Characterization of Wax Precipitation and Deposition Behavior of Condensate Oil in Wellbore: A Comprehensive Review of Modeling, Experiment, and Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Modeling Characterization of Wax Precipitation and Deposition Behavior
2.1. Modeling Characterization of Wax Precipitation Behavior
2.2. Modeling Characterization of Wax Deposition Behavior
3. Experiment Characterization of Wax Precipitation and Deposition Behavior
3.1. Experiment Characterization of Wax Precipitation Behavior
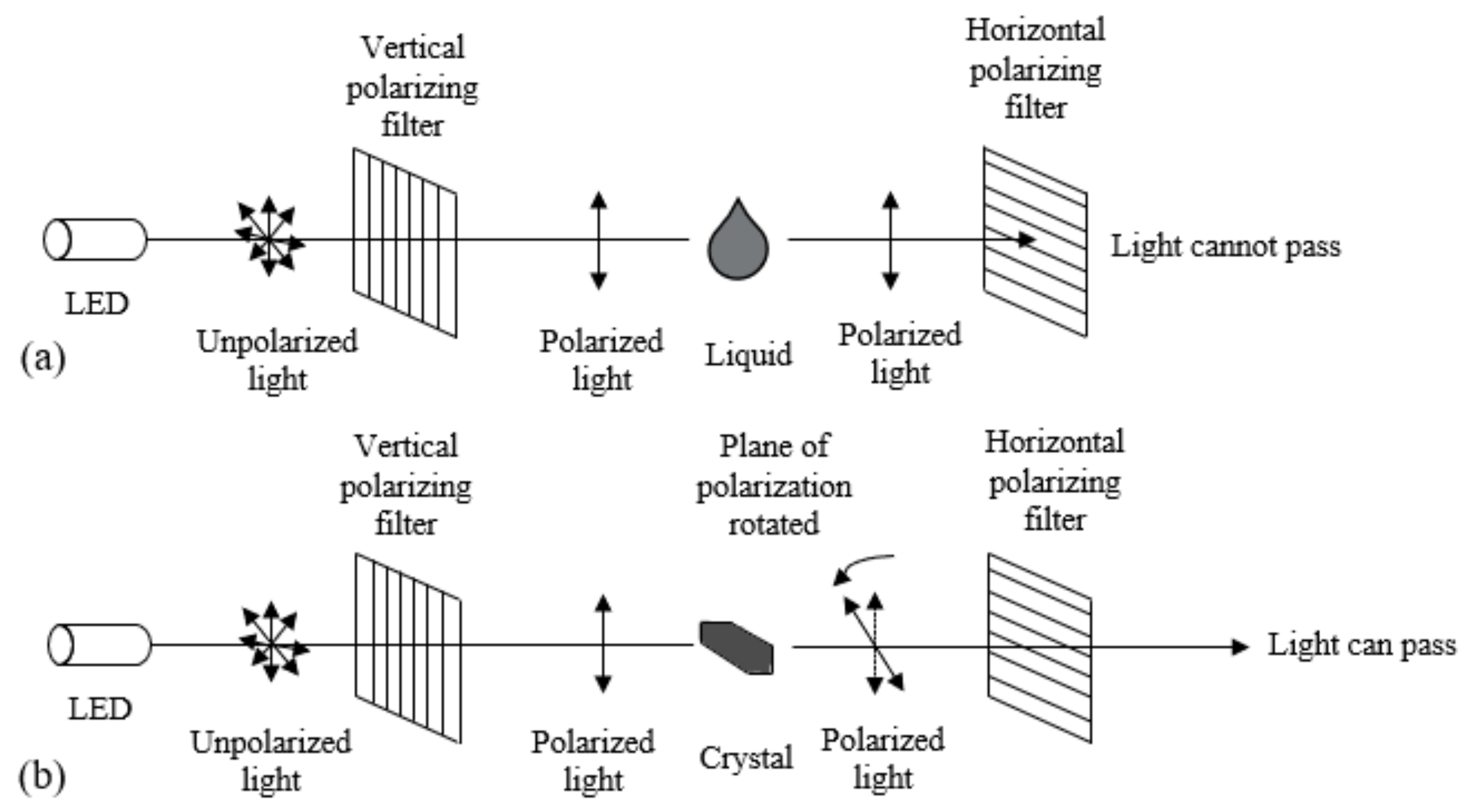
3.1.1. Microscopic Observation Method
3.1.2. Viscometry Method
3.1.3. Differential Scanning Calorimetry Method
3.1.4. Laser Scattering Method
3.1.5. Ultrasonic Method
3.2. Experiment Characterization of Wax Deposition Behavior
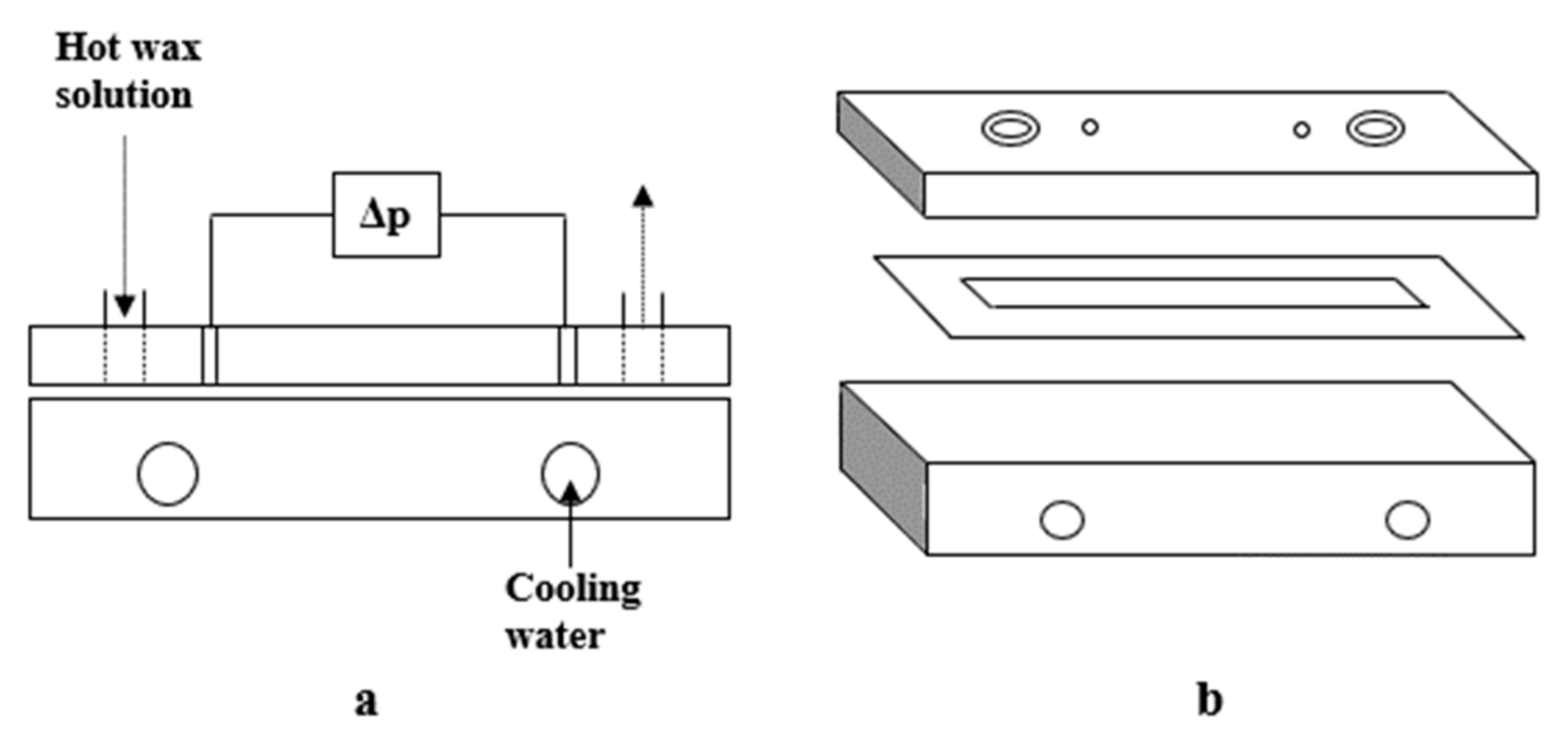
3.2.1. Cold Plate Method
3.2.2. Cold Finger Method
3.2.3. Rotating Disk Method
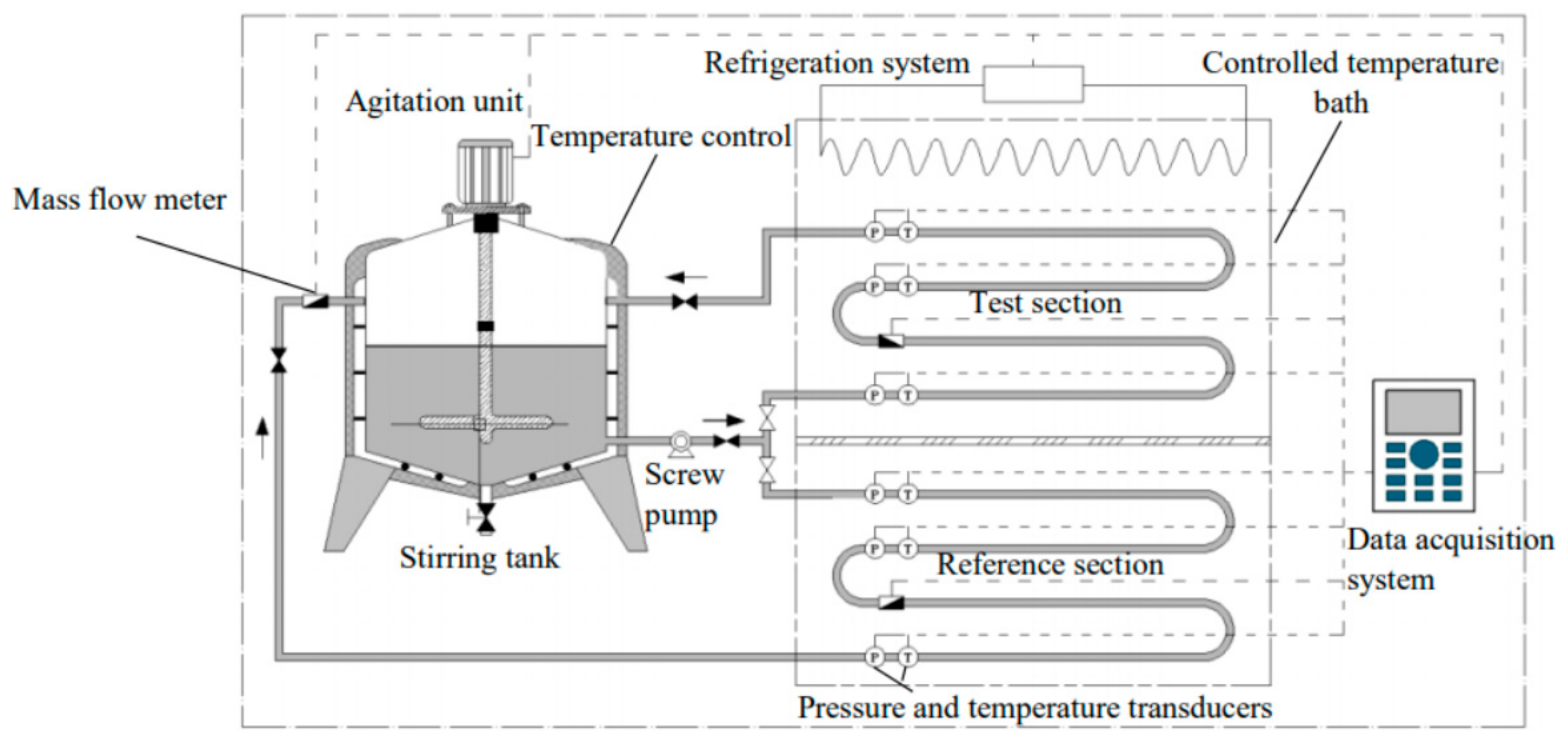
3.2.4. Flow Loop Method
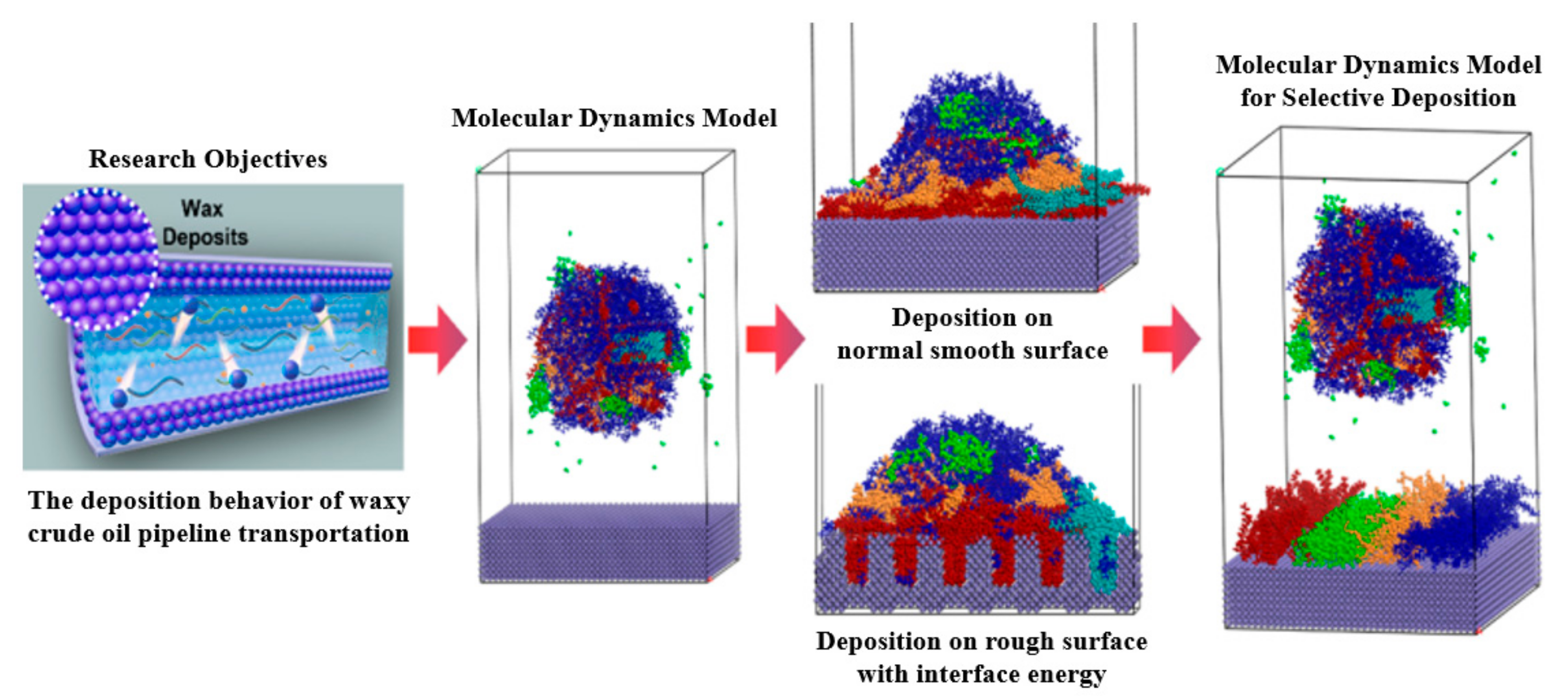
4. Molecular Dynamics Characterization of Wax Precipitation and Deposition Behavior
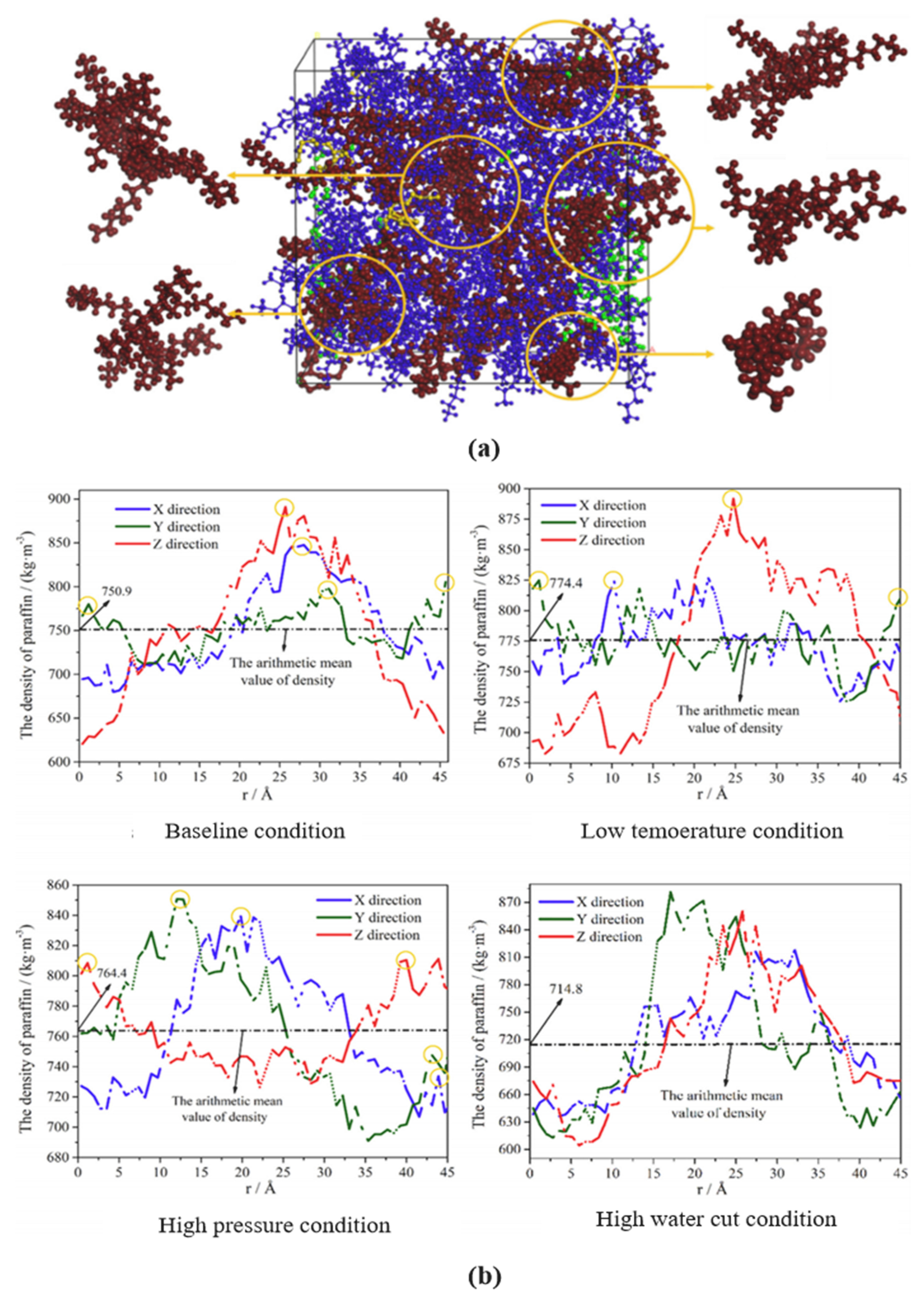
4.1. MD Simulation of Wax Precipitation Behavior
4.2. MD Simulation of Wax Deposition Behavior
5. Challenges and Prospects
- (i)
- The above models can characterize the wax precipitation behavior of the condensate oil system with better accuracy. However, the models to predict the wax precipitation temperature and amount of the condensate oil may not give ideal results under certain extreme temperature and pressure conditions, such as deep reservoir forming environment at high temperature and high pressure.
- (ii)
- There are few experimental studies on the multi-phase flow wax deposition, and the experimental data obtained under different conditions are also scarce. It is also difficult to obtain the relevant data of wax deposition in the wellbore during the condensate oil production, which makes it difficult to provide sufficient data support for modeling and verification of wax deposition in condensate oil.
- (iii)
- Currently, the common experimental methods for characterizing wax deposition are still the cold finger method and the flow loop method. Although these characterization methods can be performed under the system with pressure, however, the maximum temperature and pressure values of the experimental apparatus are limited, and the shear flow field cannot also be truly reflected. Hence, the experimental method for characterizing the wax deposition behavior of condensate in the wellbore has certain drawbacks.
- (iv)
- At present, most of the proven condensate oil is buried in deep and ultra-deep formations, and it is difficult to directly understand the wax precipitation and deposition behavior in the wellbore during development. Considering the safety and economy of working under high temperature and high pressure, it is also difficult to conduct large-scale experimental research in the laboratory. MD simulation technology is expected to become a new means to characterize the wax precipitation and deposition behavior of condensate oil in the wellbore, which can supplement and guide the predictive models and experimental studies, help to analyze the microscopic properties, and explain the macroscopic phenomena.
- (v)
- Considering the parallel calculation and calculation speed, it is inevitable to make appropriate simplifications to build the model, but the actual oil phase components are diverse, and the models containing a single hydrocarbon component are not conducive to the characterization of MD method to the microscopic behavior of wax precipitation and deposition of condensate oil in wellbore.
- (vi)
- During the condensate oil development, massive wax crystals are often precipitated and adhere to the wellbore wall due to variations in oil phase composition and external environment. Along with the existence of sand and scale, and the possibility of hydrate formation, the wellbore blockage is increasingly becoming serious. Therefore, how to effectively construct a method to characterize the coupled deposition behavior of wax and asphaltene, sand, scale, and hydrate at the microscale is the direction for further investigation and application of MD simulation in the future.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGlade, C.; Speirs, J.; Sorrell, S. Unconventional gas—A review of regional and global resource estimates. Energy 2013, 55, 571–584. [Google Scholar] [CrossRef]
- Wang, H.; Ma, F.; Tong, X.; Liu, Z.; Zhang, X.; Wu, Z.; Li, D.; Wang, B.; Xie, Y.; Yang, L. Assessment of global unconventional oil and gas resources. Pet. Explor. Dev. 2016, 43, 925–940. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Beagle, J.R.; Liao, L.; Shi, S.; Deng, R. Reconstruction of the evolution of deep fluids in light oil reservoirs in the central tarim basin by using PVT simulation and basin modeling. Mar. Pet. Geol. 2019, 107, 116–126. [Google Scholar] [CrossRef]
- Sheng, J.; Mody, F.; Griffith, P.J.; Barnes, W.N. Potential to increase condensate oil production by Huff-n-Puff gas injection in a shale condensate reservoir. J. Nat. Gas Sci. Eng. 2016, 28, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, S.; Wang, N.; Li, W.; Qin, S.; Ma, W. Analysis of condensate oil by comprehensive two-dimensional gas chromatography-sciencedirect. Pet. Explor. Dev. 2012, 39, 132–138. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Luo, H.; Peng, B.; Sun, X.; Liu, Y.; Rui, Z. Foaming properties and foam structure of produced liquid in alkali/surfactant/polymer flooding production. J. Energy Resour. Technol. 2021, 143, 103005. [Google Scholar] [CrossRef]
- Chen, J.; Deng, C.; Wang, X.; Ni, Y.; Sun, Y.; Zhao, Z.; Wang, P.; Liao, J.; Zhang, D.; Liang, D. Source of condensate oil in the middle of southern margin, Junggar Basin, NW China. Pet. Explor. Dev. 2016, 43, 902–913. [Google Scholar] [CrossRef]
- Weijermars, R.; Al-Shehri, D. Regulation of oil and gas reserves reporting in Saudi Arabia: Review and recommendations. J. Pet. Sci. Eng. 2022, 210, 109806. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, B.; Chen, W.; Zhang, K.; Ye, Y. Simulation-assisted gas tracer test study of Tarim Yaha gas-condensate reservoir in China. J. Nat. Gas Sci. Eng. 2017, 48, 77–84. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Liu, P.; Li, Y.; Wu, S.; Dai, P.; Yang, Q. Treatment of the complex liquid phase that contains produced water, condensate oil, and floccule from an offshore gas field: A pilot system for the South China Sea. J. Nat. Gas Sci. Eng. 2021, 94, 104125. [Google Scholar] [CrossRef]
- Zhang, A.; Fan, Z.; Zhao, L. An investigation on phase behaviors and displacement mechanisms of gas injection in gas condensate reservoir. Fuel 2020, 268, 117373. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, F.; Zhang, L.; Huang, Y.; Yao, E.; Zhang, L.; Wang, F.; Fan, F. Experimental study of wax deposition pattern concerning deep condensate gas in Bozi Block of tarim oilfield and its application. Thermochim. Acta 2018, 671, 1–9. [Google Scholar] [CrossRef]
- White, M.; Pierce, K.; Acharya, T. A review of wax-formation/mitigation technologies in the petroleum industry. SPE Prod. Oper. 2018, 33, 476–485. [Google Scholar] [CrossRef]
- Ellison, B.T.; Gallagher, C.T.; Frostman, L.M.; Lorimer, S.E. The physical chemistry of wax, hydrates, and asphaltene. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 1–4 May 2000. [Google Scholar]
- Oliveira, M.D.; Vieira, L.; Miranda, L.; Miranda, D.; Marques, L. On the influence of micro-and macro-cristalline paraffins on the physical and rheological properties of crude oil and organic solvents. Chem. Chem. Technol. 2016, 10, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Adebiyi, F.M. Paraffin wax precipitation/deposition and mitigating measures in oil and gas industry: A review. Pet. Sci. Technol. 2020, 38, 962–971. [Google Scholar] [CrossRef]
- Leontaritis, K.J. Wax flow assurance issues in gas condensate multiphase flowlines. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2007. [Google Scholar]
- Yang, F.; Chen, J.; Yao, B.; Li, C.; Sun, G. Effects of EVA additive dosage on rheolegical properties of asphaltenic waxy oils. Acta Pet. Sin. 2021, 37, 572–583. [Google Scholar]
- El-Dalatony, M.M.; Jeon, B.H.; Salama, E.S.; Eraky, M.; Kim, W.B.; Wang, J.; Ahn, T. Occurrence and characterization of paraffin wax formed in developing wells and pipelines. Energies 2019, 12, 967. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.T.; Rogachev, M.K.; Aleksandrov, A. Nikolaevich. A new approach to improving efficiency of gas-lift wells in the conditions of the formation of organic wax deposits in the Dragon field. J. Pet. Explor. Prod. Technol. 2020, 10, 3663–3672. [Google Scholar] [CrossRef]
- Labes-Carrier, C.; Ronningsen, H.P.; Kolnes, J.; Leporcher, E. Wax Deposition in North Sea Gas Condensate and Oil Systems: Comparison Between Operational Experience and Model Prediction. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 24–27 September 2002. [Google Scholar]
- Rahimpour, M.R.; Davoudi, M.; Jokar, S.M.; Khoramdel, I.; Shariati, A.; Dehnavi, M.R. Wax formation assessment of condensate in south pars gas processing plant sea pipeline (a case study). J. Nat. Gas Sci. Eng. 2013, 10, 25–40. [Google Scholar] [CrossRef]
- Gluyas, J.; Underhill, J.R. The staffa field, Block 3/8b, UK North Sea. Geol. Soc. Lond. Mem. 2003, 20, 327–333. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Lu, J. Experimental study and numerical modeling of boron transport in reservoir and its influence on seawater-breakthrough calculation. SPE Reserv. Eval. Eng. 2021, 24, 292–309. [Google Scholar] [CrossRef]
- Geest, C.; Melchuna, A.; Bizarre, L.; Bannwart, A.C.; Guersoni, V. Critical review on wax deposition in single-phase flow. Fuel 2021, 293, 120358. [Google Scholar] [CrossRef]
- Sandyga, M.S.; Struchkov, I.A.; Rogachev, M.K. Formation damage induced by wax deposition: Laboratory investigations and modeling. J. Pet. Explor. Prod. Technol. 2020, 10, 2541–2558. [Google Scholar] [CrossRef]
- Shoushtari, A.B.; Asadolahpour, S.R.; Madani, M. Thermodynamic investigation of asphaltene precipitation and deposition profile in wellbore: A case study. J. Mol. Liq. 2020, 320, 114468. [Google Scholar] [CrossRef]
- Meighani, H.M.; Ghotbi, C.; Behbahani, T.J.; Sharifi, K. A new investigation of wax precipitation in Iranian crude oils: Experimental method based on FTIR spectroscopy and theoretical predictions using PC-SAFT model. J. Mol. Liq. 2018, 249, 970–979. [Google Scholar] [CrossRef]
- Coutinho, J.A.P. Predictive UNIQUAC: A new model for the description of multiphase solid-liquid equilibria in complex hydrocarbon mixtures. Ind. Eng. Chem. Res. 1998, 37, 4870–4875. [Google Scholar] [CrossRef]
- Coutinho, J.A.P.; Andersen, S.I.; Stenby, E.H. Evaluation of activity coefficient models in prediction of alkane solid–liquid equilibria. Fluid Phase Equilibria 1995, 103, 23–39. [Google Scholar] [CrossRef]
- Yang, Y.; Lun, Z.; Wang, R.; Hu, W. Non-equilibrium phase behavior in gas condensate depletion experiments-sciencedirect. Fluid Phase Equilibria 2020, 506, 112410. [Google Scholar] [CrossRef]
- Loskutov, V.Y.; Yadrevskaya, N.N.; Yudina, N.V.; Usheva, N.V. Study of viscosity-temperature properties of oil and gas-condensate mixtures in critical temperature ranges of phase transitions. Procedia Chem. 2014, 10, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Asbaghi, E.V.; Nazari, F.; Assareh, M.; Nezhad, M.M. Toward an efficient wax precipitation model: Application of multi-solid framework and PC-SAFT with focus on heavy end characterization for different crude types. Fuel 2022, 310, 122205. [Google Scholar] [CrossRef]
- Góes, M.R.R.T.; Teixeira, R.G.D.; Tavares, F.W.; Secchi, A.R. Wax appearance and prevention in two-phase flow using the multi-solid and drift-flux model. J. Pet. Sci. Eng. 2019, 177, 374–383. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Huang, H.; Shi, G.; Shi, B.; Cheng, B.; Gong, J. Prediction of solid-liquid equilibrium in paraffinic systems with new solid solution model. Fluid Phase Equilibria 2016, 427, 504–512. [Google Scholar] [CrossRef]
- Won, K.W. Thermodynamics for solid solution-liquid-vapor equilibria: Wax phase formation from heavy hydrocarbon mixtures. Fluid Phase Equilibria 1986, 30, 265–279. [Google Scholar] [CrossRef]
- Thomas, F.B.; Bennion, D.B.; Hunter, B.E. Experimental and theoretical studies of solids precipitation from reservoir fluid. J. Can. Pet. Technol. 1992, 31, 22–31. [Google Scholar] [CrossRef]
- Lira-Galeana, C.; Firoozabadi, A.; Prausnitz, J.M. Thermodynamics of wax precipitation in petroleum mixtures. AIChE J. 1996, 42, 239–248. [Google Scholar] [CrossRef]
- Pedersen, K.S. Prediction of cloud-point temperatures and amount of wax precipitation. SPE Prod. Facil. 1995, 10, 46–49. [Google Scholar] [CrossRef]
- Pan, H.; Firoozabadi, A.; Fotland, P. Pressure and composition effect on wax precipitation: Experimental data and model results. SPE Prod. Facil. 1997, 12, 250–258. [Google Scholar] [CrossRef]
- Nichita, D.V.; Goual, L.; Firoozabadi, A. Wax precipitation in gas condensate mixtures. SPE Prod. Facil. 1999, 16, 250–259. [Google Scholar] [CrossRef]
- Nichita, D.V.; Pauly, J.; Montel, F.; Daridon, J.L. Pseudocomponent delumping for multiphase systems with waxy solid phase precipitation. Energy Fuels 2007, 22, 775–783. [Google Scholar] [CrossRef]
- Daridon, J.L.; Pauly, J.; Coutinho, J.A.P.; Montel, F. Solid-liquid-vapor phase boundary of a North Sea waxy crude: Measurement and modeling. Energy Fuels 2001, 15, 730–735. [Google Scholar] [CrossRef]
- Sansot, J.M.; Pauly, J.; Daridon, J.L.; Coutinho, J.A.P. Modeling high-pressure wax formation in petroleum fluids. AIChE J. 2005, 51, 2089–2097. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, J.A.P.; Edmonds, B.; Moorwood, T.; Szczepanski, R.; Zhang, X. Reliable wax predictions for flow assurance. Energy Fuels 2006, 20, 1081–1088. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, D. Wax formation from synthetic oil systems and reservoir fluids. Energy Fuels 2008, 22, 2390–2395. [Google Scholar] [CrossRef]
- Burger, E.D.; Perkins, T.K.; Striegler, J.H. Studies of wax deposition in the Trans Alaska pipeline. J. Pet. Technol. 1981, 33, 1075–1086. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Ravneoy, J.M. Prediction of wax deposition in pipelines and field experience on the influence of wax on drag-reducer performance. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 1992. [Google Scholar]
- Hsu, J.J.C.; Santamaria, M.M.; Brubaker, J.P. Wax deposition of waxy live crudes under turbulent flow conditions. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 26–28 September 1994. [Google Scholar]
- Ramirez-Jaramillo, E.; Lira-Galeana, C.; Brito, O.M. Numerical model for wax deposition in oil wells. Pet. Sci. Technol. 2001, 19, 587–608. [Google Scholar] [CrossRef]
- Singh, P.; Fogler, H.S.; Nagarajan, N. Prediction of the wax content of the incipient wax-oil gel in a pipeline: An application of the controlled-stress rheometer. J. Rheol. 1999, 43, 1437–1459. [Google Scholar] [CrossRef]
- Singh, P.; Youyen, A.; Fogler, H.S. Existence of a critical carbon number in the aging of a wax-oil gel. AIChE J. 2001, 47, 2111–2124. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Lee, H.S.; Singh, P.; Sarica, C. Flow assurance: Validation of wax deposition models using field data from a subsea pipeline. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2011. [Google Scholar]
- Huang, Z.; Lu, Y.; Hoffmann, R.; Amundsen, L.; Fogler, H.S. The effect of operating temperatures on wax deposition. Energy Fuels 2011, 25, 5180–5188. [Google Scholar] [CrossRef]
- Eskin, D.; Ratulowski, J.; Akbarzadeh, K. Modelling wax deposition in oil transport pipelines. Can. J. Chem. Eng. 2014, 92, 973–988. [Google Scholar] [CrossRef]
- Quan, Q.; Wu, H.; Gao, G. Wax deposition of single-phase waxy crude oil at different temperatures. Oil Gas Storage Transport. 2014, 33, 852–856. [Google Scholar]
- Lin, X.; Wang, Z.; Feng, Q.; Zhang, L.; Xu, Y. The role of emulsified water in the wax deposition path of a waxy crude oil multiphase transportation pipeline. In Proceedings of the ASME Asia Pacific Pipeline Conference, Qingdao, China, 15–19 May 2019. [Google Scholar]
- Wang, Z.; Wang, H.; Zhu, C.; Rui, Z.; Liu, Y. A novel method for characterizing the aggregation of wax crystals and an improvement in wax deposition modeling. J. Energy Resour. Technol. 2020, 142, 103003. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, Y.; Zhang, H.; Liu, Y. Investigation on gelation nucleation kinetics of waxy crude oil emulsions by their thermal behavior. J. Pet. Sci. Eng. 2019, 181, 106230. [Google Scholar] [CrossRef]
- Mansourpoor, M.; Azin, R.; Osfouri, S.; Izadpanah, A.A.; Saboori, R. Experimental investigation of rheological behavior and wax deposition of waxy oil−disulfide oil systems. Nat. Resour. Res. 2019, 28, 1609. [Google Scholar] [CrossRef]
- Elphingstone, G.M.; Greenhill, K.L.; Hsu, J.J.C. Modeling of multiphase wax deposition. J. Energy Resour. Technol. 1999, 121, 81. [Google Scholar] [CrossRef]
- Apte, M.S.; Matzain, A.; Zhang, H.Q.; Volk, M.; Brill, J.P.; Creek, J.L. Investigation of paraffin deposition during multiphase flow in pipelines and wellbores-part 2: Modeling. J. Energy Resour. Technol. 2001, 123, 150–157. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Y.; Liao, L.; Duan, J.; Wang, P.; Zhou, J. Wax deposition in the oil/gas two-phase flow for a horizontal pipe. Energy Fuels 2011, 25, 1624–1632. [Google Scholar] [CrossRef]
- Duan, J.; Liu, H.; Guan, J.; Hua, W.; Jiao, G.; Gong, J. Wax deposition modeling of oil/gas stratified smooth pipe flow. AIChE J. 2016, 62, 2550–2562. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Liu, H.; Gu, K.; Guan, J.; Xu, S.; Gong, J. A model of wax deposition under oil-gas two-phase stratified flow in horizontal pipe. Oil Gas Sci. Technol. 2018, 73, 80. [Google Scholar] [CrossRef]
- Couto, G.H.; Chen, H.; Dellecase, E.; Sarica, C.; Volk, M. An investigation of two-phase oil/water paraffin deposition. SPE Prod. Oper. 2008, 23, 49–55. [Google Scholar] [CrossRef]
- Bruno, A.; Sarica, C.; Chen, H.; Volk, M. In paraffin deposition during the flow of water-in-oil and oil-in-water dispersions in pipes. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar]
- Wang, Z.; Liu, Y.; Li, J.; Zhuge, X.; Zhang, L. Study on two-phase oil-water gelling deposition behavior in low temperature transportation. Energy Fuels 2016, 30, 4570–4582. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Zhao, Y.; Li, Z.; Liu, Y.; Hong, J. Role of shearing dispersion and stripping in wax deposition in crude oil pipelines. Energies 2019, 22, 4325. [Google Scholar] [CrossRef] [Green Version]
- Obaseki, M.; Paul, E. Dynamic modeling and prediction of wax deposition thickness in crude oil pipelines. J. King Saud Univ.-Eng. Sci. 2021, 33, 437–445. [Google Scholar] [CrossRef]
- Kelechukwu, E.M.; Al-Salim, H.S.; Saadi, A. Prediction of wax deposition problems of hydrocarbon production system. J. Pet. Sci. Eng. 2013, 108, 128–136. [Google Scholar] [CrossRef]
- Quan, Q.; Wang, W.; Duan, J.; Li, Q.; Ruan, C.; Gong, J. A study on wax deposition model in oil-gas stratified flows. In Proceedings of the 5th Asian Symposium on Computational Heat Transfer and Fluid Flow, Busan, Korea, 22–25 November 2015. [Google Scholar]
- Xu, Q.; Wang, Y.; Jiang, Y.; Zhang, Q.; Bo, L.; Liu, Z. Application of coiled tubing paraffin removal technique in high pressure oil and gas well. Petrochem. Ind. Appl. 2017, 36, 83–85. [Google Scholar]
- Zhu, C.; Liu, X.; Xu, Y.; Liu, W.; Wang, Z. Determination of boundary temperature and intelligent control scheme for heavy oil field gathering and transportation system. J. Pipeline Sci. Eng. 2022, 1, 407–418. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Shi, B.; Zhu, C.; Wang, Z. Optimization and intelligent control for operation parameters of multiphase mixture transportation pipeline in oilfield: A case study. J. Pipeline Sci. Eng. 2022, 1, 367–378. [Google Scholar] [CrossRef]
- Xu, J.; Xing, S.; Qian, H.; Chen, S.; Wei, X.; Zhang, R.; Li, L.; Guo, X. Effect of polar/nonpolar groups in comb-type copolymers on cold flowability and paraffin crystallization of waxy oils. Fuel 2013, 103, 600–605. [Google Scholar] [CrossRef]
- Shi, J. Application of thermal analysis in the study of wax precipitation process of crude oil. Oil Gas Storage Transp. 1993, 12, 6. [Google Scholar]
- Li, H.; Zhang, J.; Chen, J. Comparison of different methods on determining wax appearance temperature of crude oils. Oil Gas Storage Transp. 2003, 22, 4. [Google Scholar]
- Japper-Jaafar, A.; Bhaskoro, P.T.; Mior, Z.S. A new perspective on the measurements of wax appearance temperature: Comparison between DSC, thermomicroscopy and rheometry and the cooling rate effects. J. Pet. Sci. Eng. 2016, 147, 672–681. [Google Scholar] [CrossRef]
- Yang, W.; Fu, C.; Du, Y.; Xu, K.; Balhoff, M.T.; Weston, J.; Lu, J. Dynamic contact angle reformulates pore-scale fluid-fluid displacement at ultralow interfacial tension. SPE J. 2020, 26, 1278–1289. [Google Scholar] [CrossRef]
- Japper-Jaafar, A.; Bhaskoro, P.T.; Sean, L.L.; Sariman, M.Z.; Nugroho, H. Yield stress measurement of gelled waxy crude oil: Gap size requirement. J. Non-Newton. Fluid Mech. 2015, 218, 71–82. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, J. Effect of carbon number distribution of wax on the yield stress of waxy oil gels. Ind. Eng. Chem. Res. 2013, 52, 2732–2739. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, S.; Fogler, H.S. Wax Deposition: Experimental Characterizations, Theoretical Modeling, and Field Practices; CRC Press: Hoboken, NJ, USA, 2016. [Google Scholar]
- Escobedo, J.; Mansoori, G.A. Theory of viscosity as a criterion for detection of onset of asphaltene flocculation. In Proceedings of the Society of Petroleum Engineers, Richardson, TX, USA, 6 January 1996. [Google Scholar]
- Hansen, A.B.; Larsen, E.; Pedersen, W.B.; Nielsen, A.B.; Rønningsen, H.P. Wax precipitation from North Sea crude oils. 3. Precipitation and dissolution of wax studied by differential scanning calorimetry. Energy Fuels 1991, 5, 914–923. [Google Scholar] [CrossRef]
- Claudy, P.; Létoffé, J.M.; Chagué, B.; Orrit, J. Crude oils and their distillates: Characterization by differential scanning calorimetry. Fuel 1988, 67, 58–61. [Google Scholar] [CrossRef]
- Cui, S.; Tang, Y.; Zhao, Y.; Yan, Z.; Wang, H. Study on prediction of wax deposition conditions in high temperature and high pressure condensate gas reservoir. Pet. Reserv. Eval. Dev. 2017, 7, 5. [Google Scholar]
- Jiang, Z.; Hutchinson, J.M.; Imrie, C.T. Measurement of the wax appearance temperatures of crude oils by temperature modulated differential scanning calorimetry. Fuel 2001, 80, 367–371. [Google Scholar] [CrossRef]
- Roenningsen, H.P.; Bjoerndal, B.; Hansen, A.B.; Pedersen, W.B. Wax precipitation from North Sea crude oils: 1.Crystallization and dissolution temperatures, and newtonian and non-newtonian flow properties. Energy Fuels 1991, 5, 895–908. [Google Scholar] [CrossRef]
- Cai, H.; Huang, C. Experimental study on wax deposit of high-waxy condensate gas well. Petrochem. Ind. Appl. 2011, 30, 3. [Google Scholar]
- Zhong, C.; Wang, J.; Liu, J.; Huang, Y.; Zhou, F.; Yang, X. Software simulation and experimental study on the law of wax deposition pattern in deep condensate gas. J. Petrochem. Univ. 2019, 32, 96–100. [Google Scholar]
- Chen, H.; Yang, S.; Nie, X.; Wu, Y.; Ding, J.; Wang, Z. Ultrasonic detection and analysis of the wax participation in high waxy oilfield. Chin. Sci. Bull. 2015, 60, 8. [Google Scholar]
- Pedersen, W.B.; Hansen, A.B.; Larsen, E.; Nielsen, A.B.; Roenningsen, H.P. Wax precipitation from North Sea crude oils. 2. Solid-phase content as function of temperature determined by pulsed NMR. Energy Fuels 1991, 5, 908–913. [Google Scholar] [CrossRef]
- Cazaux, G.; Barre, L.; Brucy, F. Waxy crude cold start: Assessment through gel structural properties. In Proceedings of the SPE Annual Technical Conference and Exhition, New Orleans, LA, USA, 27–30 September 1998. [Google Scholar]
- Cole, R.J.; Jessen, F.W. Paraffin deposition. Oil Gas J. 1960, 58, 87–91. [Google Scholar]
- Hunt, E.B. Laboratory study of paraffin deposition. J. Pet. Technol. 1962, 4, 1259–1269. [Google Scholar] [CrossRef]
- Leontaritis, K.J.; Geroulis, E. Wax deposition correlation-application in multiphase wax deposition models. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2011. [Google Scholar]
- Tinsley, J.F.; Prud’homme, R.K. Deposition apparatus to study the effects of polymers and asphaltenes upon wax deposition. J. Pet. Sci. Eng. 2010, 72, 166–174. [Google Scholar] [CrossRef]
- Bern, P.A.; Withers, V.R.; Cairns, R.J.R. Wax deposition in crude oil pipelines. In Proceedings of the Offshore Technology Conference and Exhibition, London, UK, 3–5 October 1980. [Google Scholar]
- Weispfennig, K. Advancements in paraffin testing methodology. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001. [Google Scholar]
- Hamouda, A.A.; Viken, B.K. Wax deposition mechanism under high-pressure and in presence of light hydrocarbons. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 2–5 March 1993. [Google Scholar]
- Zhang, Y.; Gong, J.; Ren, Y.; Wang, P. Effect of emulsion characteristics on wax deposition from water-in-waxy crude oil emulsions under static cooling conditions. Energy Fuels 2010, 24, 1146–1155. [Google Scholar] [CrossRef]
- Kasumu, A.S.; Mehrotra, A.K. Solids deposition from wax-solvent-water “Waxy” mixtures using a cold finger apparatus. Energy Fuels 2015, 29, 501–511. [Google Scholar] [CrossRef]
- Mahir, L.H.A.; Vilas, B.F.C.; Ketjuntiwa, T.; Fogler, H.S.; Larson, R.G. Mechanism of wax deposition on cold surfaces: Gelation and deposit aging. Energy Fuels 2019, 33, 3776–3786. [Google Scholar] [CrossRef]
- Zougari, M.; Jacobs, S.; Ratulowski, J.; Hammami, A.; Broze, G.; Flannery, M.; Stankiewicz, A.; Karan, K. Novel organic solids deposition and control device for live-oils: Design and applications. Energy Fuels 2006, 20, 1656–1663. [Google Scholar] [CrossRef]
- Matlach, W.J.; Newberry, M.E. Paraffin deposition and rheological evaluation of high wax content altamont crude oils. In Proceedings of the SPE Rocky Mountain Regional Meeting, Salt Lake City, UT, USA, 22 May 1983. [Google Scholar]
- Ahn, S.; Wang, K.S.; Shuler, P.J.; Creek, J.L.; Tang, Y. Paraffin crystal and deposition control by emulsification. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 2–4 February 2005. [Google Scholar]
- Hoffmann, R.; Amundsen, L. Single-phase wax deposition experiments. Energy Fuels 2010, 24, 1069–1080. [Google Scholar] [CrossRef]
- Rittirong, A.; Panacharoensawad, E.; Sarica, C. An experimental study of paraffin deposition under two-phase gas-oil slug flow in horizontal pipes. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2015. [Google Scholar]
- Fang, T.; Zhang, Y.; Ma, R.; Yan, Y.; Dai, C.; Zhang, J. Oil extraction mechanism in CO2 flooding from rough surface: Molecular dynamics simulation. Appl. Surf. Sci. 2019, 494, 80–86. [Google Scholar] [CrossRef]
- Fang, T.; Zhang, Y.; Liu, J.; Ding, B.; Yan, Y.; Zhang, J. Molecular insight into the miscible mechanism of CO2/C10 in bulk phase and nanoslits. Int. J. Heat Mass Transf. 2019, 141, 643–650. [Google Scholar] [CrossRef]
- Yan, Y.; Dong, Z.; Zhang, Y.; Wang, P.; Fang, T.; Zhang, J. CO2 activating hydrocarbon transport across nanopore throat: Insights from molecular dynamics simulation. Phys. Chem. Chem. Phys. 2017, 19, 30439–30444. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Zhang, J.; Xiao, S.; Zhang, Z.; Shen, Y.; Sun, B.; He, J. Displacement mechanism of oil in shale inorganic nanopores by supercritical carbon dioxide from molecular dynamics simulations. Energy Fuels 2017, 31, 738–746. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Liu, Y.; Liu, X.; Rui, Z. Molecular dynamics-based simulation on chemical flooding produced emulsion formation and stabilization: A critical review. Arab. J. Sci. Eng. 2020, 45, 7161–7173. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Wang, Z.; Xu, Z.; Hong, J.; Sun, W. Microscopic mechanism of asphaltene and resin aggregation behavior to the stability of oil water interface. J. Northeast. Pet. Univ. 2021, 45, 90–101. [Google Scholar]
- Gamba, Z.; Hautman, J.; Shelley, J.C.; Klein, M.L. Molecular dynamics investigation of a newtonian black film. Langmuir 1992, 8, 3155–3160. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Wang, H.; Li, Q.; Yuan, S.; Xu, G.; Liu, C. Molecular dynamics simulation of anionic surfactant aggregation at the oil/water interface. Acta Phys. Chim. Sin. 2009, 25, 6–12. [Google Scholar]
- Li, K.; Chen, B.; Song, Y.; Yang, M. Molecular dynamics simulation of the effects of different thermodynamic parameters on methane hydrate dissociation: An analysis of temperature, pressure and gas concentrations. Fluid Phase Equilibria 2020, 516, 112606. [Google Scholar] [CrossRef]
- Gan, Y.; Cheng, Q.; Wang, Z.; Yang, J.; Sun, W.; Liu, Y. Molecular dynamics simulation of the microscopic mechanisms of the dissolution, diffusion and aggregation processes for waxy crystals in crude oil mixtures. J. Pet. Sci. Eng. 2019, 179, 56–69. [Google Scholar] [CrossRef]
- Chen, X.; Hou, L.; Wei, X.; Bedrov, D. Transport properties of waxy crude oil: A molecular dynamics simulation study. ACS Omega 2020, 5, 18557–18564. [Google Scholar] [CrossRef]
- Cao, J.; Liu, L.; Liu, C.; He, C. Phase transition mechanisms of paraffin in waxy crude oil in the absence and presence of pour point depressant. J. Mol. Liq. 2021, 345, 116989. [Google Scholar] [CrossRef]
- San-Miguel, M.A.; Rodger, P.M. Simulation of deposition of wax to iron oxide surfaces. Mol. Simul. 2001, 26, 193–216. [Google Scholar] [CrossRef]
- Gan, Y.; Cheng, Q.; Chu, S.; Wang, Z.; Luan, G.; Sun, W.; Liu, C.; Li, Q.; Liu, Y. Molecular dynamics simulation of waxy crude oil multiphase system depositing and sticking on pipeline inner walls and the micro influence mechanism of surface physical–chemical characteristics. Energy Fuels 2021, 35, 4012–4028. [Google Scholar] [CrossRef]
- Li, Q.; Deng, X.; Liu, Y.; Cheng, Q.; Liu, C. Gelation of waxy crude oil system with Ethylene-Vinyl Acetate on solid surface: A molecular dynamics study. J. Mol. Liq. 2021, 331, 115816. [Google Scholar] [CrossRef]









| Researcher | Mechanism | Mathematical Model | Establishment and Characteristics of the Model |
|---|---|---|---|
| Burger [47] | MD, BD, SD | A predictive model considering molecular diffusion, shear dispersion, and Brownian diffusion was developed to determine the wax deposition behavior in wellbores. It was demonstrated that at high temperature, molecular diffusion was dominant, while at low temperature, shear dispersion was dominant and the contribution of Brownian diffusion was smaller. | |
| Hamouda [48] | MD, SD, ID | The wax deposition model considering molecular diffusion and shear stripping was established, and the wax deposition tendency coefficient was also introduced, which proved that the deposition rate was the largest in the stable range of wax precipitation temperature and ambient temperature. | |
| Hsu [49] | MD, SD | A semi-empirical model considering molecular diffusion and shear effects was proposed to predict the wax deposition process and distribution. It is proved that the turbulence effect had a significant impact on wax deposition. The concept of critical wax strength was proposed and it was suggested that it can be used as a reasonable scalar. | |
| Ramirez-Jaramillo [50] | MD, SD, ID | Based on the Svendsen model, the author believed that molecular diffusion, shear dispersion, and aging would affect the wax deposition process, but he noted that the effect of shear dispersion was small and could be ignored in the modeling process. The prediction results show that there were a lot of heavy components in the sedimentary layer, which was related to the prediction time and inlet distance. | |
| Singh [51,52,53] | MD, SS | A model applicable to predict the rate and amount of wax deposition under laminar flow conditions was developed based on the law of mass conservation. The results show that the driving force required for wax deposition was the main factor contributing to the temperature difference between deposited layers, which led to aging. |
| Researcher | Mechanism | Mathematical Model | Establishment and Characteristics of the Model |
|---|---|---|---|
| Elphingstone [61] | MD, SD | Based on single-phase flow wax deposition, a model for predicting gas-liquid two-phase flow wax deposition was developed by considering the effects of diffusion and shear, which may be conservative in predicting wax deposition amount, although field data were not sufficient to draw reliable conclusions. | |
| Apte [62] | SS | The effects of wax deposition layer condensate content, shear stripping, and flow pattern on the wax deposition rate were considered, and a two-phase flow wax deposition model was established. The applicability and accuracy of the model for the high wax content system need further validation. | |
| Gong [63] | MD | Based on Fick’s law, the wax deposition model of gas-liquid two-phase flow was proposed considering the superficial velocity of gas and liquid phases, flow pattern, and Reynolds number. The experimental verification was carried out with high waxy crude oil and air as the medium, and the conclusion that the thickness of wax deposition layer under stratified flow and intermittent flow varied with the change of velocity of gas and liquid phases was obtained. | |
| Duan [64] | MD, SD | A mathematical model has been developed to predict the deposit thickness and the wax fraction of deposit in oil/gas stratified pipe flow using a unidirectional flow analysis of non-isothermal hydrodynamics and heat/mass transfer. Based on diffusivity and the solubility gradient at the oil–deposit interface at a different time, the reason that the deposit forming a crescent shape at the cross-section of pipe observed in different experiments was revealed. | |
| Duan [65] | MD, SD, IG | A wax deposition model of oil-gas two-phase stratified pipe flow based on molecular diffusion mechanism is developed. In the model, unidirectional flow analyses of momentum, heat, and mass transfer are presented. The cause of forming a crescent shape at the cross-section of the pipe was given, which was observed in different experiments. |
| Characterization Category | Name | Working Principle | Advantages | Disadvantages |
|---|---|---|---|---|
| Wax precipitation behavior | Microscopic observation method | Different media have different degrees of light absorption. |
| The effect of stress is not considered. |
| Viscometry method | When the wax is precipitated, the viscosity of the oil sample will suddenly change. |
| Wax precipitation temperature can only be measured under normal pressure. | |
| Differential scanning calorimetry method | The precipitation of wax crystals is determined by the change in heat flow rate of the oil sample and the reference material. |
|
| |
| Laser Scattering method | The laser will show different energy attenuation after passing through different media. |
|
| |
| Ultrasonic method | Ultrasonic waves propagate at different speeds in different media. |
|
| |
| Wax deposition behavior | Cold plate method | Using temperature difference of oil-plate. |
|
|
| Cold finger method | Using temperature difference of oil-wall. |
|
| |
| Rotating disk method | Using temperature difference of oil-plate. | The shear rate was used as a sensitive factor. | Does not reflect actual flow. | |
| Flow Loop method | Controlling the oil-wall temperature difference by using the cooling medium in the air of the loop |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, X.; Huang, Z.; Wang, Z.; Liu, Y. Characterization of Wax Precipitation and Deposition Behavior of Condensate Oil in Wellbore: A Comprehensive Review of Modeling, Experiment, and Molecular Dynamics Simulation. Energies 2022, 15, 4018. https://doi.org/10.3390/en15114018
Wang Y, Liu X, Huang Z, Wang Z, Liu Y. Characterization of Wax Precipitation and Deposition Behavior of Condensate Oil in Wellbore: A Comprehensive Review of Modeling, Experiment, and Molecular Dynamics Simulation. Energies. 2022; 15(11):4018. https://doi.org/10.3390/en15114018
Chicago/Turabian StyleWang, Yong, Xiaoyu Liu, Zuonan Huang, Zhihua Wang, and Yang Liu. 2022. "Characterization of Wax Precipitation and Deposition Behavior of Condensate Oil in Wellbore: A Comprehensive Review of Modeling, Experiment, and Molecular Dynamics Simulation" Energies 15, no. 11: 4018. https://doi.org/10.3390/en15114018
APA StyleWang, Y., Liu, X., Huang, Z., Wang, Z., & Liu, Y. (2022). Characterization of Wax Precipitation and Deposition Behavior of Condensate Oil in Wellbore: A Comprehensive Review of Modeling, Experiment, and Molecular Dynamics Simulation. Energies, 15(11), 4018. https://doi.org/10.3390/en15114018






