Experimental Investigation of Gaseous Sodium Release in Slag-Tapping Coal-Fired Furnaces by Spontaneous Emission Spectroscopy
Abstract
:1. Introduction
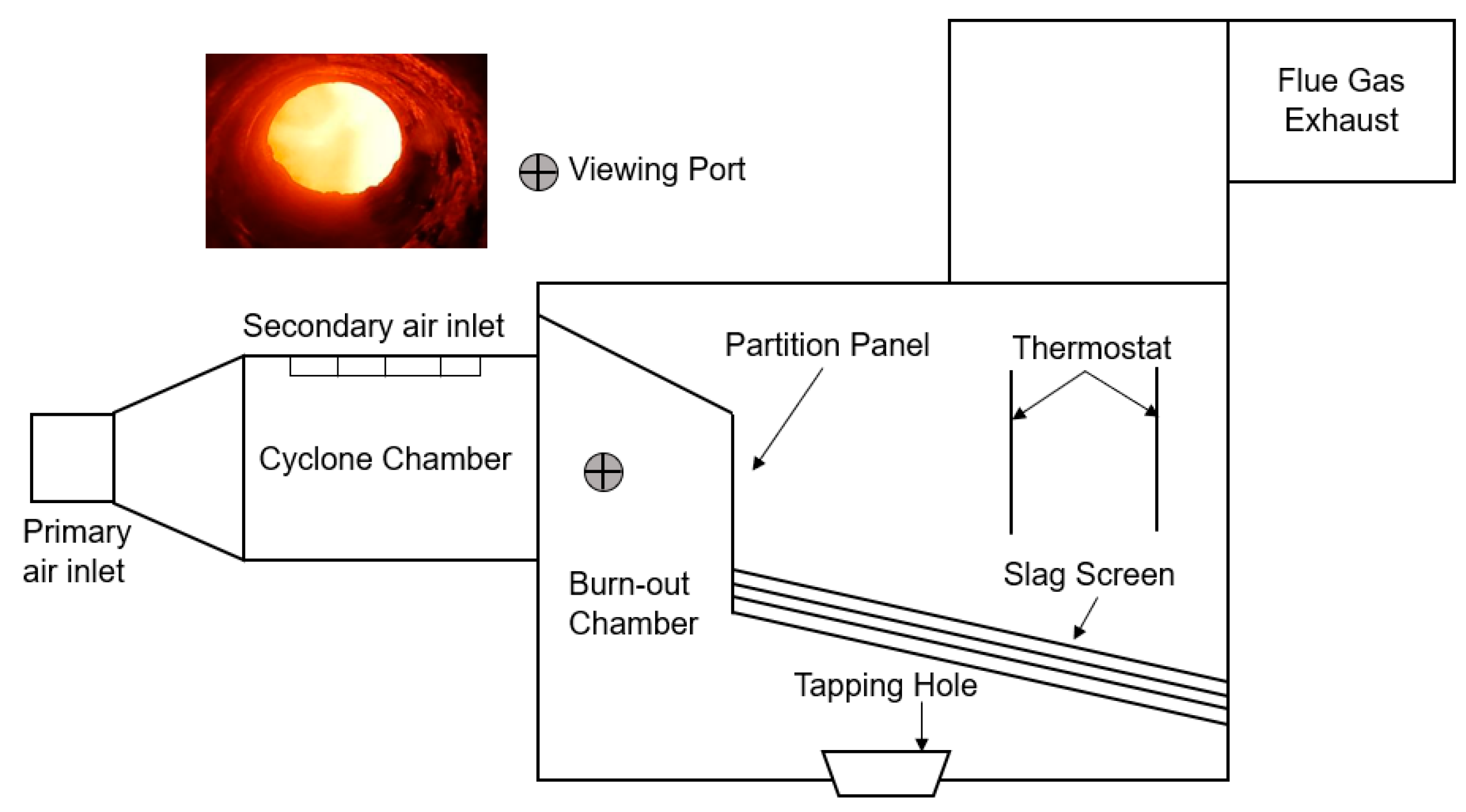
2. Method and Experimental Setup
3. Experiment in Slag-Tapping Combustor
3.1. Experimental Conditions
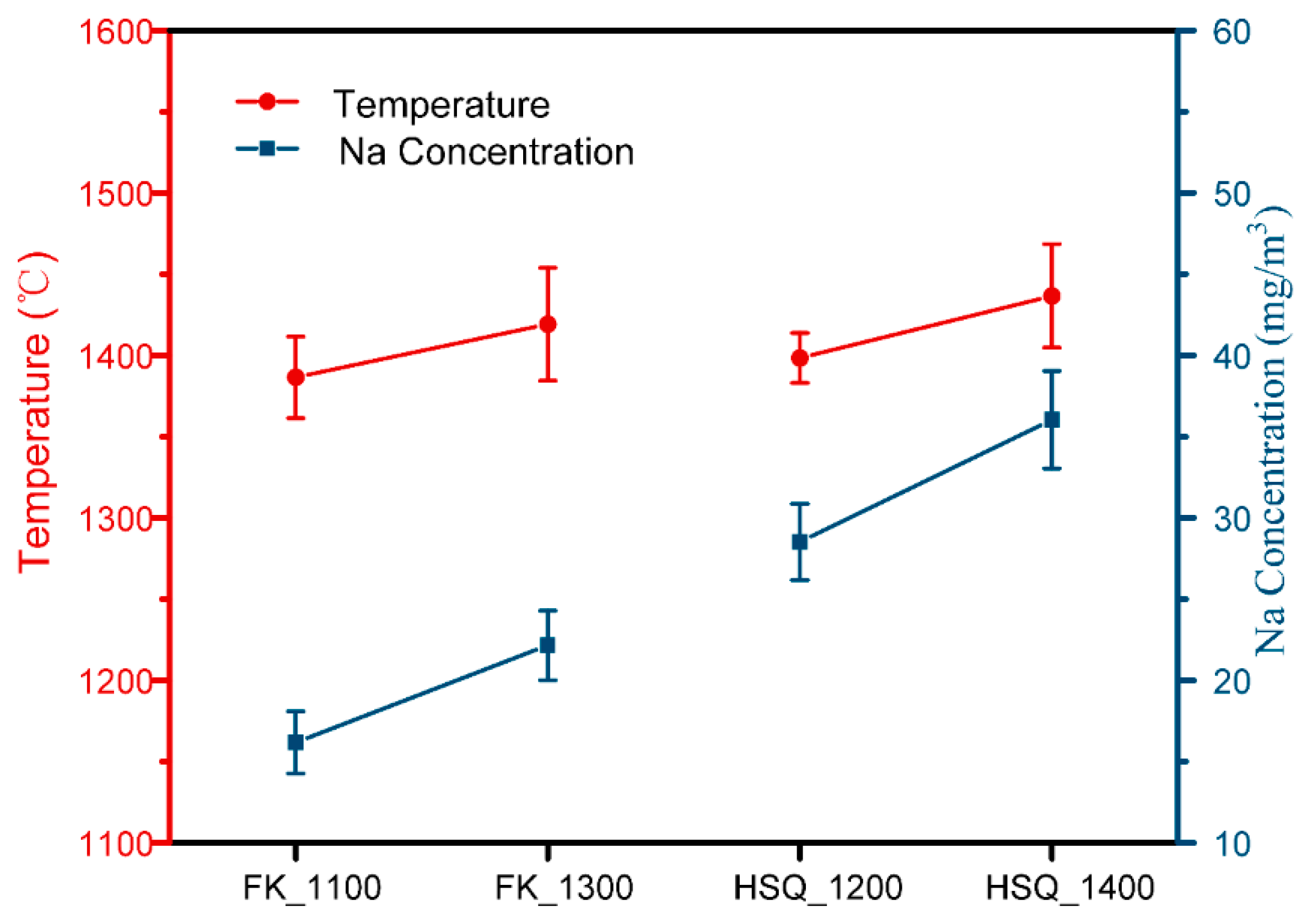
3.2. Results and Analysis
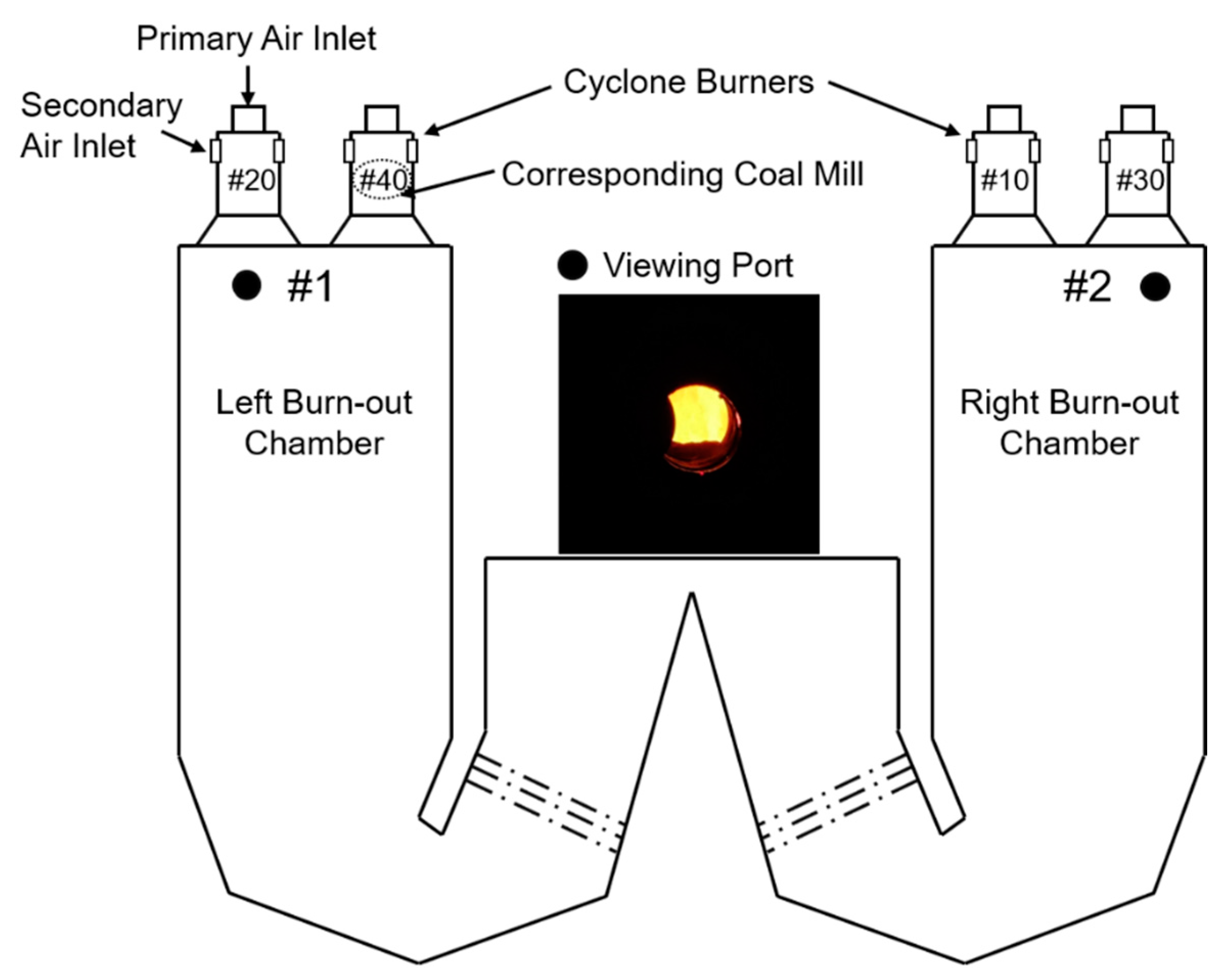
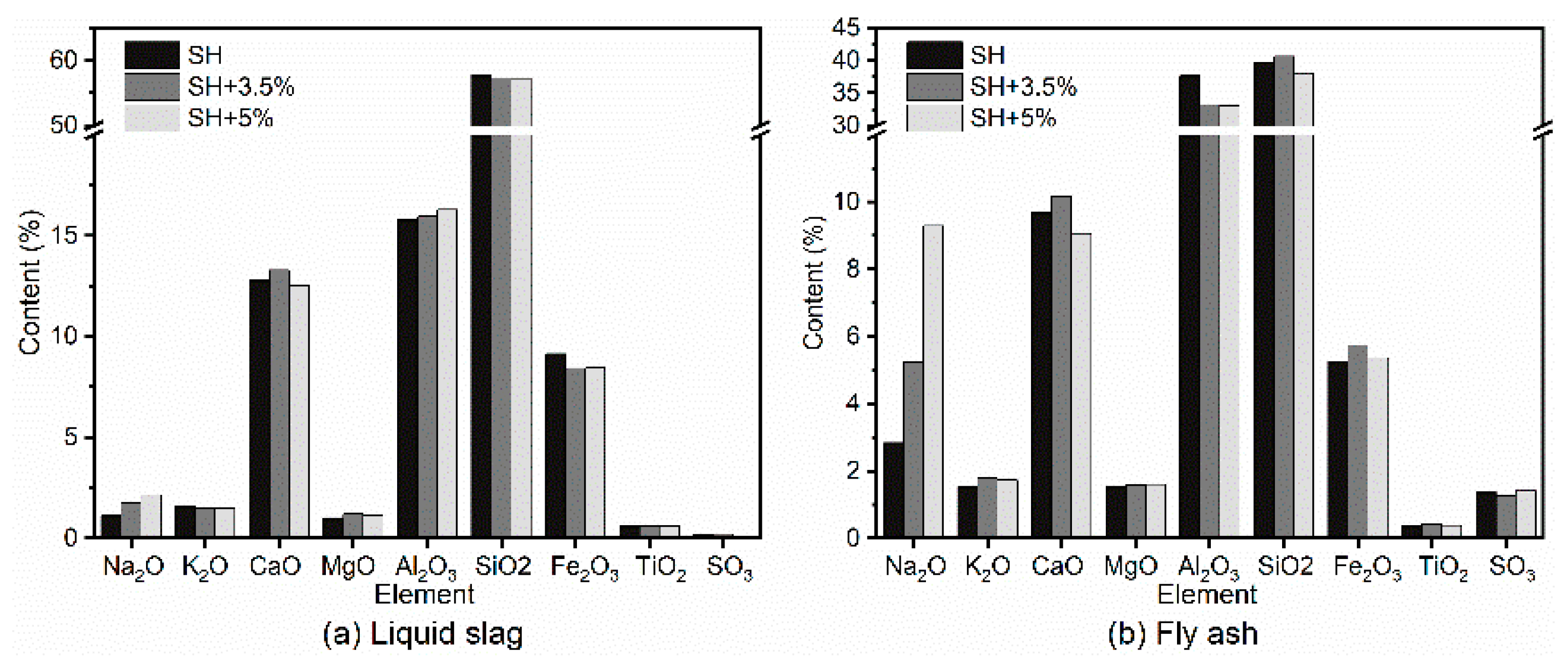
4. Experiment in Slagging Boiler Furnace
4.1. Experimental Conditions
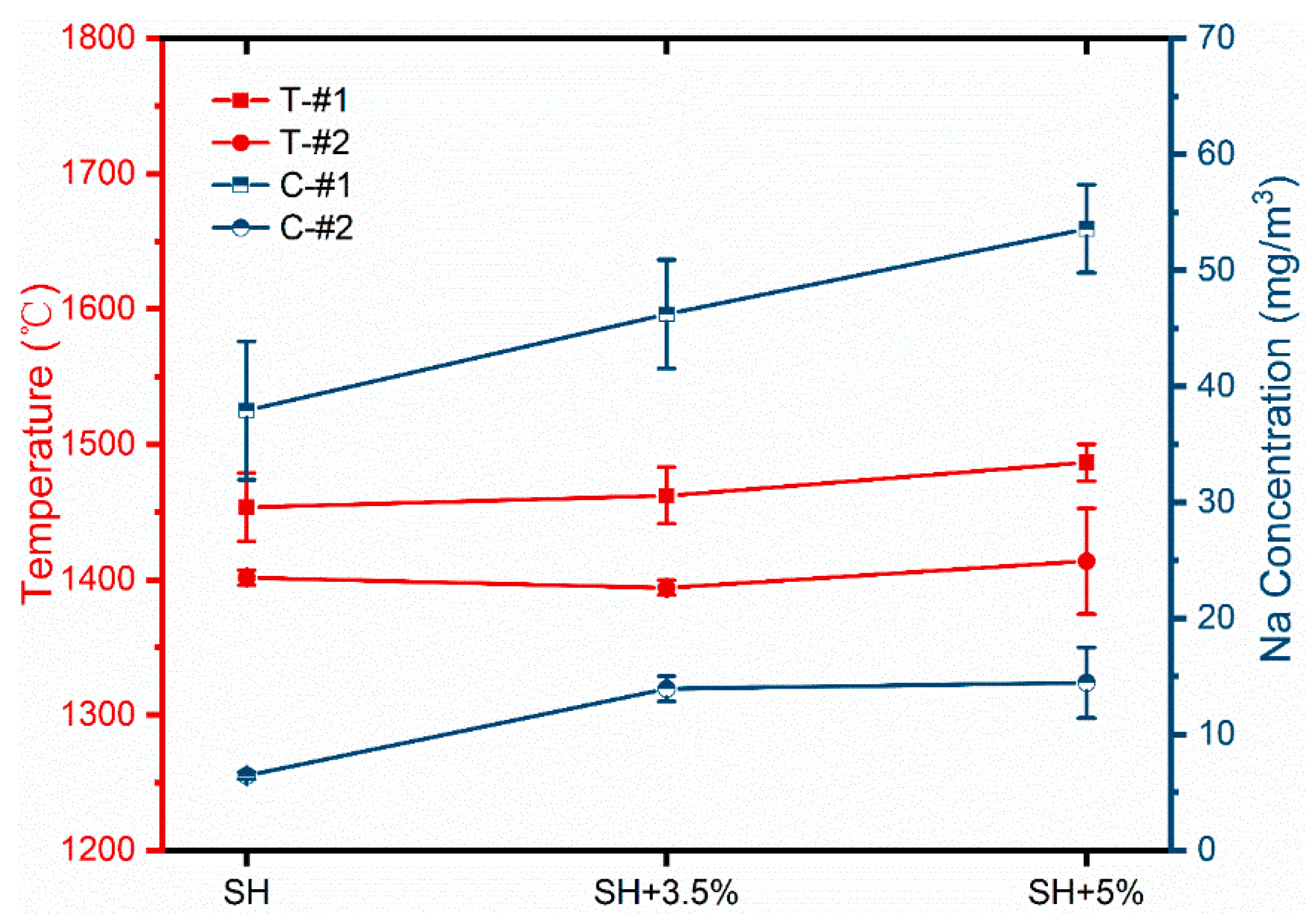
4.2. Results and Analysis
5. Conclusions
- (1)
- The gas-phase Na concentration released from combustion different kinds of coal, and has a strong correlation with the combustion temperature and the content of organic Na in the coal. Higher concentrations of gaseous sodium were still present in the cyclone burner outlet area. The gaseous phase Na concentration can reflect the change of load. Under a higher unit load, the flame temperature and gaseous phase Na concentration increase significantly at the outlet area cyclone burner.
- (2)
- The Na content in liquid slag is lower than that in fly ash. The gas phase Na released from the coal is more condensed in the low temperature region of the flue gas and migrates into the fly ash. Si and Fe are more enriched in liquid slag, while S is almost in fly ash;
- (3)
- Through the measurement of flame temperature and gas Na concentration at the outlet of the cyclone of the liquid slag discharge furnace by FES approach, combined with the analysis of fly ash and liquid slag samples, a closed-loop analysis of the Na migration path can be established.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Li, J.; Wu, G.G.; Bai, Z.Q.; Li, W. Clean and efficient utilization of sodium-rich Zhundong coals in China: Behaviors of sodium species during thermal conversion processes. Fuel 2018, 218, 162–173. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Tan, H.Z.; Hui, S.E. Ash-related issues during biomass combustion: Alkali induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Wen, C.S.; Cowell, L.H.; Smit, F.J.; Boyd, J.D.; LeCren, R.T. Coal alkali retention in a slagging combustor. Fuel 1992, 72, 219–224. [Google Scholar] [CrossRef]
- Ji, H.S.; Wu, X.J.; Dai, B.Q.; Zhang, L. Xinjiang lignite ash slagging and flow under the weak reducing environment at 1300 °C—Release of sodium out of slag and its modelling from the mass transfer perspective. Fuel Process. Technol. 2018, 170, 32–43. [Google Scholar] [CrossRef]
- Hu, X.L.; Wu, X.J.; Zhang, Z.X.; Fan, H.J.; Fan, J.J.; Chen, S.L.; Lan, D.H. Sodium retention behavior of Xinjiang high-alkali coal in a 20 kW slag-tapping combustor test. Fuel 2022, 317, 123298. [Google Scholar] [CrossRef]
- Hu, S.H.; Ni, Y.G.; Qi, Y.; Wang, J.K.; Lv, L.Q.; Cen, K.F.; Zhou, H. Research on element migration and ash deposition characteristics of high-alkali coal in horizontal liquid slagging cyclone furnace. Fuel 2022, 308, 121962. [Google Scholar] [CrossRef]
- Hesameddin, F.; Weng, W.B.; Li, Z.S.; Bai, X.S.; Marcus, A. Recent Development in Numerical Simulations and Experimental Studies of Biomass Thermochemical Conversion. Energy Fuels 2021, 35, 6940–6963. [Google Scholar]
- Lou, C.; Zang, L.D.; Pu, Y.; Zhang, Z.N.; Li, Z.C.; Chen, P.F. Research advances in passive techniques for combustion diagnostics based on analysis of spontaneous emission radiation. J. Exp. Fluid Mech. 2021, 35, 1–17. [Google Scholar]
- Jones, J.M.; Darvell, L.I.; Bridgeman, T.G.; Pourkashanian, M.; Williams, A. An investigation of the thermal and catalytic behaviour of potassium in biomass combustion. Proc. Combust. Inst. 2007, 31, 1955–1963. [Google Scholar] [CrossRef]
- Mason, P.E.; Darvell, L.I.; Jones, J.M.; Williams, A. Observations on the release of gas-phase potassium during the combustion of single particles of biomass. Fuel 2016, 182, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Mason, P.E.; Jones, J.M.; Darvell, L.I.; Williams, A. Gas phase potassium release from a single particle of biomass during high temperature combustion. Proc. Combust. Inst. 2017, 36, 2207–2215. [Google Scholar] [CrossRef] [Green Version]
- Mooktzeng, L.; Ahmad, Z.S.Z.; Hamdan, H. Biomass Combustion: Potassium and Sodium Flame Emission Spectra and Composition in Ash. J. Jpn. Inst. Energy 2017, 96, 367–371. [Google Scholar]
- Sun, Y.P.; Lou, C.; Zhou, H.C. A simple judgment method of gray property of flames based on spectral analysis and the two-color method for measurements of temperatures and emissivity. Proc. Combust. Inst. 2011, 33, 735–741. [Google Scholar] [CrossRef]
- Parameswaran, T.; Hughes, R.; Gogolek, P.; Hughes, P. Gasification temperature measurement with flame emission spectroscopy. Fuel 2014, 134, 579–587. [Google Scholar] [CrossRef]
- He, Z.L.; Lou, C.; Fu, J.T.; Lim, M. Experimental investigation on temporal release of potassium from biomass pellet combustion by flame emission spectroscopy. Fuel 2019, 253, 1378–1384. [Google Scholar] [CrossRef]
- He, X.H.; Lou, C.; Qiao, Y.; Lim, M. In-situ measurement of temperature and alkali metal concentration in municipal solid waste incinerators using flame emission spectroscopy. Waste Manag. 2020, 102, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Pu, Y.; Zhao, Y.G.; Bai, Y.; Yao, B.; Yu, D.X. An in-situ method for time-resolved sodium release behaviour during coal combustion and its application in industrial coal-fired boilers. Proc. Combust. Inst. 2021, 38, 4199–4206. [Google Scholar] [CrossRef]
- Li, K.Y.; Yan, W.J.; Huang, X.L.; Yu, L.B.; Chen, Y.M.; Lou, C. In-situ measurement of temperature and potassium concentration during the combustion of biomass pellets based on the emission spectrum. Fuel 2021, 289, 119863. [Google Scholar] [CrossRef]
- Li, K.Y.; Yan, W.J.; Yu, L.B.; Huang, X.L.; Chen, Y.M.; Zhou, H.C.; Zheng, S.; Lou, C. Simultaneous Determination of Na Concentration and Temperature during Zhundong Coal Combustion using the Radiation Spectrum. Energy Fuels 2021, 35, 3348–3359. [Google Scholar] [CrossRef]
- Paulauskas, R.; Striūgas, N.; Sadeckas, M.; Sommersacher, P.; Retschitzegger, S.; Kienzl, N. Online determination of potassium and sodiumrelease behaviour during single particle biomass combustion by FES and ICP-MS. Sci. Total Environ. 2020, 746, 141162. [Google Scholar] [CrossRef]
- Li, X.L.; Han, C.X.; Lu, G.; Yan, Y. Online dynamic prediction of potassium concentration in biomass fuels through flame spectroscopic analysis and recurrent neural network modelling. Fuel 2021, 304, 121376. [Google Scholar] [CrossRef]
- Arias, L.; Torres, S.; Ngendakumana, D.S.P. On the spectral bands measurements for combustion monitoring. Combust. Flame 2011, 158, 423–433. [Google Scholar] [CrossRef]
- Gaydon, A.G. The Spectroscopy of Flames, 2nd ed.; Chapman and Hall: London, UK, 1974. [Google Scholar]
- Ebdon, L.; Evans, E.H.; Fisher, A.; Hill, S.J. An Introduction to Analytical Atomic Spectrometry; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Li, J.B.; Zhu, M.M.; Zhang, Z.Z.; Zhang, K.; Shen, G.Q.; Zhang, D.K. Characterisation of ash deposits on a probe at different temperatures during combustion of a Zhundong lignite in a drop tube furnace. Fuel Process. Technol. 2016, 144, 155–163. [Google Scholar] [CrossRef]



| Coal | Proximate Analyses (Air Dried Basis, wt%) | Ultimate Analyses (Air Dried Basis, wt%) | |||||||
| Moisture | Volatility | Ash | Fixed Carbon | C | H | O | N | S | |
| ZD-FK | 16.72 | 23.77 | 5.59 | 53.92 | 74.42 | 1.96 | 15.69 | 0.62 | 0.49 |
| ZD-HSQ | 14.63 | 32.82 | 7.52 | 45.03 | 61.52 | 3.59 | 11.48 | 0.84 | 0.42 |
| Ash Composition (wt%) | |||||||||
| Coal | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | TiO2 |
| ZD-FK | 10.37 | 5.27 | 12.36 | 35.59 | 10.01 | 0.39 | 1.90 | 19.9 | 0.72 |
| ZD-HSQ | 31.52 | 13.32 | 18.68 | 16.63 | 6.19 | 0.69 | 3.18 | 7.66 | 0.68 |
| Case | 1.1 | 1.2 | 1.3 | 1.4 |
|---|---|---|---|---|
| Coal | ZD-FK | ZD-FK | ZD-HSQ | ZD-HSQ |
| Combustion temperature | 1100 °C | 1300 °C | 1200 °C | 1400 °C |
| Coal | Proximate Analyses (Air Dried Basis, wt%) | Ultimate Analyses (Air Dried Basis, wt%) | |||||||
| Moisture | Volatility | Ash | Fixed Carbon | C | H | O | N | S | |
| SH | 2.21 | 29.86 | 13.42 | 54.51 | 68.62 | 4.19 | 10.29 | 0.83 | 0.44 |
| Ash Composition (wt%) | |||||||||
| Coal | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | TiO2 |
| SH | 57.67 | 21.87 | 5.17 | 5.76 | 1.46 | 2.23 | 2.00 | 0.16 | 0.87 |
| Case | Coal Mill On | Load (MW) |
|---|---|---|
| SH | #20, #40, #10 | 240 |
| SH+3.5%Na2CO3 | #20, #40, #10 | 251 |
| SH+5%Na2CO3 | #20, #40, #10 | 224 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, X.; Pu, Y.; Li, Z.; Tang, Q.; Yao, B.; Fu, P.; Lou, C.; Lim, M. Experimental Investigation of Gaseous Sodium Release in Slag-Tapping Coal-Fired Furnaces by Spontaneous Emission Spectroscopy. Energies 2022, 15, 4165. https://doi.org/10.3390/en15114165
Jing X, Pu Y, Li Z, Tang Q, Yao B, Fu P, Lou C, Lim M. Experimental Investigation of Gaseous Sodium Release in Slag-Tapping Coal-Fired Furnaces by Spontaneous Emission Spectroscopy. Energies. 2022; 15(11):4165. https://doi.org/10.3390/en15114165
Chicago/Turabian StyleJing, Xuehui, Yang Pu, Zhaoyu Li, Quanli Tang, Bin Yao, Peifang Fu, Chun Lou, and Mooktzeng Lim. 2022. "Experimental Investigation of Gaseous Sodium Release in Slag-Tapping Coal-Fired Furnaces by Spontaneous Emission Spectroscopy" Energies 15, no. 11: 4165. https://doi.org/10.3390/en15114165






