Optimization Study on Microwave-Assisted Hydrothermal Liquefaction of Malaysian Macroalgae Chaetomorpha sp. for Phenolic-Rich Bio-Oil Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Multi-Objective Optimization Study Using RSM, Design Expert Software
2.3. Microwave-Assisted Hydrothermal Liquefaction
2.4. Phenolic Content Determination through Folin-Ciocalteu Method
2.5. Construction of Calibration Curve for Phenolic Content Determination
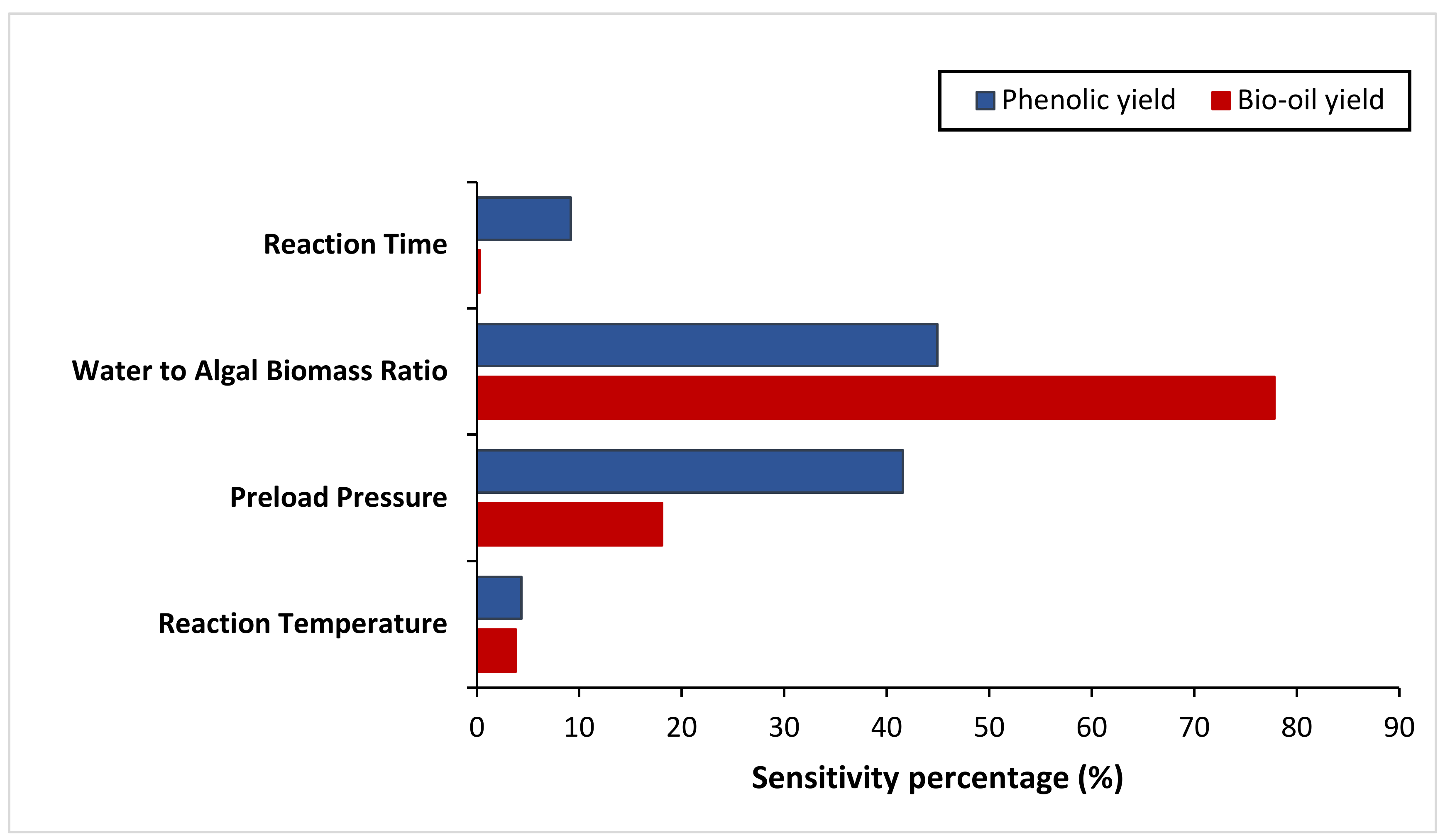
2.6. Sensitivity Analysis
2.7. Fourier Transform Infrared Spectrophotometer (FTIR)
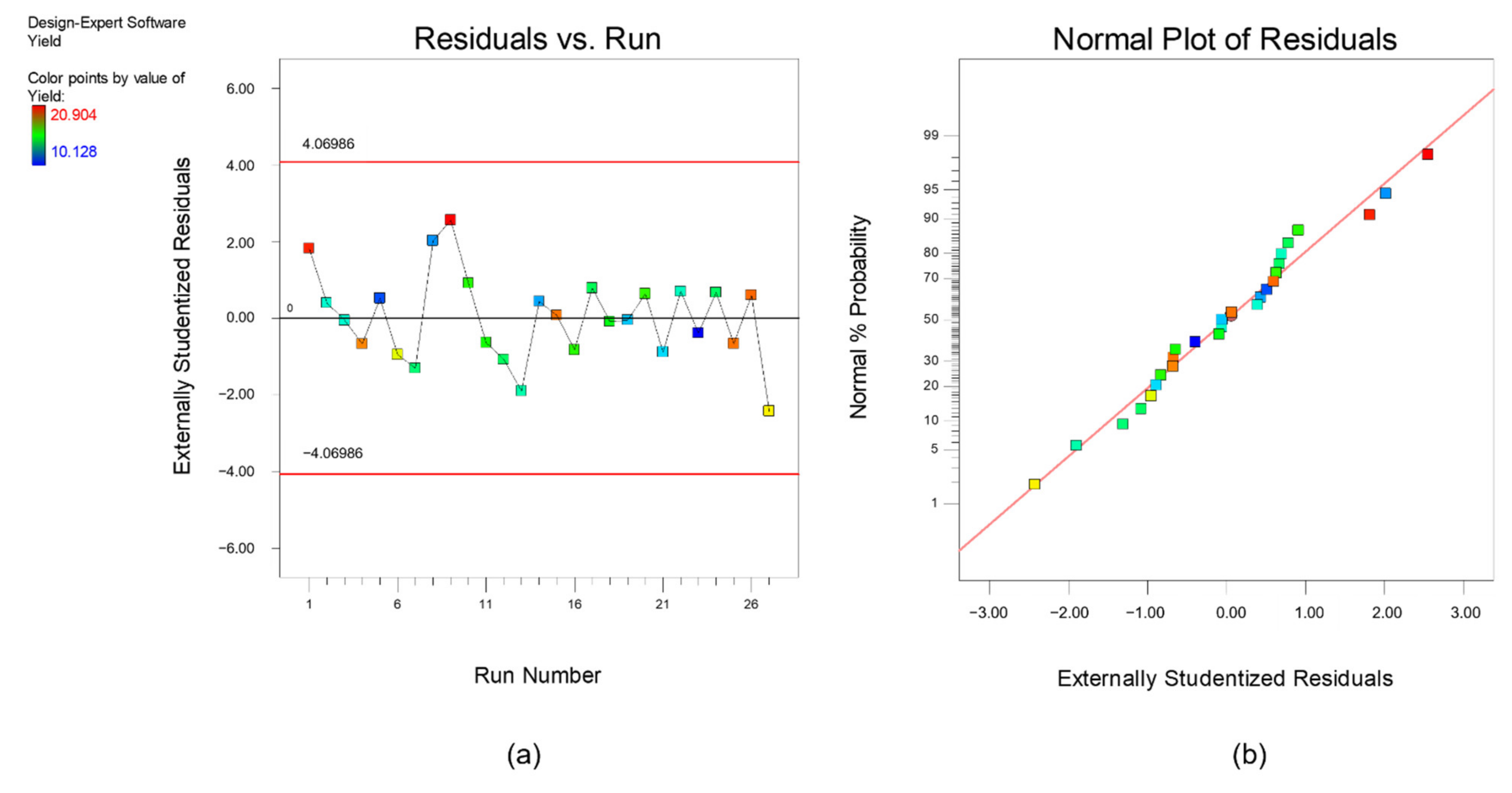
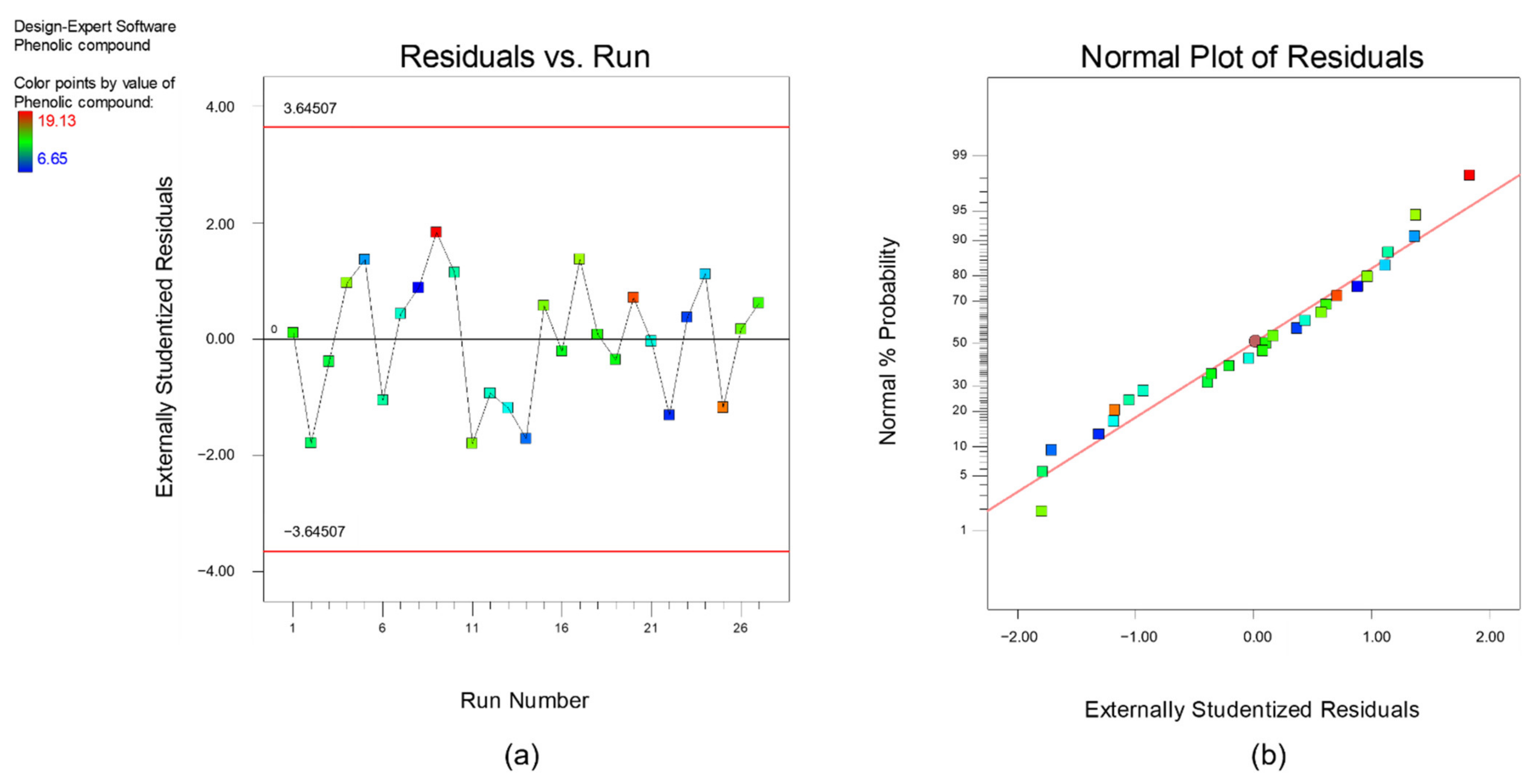
3. Results and Discussion
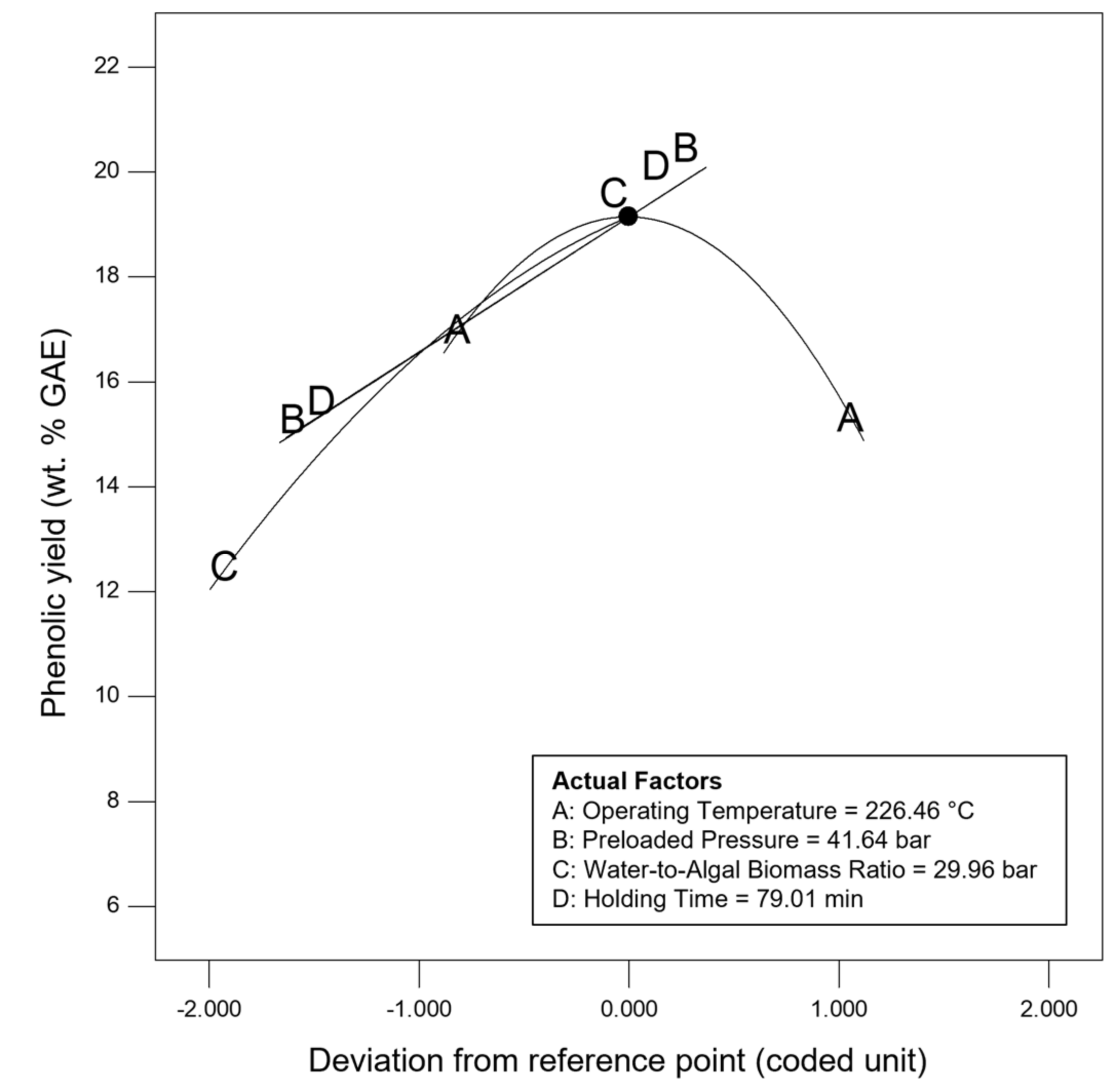
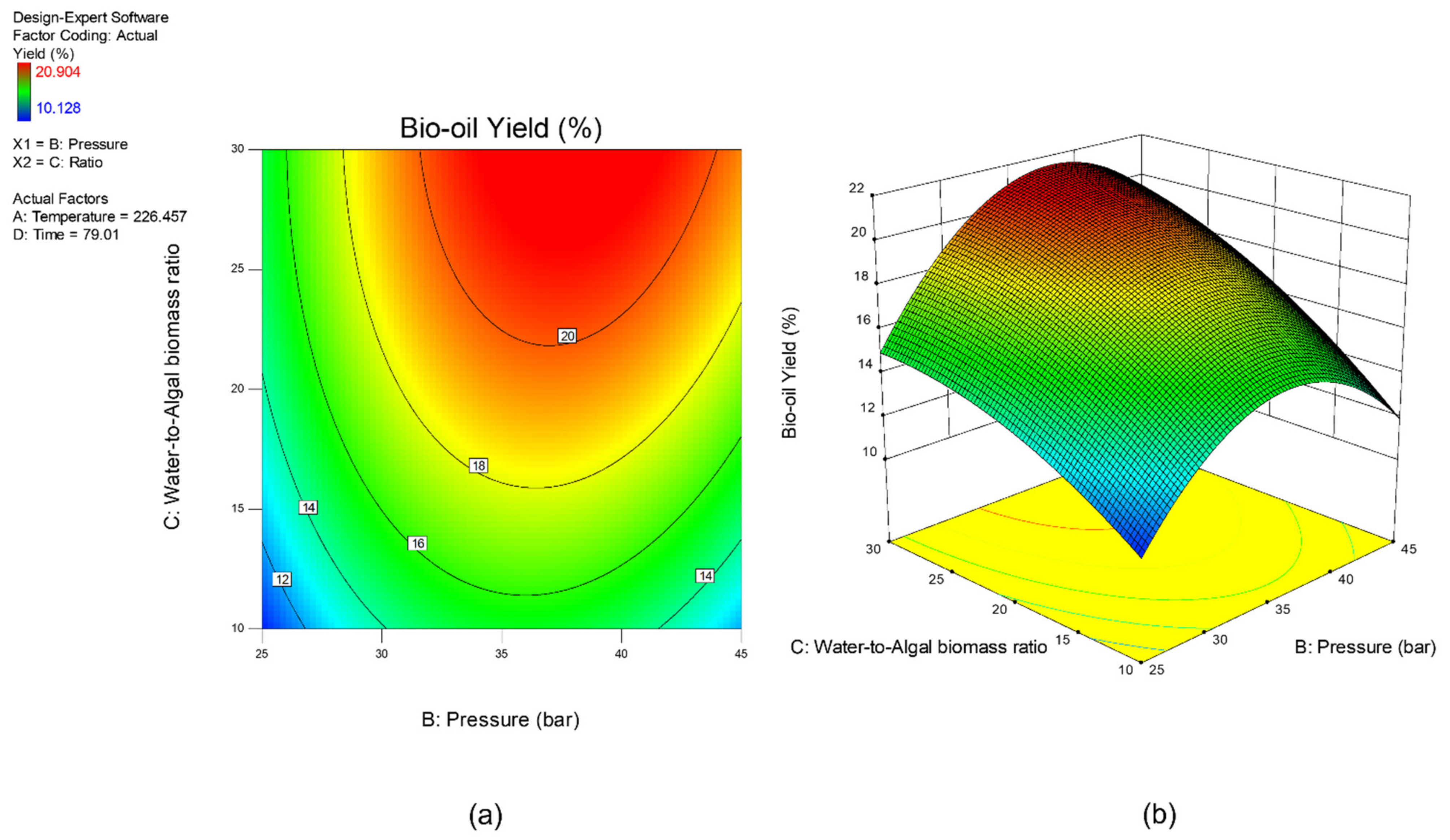
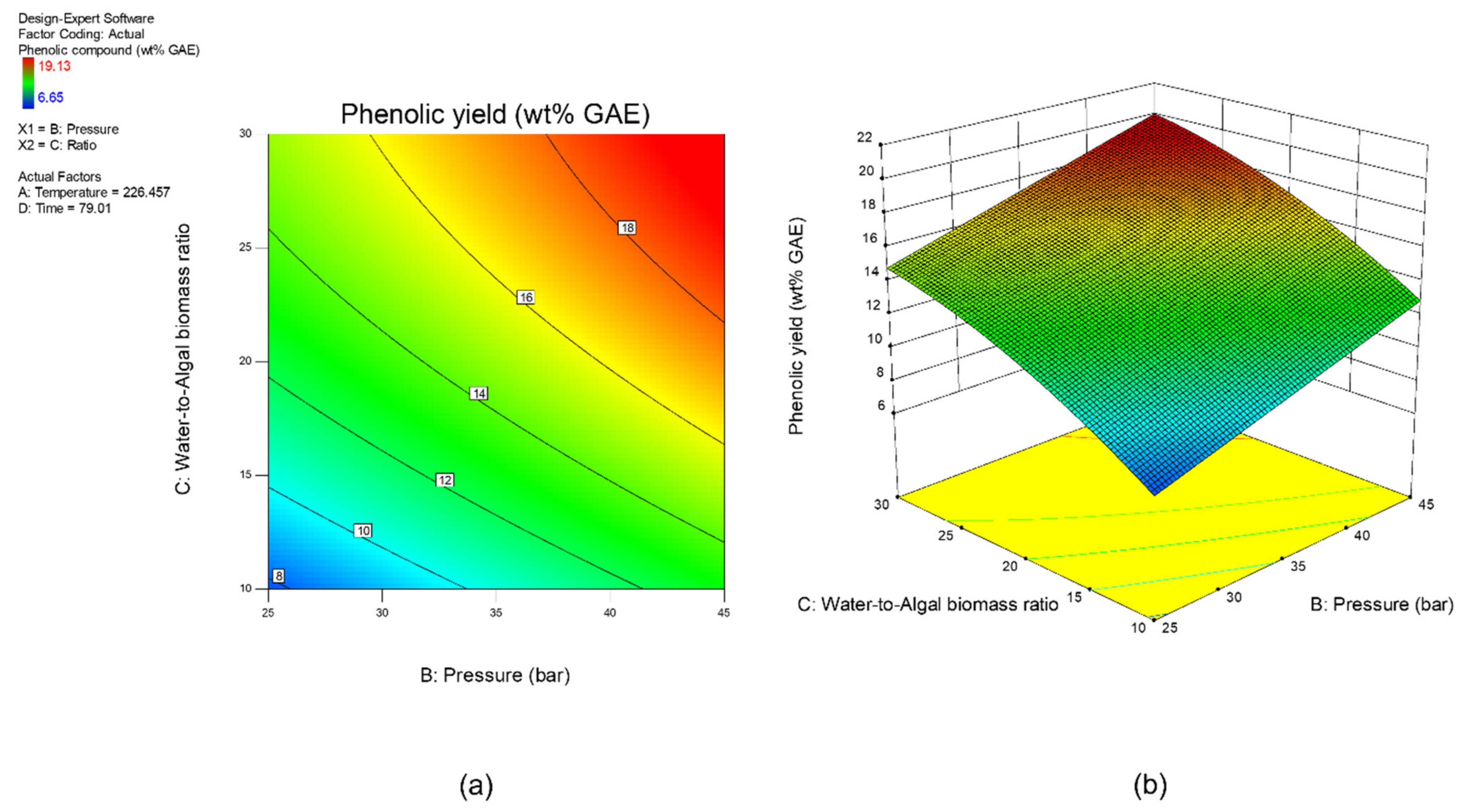
3.1. Multi-Objectuve Optimization Study
3.2. Effect of Studied Parameters on the Results & Sensitivity Analysis
3.3. FTIR Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- IEA. 2020 Regional Focus: Africa—Electricity Market Report. Available online: https://www.iea.org/reports/electricity-market-report-december-2020/2020-regional-focus-southeast-asia (accessed on 8 April 2022).
- Chen, W. Oil and Gas and Then? Available online: https://fdocuments.net/document/oil-and-gas-and-then-wong-petronas-dagangan-bhd-and-petronas-gas-bhd-a-feature.html (accessed on 5 May 2022).
- Sánchez-Borrego, F.J.; Álvarez-Mateos, P.; García-Martín, J.F. Biodiesel and other value-added products from bio-oil obtained from agrifood waste. Processes 2021, 9, 797. [Google Scholar] [CrossRef]
- Abdul Malek, A.B.M.; Hasanuzzaman, M.; Rahim, N.A. Prospects, progress, challenges and policies for clean power generation from biomass resources. Clean Technol. Environ. Policy 2020, 22, 1229–1253. [Google Scholar] [CrossRef]
- Nima, S.; Sutheravut, P.; Homket, Y. A Novel Community Health Impact Assessment towards a Public Policy: A Case Study of Biomass Power Plants in Southern Thailand. J. Southwest Jiaotong Univ. 2021, 56, 361–371. [Google Scholar] [CrossRef]
- Salinas, L.F.C.; Ochoa, G.V.; Escorcia, Y.C. A Scientometric Analysis of the Investigation of Biomass Gasification Environmental Impacts from 2001 to 2017. Int. J. Energy Econ. Policy 2018, 8, 223–229. [Google Scholar]
- He, W.; Luo, J.; Xing, L.; Yu, X.; Zhang, J.; Chen, S. Effects of temperature-control curtain on algae biomass and dissolved oxygen in a large stratified reservoir: Sanbanxi Reservoir case study. J. Environ. Manag. 2019, 248, 109250. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Wendt, L.M.; Murphy, J.A.; Seibel, F. Mitigation of variable seasonal productivity in algae biomass through blending and ensiling: An assessment of compositional changes in storage. Algal Res. 2019, 42, 101584. [Google Scholar] [CrossRef]
- Carpio, R.B.; Zhang, Y.; Kuo, C.T.; Chen, W.T.; Schideman, L.C.; de Leon, R.L. Characterization and thermal decomposition of demineralized wastewater algae biomass. Algal Res. 2019, 38, 101399. [Google Scholar] [CrossRef]
- Teo, S.H.; Islam, A.; Taufiq-Yap, Y.H. Algae derived biodiesel using nanocatalytic transesterification process. Chem. Eng. Res. Des. 2016, 111, 362–370. [Google Scholar] [CrossRef]
- Chamola, R.; Khan, M.F.; Raj, A.; Verma, M.; Jain, S. Response surface methodology based optimization of in situ transesterification of dry algae with methanol, H2SO4 and NaOH. Fuel 2019, 239, 511–520. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, C. Catalytic thermochemical conversion of algae and upgrading of algal oil for the production of high-grade liquid fuel: A review. Catalysts 2020, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Hadhoum, L.; Loubar, K.; Paraschiv, M.; Burnens, G.; Awad, S.; Tazerout, M. Optimization of oleaginous seeds liquefaction using response surface methodology. Biomass Convers. Biorefin. 2021, 11, 2655–2667. [Google Scholar] [CrossRef]
- Erdem, M.; Akdogan, E.; Bekki, A. Optimization and characterization studies on ecopolyol production from solvothermal acid-catalyzed liquefaction of sugar beet pulp using response surface methodology. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Jazie, A.A.; Hydary, J.; Abed, S.A.; Al-Dawody, M.F. Hydrothermal liquefaction of Fucus vesiculosus algae catalyzed by Hβ zeolite catalyst for Biocrude oil production. Algal Res. 2022, 61, 102596. [Google Scholar] [CrossRef]
- Guo, B.; Yang, B.; Su, Y.; Zhang, S.; Hornung, U.; Dahmen, N. Screening and Optimization of Microalgae Biomass and Plastic Material Coprocessing by Hydrothermal Liquefaction. ACS EST Eng. 2021, 2, 65–77. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.S.; Niu, H.; Dalai, A.; Corscadden, K.; Zhou, N. Microwave-assisted hydrothermal liquefaction of biomass model components and comparison with conventional heating. Fuel 2020, 277, 118202. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Saleh, A.A.; Islam, M.S.; Banggan, M.A.M.A. Production of Bio-Fuels by Microwave-Assisted Rapid Hydrothermal Liquefaction of Palm Kernel Shells Biomass. 2021. Available online: https://assets.researchsquare.com/files/rs-409704/v1/dbe4fac1-900c-49d8-8821-e9b2e38492b9.pdf?c=1631880993 (accessed on 5 April 2022).
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-Y.; Cui, Z.-W. A Microwave-Sensitive Solid Acid Catalyst Prepared from Sweet Potato via a Simple Method. Catalysts 2016, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Arshanitsa, A.; Akishin, Y.; Zile, E.; Dizhbite, T.; Solodovnik, V.; Telysheva, G. Microwave treatment combined with conventional heating of plant biomass pellets in a rotated reactor as a high rate process for solid biofuel manufacture. Renew. Energy 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Salman, B.; Hussain, R.; Ong, M.Y. Microwave pyrolysis of lignocellulosic biomass—A contribution to power Africa. Energy Sustain. Soc. 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Abdul Latif, N.I.S.; Ong, M.Y.; Nomanbhay, S. Hydrothermal liquefaction of Malaysia’s algal biomass for high-quality bio-oil production. Eng. Life Sci. 2019, 19, 246–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S. Production, separation and applications of phenolic-rich bio-oil—A review. Bioresour. Technol. 2015, 178, 90–98. [Google Scholar] [CrossRef]
- Gautam, R.; Shyam, S.; Reddy, B.R.; Govindaraju, K.; Vinu, R. Microwave-assisted pyrolysis and analytical fast pyrolysis of macroalgae: Product analysis and effect of heating mechanism. Sustain. Energy Fuels 2019, 3, 3009–3020. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Q.; Lin, Y.; Hu, Y.; Wang, H.; Zhang, G. Characterization of Aqueous Products Obtained from Hydrothermal Liquefaction of Rice Straw: Focus on Product Comparison via Microwave-Assisted and Conventional Heating. Energy Fuels 2018, 32, 510–516. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, J.; Wang, C.; Zhang, Q.; Ma, L. Microwave-assisted hydrothermal liquefaction for biomass valorization: Insights into the fuel properties of biocrude and its liquefaction mechanism. Fuel 2022, 317, 123462. [Google Scholar] [CrossRef]
- Yang, J.; Niu, H.; Corscadden, K.; He, Q.; Zhou, N. MW-assisted hydrothermal liquefaction of spent coffee grounds. Can. J. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Remón, J.; Randall, J.; Budarin, V.L.; Clark, J.H. Production of bio-fuels and chemicals by microwave-assisted, catalytic, hydrothermal liquefaction (MAC-HTL) of a mixture of pine and spruce biomass. Green Chem. 2019, 21, 284–299. [Google Scholar] [CrossRef]
- Tsubaki, S.; Onda, A.; Ueda, T.; Hiraoka, M.; Fujii, S.; Wada, Y. Microwave-Assisted Hydrothermal Processing of Seaweed Biomass. In Hydrothermal Processing in Biorefineries; Ruiz, H., Hedegaard Thomsen, M., Trajano, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 443–460. [Google Scholar]
- Tsutsui, I.; Miyoshi, T.; Aue-Umneoy, D.; Songphatkaew, J.; Meeanan, C.; Klomkling, S.; Sukchai, H.; Pinphoo, P.; Yamaguchi, I.; Ganmanee, M.; et al. High tolerance of Chaetomorpha sp. to salinity and water temperature enables survival and growth in stagnant waters of central Thailand. Int. Aquat. Res. 2015, 7, 47–62. [Google Scholar] [CrossRef] [Green Version]
- Ong, M.Y.; Abdul Latif, N.I.S.; Leong, H.Y.; Salman, B.; Show, P.L.; Nomanbhay, S. Characterization and analysis of Malaysian macroalgae biomass as potential feedstock for bio-oil production. Energies 2019, 12, 3509. [Google Scholar] [CrossRef] [Green Version]
- Celikbag, Y.; Nuruddin, M.; Biswas, M.; Asafu-Adjaye, O.; Via, B.K. Bio-oil-based phenol–formaldehyde resin: Comparison of weight- and molar-based substitution of phenol with bio-oil. J. Adhes. Sci. Technol. 2020, 34, 2743–2754. [Google Scholar] [CrossRef]
- Cui, Y.; Hou, X.; Wang, W.; Chang, J. Synthesis and characterization of bio-oil phenol formaldehyde resin used to fabricate phenolic based materials. Materials 2017, 10, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, P.M.V.; Barron, A.R. UV-Visible Spectroscopy. In Physical Methods in Chemistry and Nano Science (Barron); Rice University: Houston, TX, USA, 2021. [Google Scholar]
- Lazzari, E.; Schena, T.; Marcelo, M.C.A.; Primaz, C.T.; Silva, A.N.; Ferrão, M.F.; Bjerk, T.; Caramão, E.B. Classification of biomass through their pyrolytic bio-oil composition using FTIR and PCA analysis. Ind. Crops Prod. 2018, 111, 856–864. [Google Scholar] [CrossRef]
- Sugumaran, V.; Prakash, S.; Ramu, E.; Arora, A.K.; Bansal, V.; Kagdiyal, V.; Saxena, D. Detailed characterization of bio-oil from pyrolysis of non-edible seed-cakes by Fourier Transform Infrared Spectroscopy (FTIR) and gas chromatography mass spectrometry (GC-MS) techniques. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1058, 47–56. [Google Scholar] [CrossRef]
- Wei, X.; Jie, D. Optimization to Hydrothermal Liquefaction of Low Lipid Content Microalgae Spirulina sp. Using Response Surface Methodology. J. Chem. 2018, 2018, 2041812. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.H.; Quitain, A.T.; Yusup, S.; Uemura, Y.; Sasaki, M.; Kida, T. Optimization of hydrothermal liquefaction of palm kernel shell and consideration of supercritical carbon dioxide mediation effect. J. Supercrit. Fluids 2018, 133, 640–646. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Chen, G. Optimizing the conditions for hydrothermal liquefaction of barley straw for bio-crude oil production using response surface methodology. Sci. Total Environ. 2018, 630, 560–569. [Google Scholar] [CrossRef]
- Hossain, F.M.; Kosinkova, J.; Brown, R.J.; Ristovski, Z.; Hankamer, B.; Stephens, E.; Rainey, T.J. Experimental investigations of physical and chemical properties for microalgae HTL bio-crude using a large batch reactor. Energies 2017, 10, 467. [Google Scholar] [CrossRef] [Green Version]
- Nomanbhay, S.; Ong, M.Y. A Review of Microwave-Assisted Reactions for Biodiesel Production. Bioengineering 2017, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Guzik, P.; Kulawik, P.; Zając, M.; Migdał, W. Microwave applications in the food industry: An overview of recent developments. Crit. Rev. Food Sci. Nutr. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Fang, Z.; Wang, B.; Xu, Z. Study on Microwave Sintering Process and Surface Texture Characteristics of Ceramic Materials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 677, 022078. [Google Scholar]
- Rosyadi, I.; Suyitno, S.; Ilyas, A.X.; Faishal, A.; Budiono, A.; Yusuf, M. Producing hydrogen-rich syngas via microwave heating and co-gasification: A systematic review. Biofuel Res. J. 2022, 9, 1573–1591. [Google Scholar] [CrossRef]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorization of Biobased Chemicals. ACS Sustain. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Chandra, R.; Vishal, G.; Sánchez, C.E.G.; Uribe, J.A.G. Bioreactor for algae cultivation and biodiesel production. In Bioreactors Sustainable Design and Industrial Applications in Mitigation of GHG Emission; Singh, L., Yousuf, A., Mahapatra, D.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 289–307. [Google Scholar]
- De La Hoz, A.; Alczar, J.; Carrillo, J.; Herrero, M.A.; Muñoz, J.D.M.; Prieto, P.; De Cózar, A.; Diaz-Ortiz, A. Reproducibility and Scalability of Microwave-Assisted Reactions. In Microwave Heating; Chandra, U., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Inaba, S. Catalytic role of H2O molecules in oxidation of CH3OH in water. Catalysts 2018, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Daly, H.; Xiang, H.; Yan, Y.; Zhang, H.; Hardacre, C.; Fan, X. Microwave-assisted catalyst-free hydrolysis of fibrous cellulose for deriving sugars and biochemicals. Front. Chem. Sci. Eng. 2019, 13, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Raikova, S.; Allen, M.J.; Chuck, C.J. Hydrothermal liquefaction of macroalgae for the production of renewable biofuels. Biofuels Bioprod. Biorefin. 2019, 13, 1483–1504. [Google Scholar] [CrossRef]
- Xiu, S.N.; Shahbazi, A.; Croonenberghs, J.; Wang, L.J. Oil production from duckweed by thermochemical liquefaction. Energy Sources Part A Recover. Util. Environ. Eff. 2010, 32, 1293–1300. [Google Scholar] [CrossRef]
- Venkatachalam, C.D.; Ravichandran, S.R.; Sengottian, M. Lignocellulosic and algal biomass for bio-crude production using hydrothermal liquefaction: Conversion techniques, mechanism and process conditions: A review. Environ. Eng. Res. 2022, 27, 200555. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal Liquefaction of Macroalgae Enteromorpha prolifera to Bio-oil. Energy Fuels 2010, 24, 4054–4061. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Ghavami, N.; Özdenkçi, K.; Salierno, G.; Björklund-Sänkiaho, M.; De Blasio, C. Analysis of operational issues in hydrothermal liquefaction and supercritical water gasification processes: A review. Biomass Convers. Biorefin. 2021, 1, 1–28. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Jayathilake, M.; Rudra, S.; Akhtar, N.; Christy, A.A. Characterization and evaluation of hydrothermal liquefaction char from alkali lignin in subcritical temperatures. Materials 2021, 14, 3024. [Google Scholar] [CrossRef]
- Posmanik, R.; Martinez, C.M.; Cantero-Tubilla, B.; Cantero, D.A.; Sills, D.L.; Cocero, M.J.; Tester, J.W. Acid and Alkali Catalyzed Hydrothermal Liquefaction of Dairy Manure Digestate and Food Waste. ACS Sustain. Chem. Eng. 2018, 6, 2724–2732. [Google Scholar] [CrossRef]
- Watson, J.; Wang, T.; Si, B.; Chen, W.T.; Aierzhati, A.; Zhang, Y. Valorization of hydrothermal liquefaction aqueous phase: Pathways towards commercial viability. Prog. Energy Combust. Sci. 2020, 77, 100819. [Google Scholar] [CrossRef]
- Bayat, H.; Dehghanizadeh, M.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Hydrothermal Liquefaction of Food Waste: Effect of Process Parameters on Product Yields and Chemistry. Front. Sustain. Food Syst. 2021, 5, 160. [Google Scholar] [CrossRef]
- López Barreiro, D.; Beck, M.; Hornung, U.; Ronsse, F.; Kruse, A.; Prins, W. Suitability of hydrothermal liquefaction as a conversion route to produce biofuels from macroalgae. Algal Res. 2015, 11, 234–241. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Liu, S.; Feng, L. Direct hydrothermal liquefaction of undried macroalgae Enteromorpha prolifera using acid catalysts. Energy Convers. Manag. 2014, 87, 938–945. [Google Scholar] [CrossRef]
- Kapcsándi, V.; Kovács, A.J.; Neményi, M.; Lakatos, E. Investigation of a non-thermal effect of microwave treatment. Acta Aliment. 2016, 45, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Cheng, K.; Jia, G. Microwave heating and non-thermal effects of sodium chloride aqueous solution. Mol. Phys. 2020, 118, e1662505. [Google Scholar] [CrossRef]
- Fan, J.; De Bruyn, M.; Budarin, V.L.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S.; Macquarrie, D.J.; Clark, J.H. Direct microwave-assisted hydrothermal depolymerization of cellulose. J. Am. Chem. Soc. 2013, 135, 12728–12731. [Google Scholar] [CrossRef] [PubMed]
- King, J.W.; Holliday, R.L.; List, G.R. Hydrolysis of soybean oil: In a subcritical water flow reactor. Green Chem. 1999, 1, 261–264. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B. Hydrothermal liquefaction of the brown macro-alga Laminaria Saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883. [Google Scholar] [CrossRef]
- Pińkowska, H.; Wolak, P. Hydrothermal decomposition of rapeseed straw in subcritical water. Proposal of three-step treatment. Fuel 2013, 113, 340–346. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: A state of the art review. Renew. Sustain. Energy Rev. 2017, 68, 113–125. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR spectroscopy, total phenolic content, antioxidant activity and anti-amylase activity of extracts and different tea forms of Garcinia schomburgkiana leaves. LWT 2020, 134, 110005. [Google Scholar] [CrossRef]
- Ma, C.; Geng, J.; Zhang, D.; Ning, X. Hydrothermal liquefaction of macroalgae: Influence of zeolites based catalyst on products. J. Energy Inst. 2020, 93, 581–590. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Shekhawat, D.; Smith, M.W.; Link, D.; Stiegman, A.E. Microwave-assisted pyrolysis of Mississippi coal: A comparative study with conventional pyrolysis. Fuel 2018, 217, 656–667. [Google Scholar] [CrossRef]
- Ouyang, X.; Zhu, G.; Huang, X.; Qiu, X. Microwave assisted liquefaction of wheat straw alkali lignin for the production of monophenolic compounds. J. Energy Chem. 2015, 24, 72–76. [Google Scholar] [CrossRef]
- Zou, S.; Wu, Y.; Yang, M.; Li, C.; Tong, J. Thermochemical Catalytic Liquefaction of the Marine Microalgae Dunaliella tertiolecta and Characterization of Bio-oils. Energy Fuels 2009, 23, 3753–3758. [Google Scholar] [CrossRef]
- Chen, J.; Li, S. Characterization of biofuel production from hydrothermal treatment of hyperaccumulator waste (Pteris vittata L.) in sub- and supercritical water. RSC Adv. 2020, 10, 2160–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topală, C.M.; Tătaru, L.D.; Ducu, C. ATR-FTIR Spectra Fingerprinting of Medicinal Herbs Extracts Prepared Using Microwave Extraction. Arab. J. Med. Aromat. Plants 2017, 3, 1–9. [Google Scholar]
- Zhang, Y.; Chen, D.Y.; Zhang, D.; Zhu, X.F. TG-FTIR analysis of bio-oil and its pyrolysis/gasification property. J. Fuel Chem. Technol. 2012, 40, 1194–1199. [Google Scholar] [CrossRef]
- Caunii, A.; Pribac, G.; Grozea, I.; Gaitin, D.; Samfira, I. Design of optimal solvent for extraction of bio–active ingredients from six varieties of Medicago sativa. Chem. Cent. J. 2012, 6, 123. [Google Scholar] [CrossRef] [Green Version]
- Çulcuoglu, E.; Ünay, E.; Karaosmanoglu, F.; Angin, D.; Şensöz, S. Characterization of the Bio-Oil of Rapeseed Cake. Energy Sources 2006, 27, 1217–1223. [Google Scholar] [CrossRef]




| Properties | Value |
|---|---|
| Moisture content (%) | 81.9 |
| Elemental composition | |
| Carbon, C (%) | 30.21 |
| Hydrogen, H (%) | 4.42 |
| Nitrogen, N (%) | 2.02 |
| Oxygen, O (%) | 61.73 |
| Sulphur, S (%) | 1.62 |
| Protein content (wt%) * | 12.63 |
| Lipid content (wt%) * | 8.88 |
| Ash content (wt%) * | 36.3 |
| Total Carbohydrate content (wt%) * | 42.19 |
| Crude fibre content (wt%) * | 1.7 |
| Nitrogen-Free Extract content (wt%) * | 40.49 |
| High Heating Value (MJ/kg) | 9.17 |
| Level | Operating Temperature (°C) | Preloaded Pressure (bar) | Water-to-Algal Biomass Ratio | Holding Time (min) |
|---|---|---|---|---|
| Low (−1) | 200 | 25 | 10 | 30 |
| Medium (0) | 230 | 35 | 20 | 60 |
| High (+1) | 260 | 45 | 30 | 90 |
| Experimental Run | Temperature (°C) | Preload Pressure (bar) | Water to Algal Biomass Ratio | Reaction Time (min) | Experimental Bio-oil Yield (%) | Experimental Phenolic Yield (wt% GAE) |
|---|---|---|---|---|---|---|
| 1 | 230 | 35 | 30 | 30 | 20.562 | 13.22 |
| 2 | 230 | 25 | 20 | 90 | 13.35 | 11.68 |
| 3 | 260 | 45 | 20 | 60 | 13.35 | 12.16 |
| 4 * | 230 | 35 | 20 | 60 | 19.512 | 14.62 |
| 5 | 230 | 25 | 10 | 60 | 10.956 | 8.55 |
| 6 | 260 | 35 | 30 | 60 | 17.904 | 10.96 |
| 7 | 230 | 35 | 10 | 90 | 14.316 | 10.43 |
| 8 | 260 | 35 | 10 | 60 | 11.688 | 6.65 |
| 9 | 230 | 35 | 30 | 90 | 20.904 | 19.13 |
| 10 | 200 | 35 | 20 | 30 | 15.876 | 10.91 |
| 11 | 230 | 45 | 20 | 30 | 15.684 | 14.42 |
| 12 | 260 | 35 | 20 | 90 | 14.532 | 10.48 |
| 13 | 230 | 35 | 10 | 30 | 13.722 | 9.38 |
| 14 | 200 | 25 | 20 | 60 | 11.868 | 7.96 |
| 15 * | 230 | 35 | 20 | 60 | 19.698 | 14.38 |
| 16 | 200 | 35 | 20 | 90 | 15.918 | 12.57 |
| 17 | 200 | 45 | 20 | 60 | 14.622 | 14.83 |
| 18 | 230 | 25 | 30 | 60 | 15.354 | 13.21 |
| 19 | 230 | 45 | 10 | 60 | 12.222 | 12.75 |
| 20 | 230 | 45 | 20 | 90 | 16.05 | 18.22 |
| 21 | 230 | 25 | 20 | 30 | 12.45 | 10.19 |
| 22 | 200 | 35 | 10 | 60 | 13.506 | 6.53 |
| 23 | 260 | 25 | 20 | 60 | 10.128 | 7.43 |
| 24 | 260 | 35 | 20 | 30 | 14.49 | 9.22 |
| 25 | 230 | 45 | 30 | 60 | 19.704 | 17.67 |
| 26 * | 230 | 35 | 20 | 60 | 19.83 | 14.12 |
| 27 | 200 | 35 | 30 | 60 | 18.348 | 13.56 |
| Source | Sequential p-Value | Lack of Fit p-Value | Adjusted R-Squared | Predicted R-Squared | Remarks |
|---|---|---|---|---|---|
| Linear | 0.0011 | 0.0044 | 0.4690 | 0.3967 | |
| 2 Factor Interaction (2FI) | 0.9982 | 0.0032 | 0.2888 | 0.0133 | |
| Quadratic | <0.0001 | 0.2166 | 0.9910 | 0.9767 | Suggested |
| Cubic | 0.0361 | 0.7431 | 0.9983 | 0.9897 | Aliased |
| Source | Sequential p-Value | Lack of Fit p-Value | Adjusted R-Squared | Predicted R-Squared | Remarks |
|---|---|---|---|---|---|
| Linear | <0.0001 | 0.0139 | 0.6265 | 0.5294 | |
| 2 Factor Interaction (2FI) | 0.8815 | 0.0112 | 0.5503 | 0.1997 | |
| Quadratic | <0.0001 | 0.1439 | 0.9686 | 0.9181 | Suggested |
| Cubic | 0.1697 | 0.2004 | 0.9856 | 0.7444 | Aliased |
| Source | Sum of Squares | Degree of Freedom (df) | Mean Squared | F-Value | p-Value Prob > F | Remarks |
|---|---|---|---|---|---|---|
| Model | 255.97 | 10 | 25.60 | 337.52 | <0.0001 | significant |
| A -Temperature | 5.39 | 1 | 5.39 | 71.14 | <0.0001 | |
| B -Preload Pressure | 25.60 | 1 | 25.60 | 337.52 | <0.0001 | |
| C -Water to Algal Biomass Ratio | 110.21 | 1 | 110.21 | 1453.19 | <0.0001 | |
| D -Reaction Time | 0.44 | 1 | 0.44 | 5.74 | 0.0291 | |
| AC | 0.47 | 1 | 0.47 | 6.22 | 0.0239 | |
| BC | 2.38 | 1 | 2.38 | 31.35 | <0.0001 | |
| A2 | 54.86 | 1 | 54.86 | 723.40 | <0.0001 | |
| B2 | 86.14 | 1 | 86.14 | 1135.77 | <0.0001 | |
| C2 | 6.31 | 1 | 6.31 | 83.21 | <0.0001 | |
| D2 | 8.39 | 1 | 8.39 | 110.63 | <0.0001 | |
| Residual | 1.21 | 16 | 0.076 | |||
| Lack of Fit | 1.16 | 14 | 0.083 | 3.25 | 0.2598 | not significant |
| Pure Error | 0.051 | 2 | 0.026 | |||
| Corrected Total Sum of Squares (Cor Total) | 257.18 | 26 |
| Source | Sum of Squares | Degree of Freedom (df) | Mean Squared | F-Value | p-Value Prob > F | Remarks |
|---|---|---|---|---|---|---|
| Model | 273.87 | 7 | 39.12 | 87.91 | <0.0001 | significant |
| A -Temperature | 8.43 | 1 | 8.43 | 18.95 | 0.0003 | |
| B -Preload Pressure | 80.24 | 1 | 80.24 | 180.29 | <0.0001 | |
| C -Water to Algal Biomass Ratio | 86.73 | 1 | 86.73 | 194.87 | <0.0001 | < 0.0001 |
| D -Reaction Time | 17.69 | 1 | 17.69 | 39.75 | <0.0001 | |
| CD | 7.45 | 1 | 7.45 | 16.75 | 0.0006 | |
| A2 | 72.82 | 1 | 72.82 | 163.62 | <0.0001 | |
| C2 | 5.78 | 1 | 5.78 | 13.00 | 0.0019 | |
| Residual | 8.46 | 19 | 0.45 | |||
| Lack of Fit | 8.33 | 17 | 0.49 | 7.84 | 0.1190 | not significant |
| Pure Error | 0.13 | 2 | 0.06 | |||
| Corrected Total Sum of Squares (Cor Total) | 282.32 | 26 |
| Response | Bio-Oil Yield | Phenolic Yield | ||
|---|---|---|---|---|
| Quadratic Model | Reduced Quadratic Model | Quadratic Model | Reduced Quadratic Model | |
| F-value | 204.87 | 337.52 | 58.33 | 87.91 |
| Predicted R-squared value | 0.9767 | 0.9845 | 0.9181 | 0.9352 |
| Adjusted R-squared value | 0.9910 | 0.9923 | 0.9686 | 0.9590 |
| Adequate Precision | 46.201 | 58.233 | 26.634 | 33.525 |
| Name | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
|---|---|---|---|---|---|---|
| A: Temperature | is in range | 200 | 260 | 1 | 1 | 3 |
| B: Preload Pressure | is in range | 25 | 45 | 1 | 1 | 3 |
| C: Water to Algal Biomass Ratio | is in range | 10 | 30 | 1 | 1 | 3 |
| D: Reaction Time | is in range | 30 | 90 | 1 | 1 | 3 |
| Bio-oil Yield | maximize | 10.13 | 20.91 | 1 | 1 | 3 |
| Phenolic Yield | maximize | 6.65 | 19.13 | 1 | 1 | 3 |
| Best Result before Optimization Study | After Optimization | Percentage Difference (%) | |
|---|---|---|---|
| Operating Temperature (°C) | 230 | 226 | ↓1.74 |
| Preloaded Pressure (bar) | 35 | 42 | ↑20 |
| Water-to-Algal Biomass Ratio | 30:1 | 30:1 | - |
| Holding Time (min) | 90 | 79 | ↓12.22 |
| Experimental Bio-oil Yield (%) | 20.904 | 21.47 | ↑2.71 |
| Experimental Phenolic Yield (wt% GAE) | 19.13 | 19.22 | ↑0.47 |
| Properties | Ambient Water | Subcritical Water | Supercritical Water |
|---|---|---|---|
| Temperature (°C) | 25 | 250 | 400 |
| Pressure (bar) | 1 | 50 | 250 |
| Density, ρ (g/cm3) | 1 | 0.8 | 0.17 |
| Dielectric constant, ε (F/m) | 78.5 | 27.10 | 5.9 |
| Ionic product, log10Kw | −14.0 | −11.2 | −19.4 |
| Heat capacity, Cp (kJ/kg·K) | 4.22 | 4.86 | 13.0 |
| Dynamic viscosity, η (mPA·s) | 0.89 | 0.11 | 0.03 |
| Wavenumber Range (Reference, cm−1) | Wavenumber Range (Observed in This Work, cm−1) | Possible Assignment | References | ||
|---|---|---|---|---|---|
| 3000–3600 | 3298 | O-H stretching | [75] | ||
| 2800–3000 | 2926 | CH2 and CH3 stretching vibrations | [41] | ||
| 2858 | |||||
| 1640–1680 | 1674 | Primary and secondary amide C=O stretching | [41,81] | ||
| 1600–1760 | N–H bending vibrations | ||||
| 1500–1600 | 1521 | Aromatic C=C, C=O and N–H bending vibrations | [80] | ||
| 1500–1540 | Aromatic nitro compound NO2 asymmetric stretching | [41,82] | |||
| 1380–1465 | 1460 | CH3 lipids/proteins and COO– of amino acids | [79,83] | ||
| 1388 | |||||
| Primary and secondary O-H bending (in-plane), and phenol or tertiary alcohol (O-H bend) | |||||
| 1350–1370 | 1361 | ||||
| CH3 and CH2 in rings | [84] | ||||
| 1100–1300 | 1265 | C–O stretching, vibration | [41,79] | ||
| 1201 | |||||
| 1109 | |||||
| 1003–1230 | 1018 | C–O stretch, C–C stretch, C–H bend | |||
| <1000 | 965–930 | 939 | C-H bending vibration | Oxime N–O stretching | [41,80] |
| 915–650 | 891 | O–H bending | |||
| 750–650 | 731 | Secondary amid N–H wagging | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, M.Y.; Nomanbhay, S. Optimization Study on Microwave-Assisted Hydrothermal Liquefaction of Malaysian Macroalgae Chaetomorpha sp. for Phenolic-Rich Bio-Oil Production. Energies 2022, 15, 3974. https://doi.org/10.3390/en15113974
Ong MY, Nomanbhay S. Optimization Study on Microwave-Assisted Hydrothermal Liquefaction of Malaysian Macroalgae Chaetomorpha sp. for Phenolic-Rich Bio-Oil Production. Energies. 2022; 15(11):3974. https://doi.org/10.3390/en15113974
Chicago/Turabian StyleOng, Mei Yin, and Saifuddin Nomanbhay. 2022. "Optimization Study on Microwave-Assisted Hydrothermal Liquefaction of Malaysian Macroalgae Chaetomorpha sp. for Phenolic-Rich Bio-Oil Production" Energies 15, no. 11: 3974. https://doi.org/10.3390/en15113974






