulation of potassium in the burden of SAF will require an ash removal for all feedstock materials, decreasing the chance of a partial replacement of ash free charcoal and renewable coke. A combination of leaching and briquetting slightly improves the CO
reactivity, ash content, charcoal yield and mechanical strength. However, a low mechanical strength of fresh briquettes (also called green briquettes) limits its handling and transport, and the application in metallurgy. The combination of acid leaching, briquetting and secondary heat treatment can produce renewable cokes with chemical, mechanical and physical properties approaching those of metallurgical coke. The mechanical properties are highly affected by the chosen binder, binder to charcoal ratio and post-treatment temperature of the carbonaceous material [
104,
111].
The large energy demand by secondary heat treatment results in the largest increase of additional greenhouse gas emissions by fossil fuel-based heat supply (up to 1350 kg CO
t
FC), whereas the additional greenhouse gas emissions by acid leaching are negligible under atmospheric conditions. Off-gases from SAF can be utilized to provide the heat for on-site processes such as charcoal upgrading, reducing the global warming potential possibly caused by power supply. Briquetting is useful to utilize charcoal fines produced by handling and transport, and can increase the charcoal potential by 10–20%. The largest uncertainties of briquetting are the used binder and the ratio of a charcoal to a binder, in which bio-oil has been proven as a superior binder compared to other organic binder, e.g., lignin or starch [
111]. Blends of different binders may further increase the mechanical and abrasion strengths but will concomitantly increase the indirect global warming potential [
61].
An increase in global warming potential by charcoal conditioning routes is minor when renewable energies are used for binder and power production, in which combining the different conditioning routes can minimize additional energy and solvent demand. The additional global warming potential for ash removal and secondary heat treatment was calculated to ≈5–10% compared to classical charcoal production. Such charcoal substitute would require a ≈20% larger charcoal input into open hearth SAF based on the higher volatile matter content and reactivity of charcoal. Removal of volatile matter by the secondary heat treatment for closed hearth SAF would increase the global warming potential by ≈18 kg CO
-eq. t
FC. By-product utilization from the upgrading processes in combination with renewable power supply can further decrease the global warming potential by 10–20%. Economical limitations of low value reductants outweigh the technical hurdles by inferior properties of classical and industrial charcoal. Similar results were obtained by iron and steel industry, where a carbon tax may play an important role to increase the application of tailor-made charcoal or renewable coke in SAF [
18].
The secondary heat treatment can be combined with methane densification to further improve the physicochemical properties of charcoal [
59], concomitantly reducing the energy demand for process heating. Biomethane can be produced sustainable by biogas production of manure, plant material or biowaste, in which the largest specific saving potential is stated for wet manure [
134], where the biogas is produced in closed digestates to minimize methane slip. More than 50% of the methane are decomposed to secondary char and synthesis gas (H
, CO, C
H
) at temperatures larger than 1000 °C [
59]. However, the quality of the off-gas has to be further investigated to be suitable as a synthesis gas. At current stage, unconverted methane in the off-gas must be separated and recycled or combusted with the remaining off-gas stream to provide the heat for the process. Natural gas as a methane source would increase the global warming potential by ≈17 g CO
MJ
[
134], resulting in a global warming potential of 28–36 kg CO
-eq. t
FC. Fossil methane decomposition would increase the global warming potential of charcoal briquettes and renewable coke by 55–75 kg CO
-eq. t
FC, more than the ash removal and secondary heat treatment when renewable energy sources are integrated.
A complete leaching, briquetting with bio-oil binder and secondary heat-treatment increases the global warming potential by ≈250 kg CO
-eq. t
, resulting in a total maximum global warming potential of ≈470 kg CO
-eq. t
of renewable coke produced from industrial charcoal [
135], whereas non-sustainable charcoal production can increase the global warming potential up to 2000–3000 kg CO
-eq. t
FC [
136,
137]. Renewable coke production from unsustainable produced classical charcoal would result in a global warming potential similar to that of metallurgical coke [
121,
138,
139]. A fully replacement of metallurgical coke by renewable coke would decrease the direct global warming potential by 70–80%, whereas further measures are required for a net zero industry. About 50% of the remaining emissions are related to the production of bio-oil and can partially be reduces by by-product utilization. However, additional processes are required for net zero emission metallurgy.
Thus, it is required to optimize the production chain of ash reduced charcoal briquettes and renewable coke, mainly by the production of charcoal in industrial retorts and the usage of bio-oil from pyrolysis, and from sustainable binder production. Due to the accumulation of alkali metals in closed SAF, it is assumed that the whole carbon feedstock should be washed or leached. However, it is not required for open hearth furnaces that the volatile matter content of the carbon feedstock is reduced to less than 3%, decreasing the necessity of the secondary heat treatment. For closed hearth furnaces, it is assumed that ≈30% of the charcoal should be compacted to briquettes, whereas the volatile matter content of the ash reduced charcoal and renewable coke should be reduced to less than 3%. A heat treatment temperature of 1300 °C is sufficient to reduce the volatile matter to this level, concurrently decreasing the CO
reactivity. A selective post-treatment of the remaining high value charcoal at a ratio of 40% leached charcoal, 30% leached and heat treated charcoal and 30% of leached, briquetted and heat-treated charcoal for open hearth SAF would increase the global warming potential by 80 kg CO
-eq. t
FC to ≈300 kg CO
-eq. t
FC. Closed hearth furnaces with 50% ash removed and heat treated charcoal, and 50% renewable coke would increase the global warming potential to ≈350 kg CO
-eq. t
FC. This renewable reductant blend can reduce the global warming potential by 80–87% compared to metallurgical coke if renewable power supply is available. Similar emission savings are reported for ultra-low carbon technologies in iron and steel production [
43] and current technologies combined with CCS [
11].
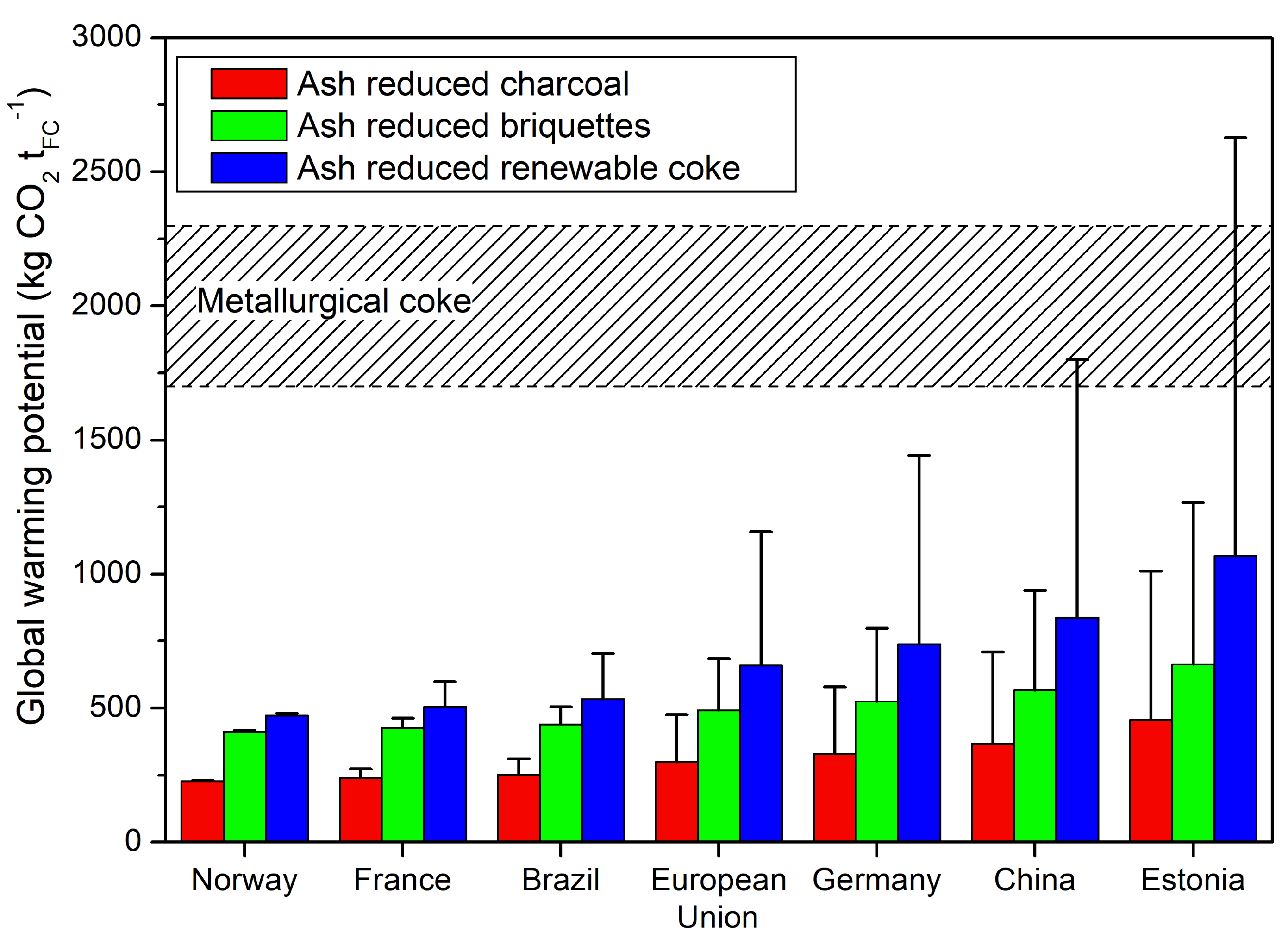
4.7. Country Depending Upgrading
National regulations and access to renewable power supply will affect the global warming potential of the ash reduced charcoal, briquettes and renewable coke, especially for coal fired power supply. A comparison of different regions based on their power supply is shown in
Figure 7.
The difference between countries can be reduced by a sustainable heat supply, for example, by the utilization of side streams, the combustion of biomass or other renewable sources. However, a reduced biomass potential may balance the savings in power supply. In long term, biomass potential may be the limiting variable for the fully replacement of fossil fuel-based reductants by ash reduced charcoal, briquettes or renewable coke. Hydrocarbon discharge by wastewater and volatile matter can increase the overall global warming potential, which were neglected in the current study. In summary, the increase in global warming potential is small for renewable power supply, whereas coal fired power supply can result in a global warming potential similar to that of fossil fuel-based reductants.
The combination of ash reduced charcoal, briquettes, and renewable coke can minimize the global warming potential. While ash removal is required for all renewable reductants with high alkali and alkali–earth contents, it is assumed that only 30–50% can be compacted and heat treated to provide properties similar to that in coke bed. However, inferior mechanical properties of charcoal necessitate the compaction and briquetting to decrease dust emissions and charcoal losses. The consecutive heat treatment of charcoal briquettes made with bio-oil binder increases the mechanical and abrasion strengths of the renewable coke. Furthermore, volatile matter release by bio-oil binder can be recovered or used as a sustainable heat source, decreasing the risk of local pollution. However, the more complex recovery and production of bio-oil may hamper an on-site upgrading at the pyrolysis plant, and increasing local emissions.