An Overview on Co-Pyrolysis of Biodegradable and Non-Biodegradable Wastes
Abstract
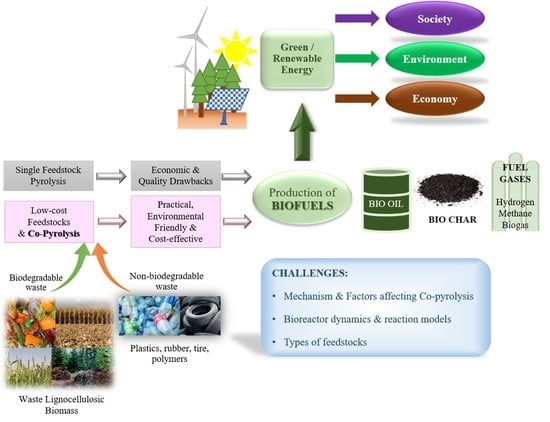
:1. Introduction
2. Co-Pyrolysis: An Alternative Technique to Upgrade Pyrolysis Oil
3. Mechanism of Co-Pyrolysis
3.1. Selection of Feedstocks for Co-Pyrolysis
| Biodegradable Wastes | ||||||
|---|---|---|---|---|---|---|
| Type of Biomass | Raw Material | Lignin | Hemicellulose | Cellulose | References | |
| Agriculture Residue | Almond Shell | 27.7–35% | 28.0–38.0% | 29.0–31.1% | [40] | |
| Banana Waste | 24.28% | 10.50% | 40.15% | [41] | ||
| Barley Straw | 13.8% | 21.9% | 33.8% | [42] | ||
| Corn Stalk | 15.59% | 43.01% | 22.82% | [40] | ||
| Corn Stover | 13 (mf * wt.%) | 43 (mf * wt.%) | 31 (mf * wt.%) | [43] | ||
| Cotton Stalk | 20.88% | 32% | 38% | [44] | ||
| Date Palm Waste | 1–25% | 19–33% | 22–40% | [45] | ||
| Flax Straw | 28.90% | 34.40% | 36.70% | [40] | ||
| Groundnut Husk | 28% | 46% | 34% | [46] | ||
| Jatropha De-oiled Cake | 24.9% | 16.6% | 53.5% | [47] | ||
| Millet Husk | 14% | 26.9% | 33.3% | [40] | ||
| Oat Straw | 12.9% | 23.3% | 37.6% | [42] | ||
| Palm Oil Empty Fruit Bunches | 12.09% DB a | 12.78% DB | 25.16% DB | [48] | ||
| Rice Husk | 14.3% | 24.3% | 31.3% | [40] | ||
| Rice Straw | 14.23% DB | 22.20% DB | 38.30% DB | [49] | ||
| Sugarcane Bagasse | 9.6% | 28.2% | 30.9% | [50] | ||
| Wheat Straw | 15–16.4% | 27.3–50% | 27.3–30% | [40] | ||
| Biodegradable Industry Waste | Fruit Industry Waste | Apple Pomace | 24.72% | 27.77% | 47.49% | [51] |
| Pineapple Peels | 10.06% | Not Mentioned | 21.66% | [52] | ||
| Orange Peels | 12.04% | 30.63 | 33.26 | [53] | ||
| Mango Endocarp | 25.9% | 21.4% | 50.13 | |||
| Apricot Kernel Shell | 47.97% | 17.01% | 29.57% | |||
| Date Pits | 16.68% | 18.67% | 45.88% | |||
| Coffee Industry waste | Spent Coffee Grounds | 25% | 42% | 13% | [54,55] | |
| Coffee Pulp | - | 21–32% of carbohydrates | ||||
| Coffee Cherry Husk | - | 58–85% of carbohydrates | ||||
| Textile Waste | Hemp, Flax, Jute, and Abaca | Small Amount | 12-20% | 60% | [56] | |
| Coir | 41–45% | 12–20% | 36–43% | |||
| Paper Biomass | 3.3% | 15.2% | 76.5% | [57] | ||
| Forest Residue | Hardwood | 20–25% | 20–25% | 45–50% | [58] | |
| Pine Wood | 25.3% | 10.5% | 48.6% | [59] | ||
| Softwood | 25–35% | 25–30% | 40–50% | [43] | ||
| Other Wastes | MSW | 16% | 4% | 38% | [60] | |
| Sewage Sludge | 5.86% | 2.37% | 14.65% | [61] | ||
| Manure | 6–16% | 18–27% | 4-23% | [62] | ||
| Energy Crops | Switchgrass | 18–19% | 24–29% | 37–43% | [58] | |
| Non-Lignocellulosic Biomass | Raw Material | Protein | Lipid | Carbohydrates | Reference | |
| Algae (Chlorella vulgaris) | 41.51% | 15.67% | 20.99% | [63] | ||
| Hair Waste | 65–95% | 1–9% | - | [63,64] | ||
| Feather Waste | 82% | 0.8% | 2% | [65] | ||
| Meat Waste | Cattle | 1% | 74.75% | 1.45% | ||
| Pig | 13.13% | 33.25% | 13.86% | |||
| Non-Biodegradable Wastes | ||||||
| Synthetic Polymers | Raw Material | Carbon | Hydrogen | Sulfur | Reference | |
| LDPE | 86.35% | 13.58% | 0.074 | [16] | ||
| HDPE | 84.89% | 14.19% | 0.54% | [66] | ||
| PET | 57.9% | 4.13% | 0.01% | [17] | ||
| Waste Tires | 87.9% | 7.4% | 1.1% | [67] | ||
3.1.1. Biodegradable Wastes
Lignocellulose Wastes
| Raw Material | Properties of Bio-Oil Produced by Pyrolysis | Reference |
|---|---|---|
| Acacia nilotica (Babool) Seeds | Rich in hydrocarbons, alcohols, phenols but further upgradation to remove oxygen is required | [81] |
| Cedrus deodara | Rich in acids, alcohols, aromatic ethers, carbonyl compounds, hydrocarbons, phenols, but further refining is required | [82] |
| Corn Cob | Acids, furans, lignin-derived phenols, nonaromatic aldehydes, non-aromatic ketones, sugars, but further upgradation for removing moisture is required | [40] |
| Cotton Residue | Bio-oil contains phenolic compounds but is highly oxygenated | [83] |
| HDPE | Rich in aliphatic hydrocarbon (C8 to C12); lower proportion of aromatic hydrocarbons | [84] |
| LDPE | Rich in aliphatic and simple aromatic hydrocarbons | [85] |
| Mixed Plastic | Heating Value 44.40 MJ/kg | [39] |
| Oil Palm Empty Fruit Bunches | Rich in phenol, furan, ketone, alcohol, acids, pyrans | [40] |
| Palm Fronds | Rich in acids, phenols, ketones, aldehydes, alcohols, but this conversion is still not optimal | [86] |
| PE | High aromatic content having other hydrocarbon compounds and some aliphatic content; higher heating values than conventional diesel | [87] |
| PET | Rich in aromatic hydroxyl groups; lack oxygen, carboxyl, and aliphatic hydroxyl groups | [88] |
| Pinewood | Rich in acids, phenols; however, further optimization and upgradation is required. | [89] |
| Poplar | Bio-oil collected under two fractions: | [90] |
| ||
| PP | Rich in the naphtha range hydrocarbons. | [91] |
| PS | Heating value 43.0 MJ/kg; flash point: 26.1 °C | [39] |
| PVC | Heating value 43.22 MJ/kg; flash point: 40 °C | [39] |
| Rice Husk | Rich in acids, aromatic, heterocyclic compounds, but to use as vehicle fuel it requires some refining | [40] |
| Spruce | Rich in non-aromatic aldehydes, sugars, non-aromatic ketones, guaiacols, acids, furans, pyrans | [40] |
| Sugarcane Bagasse | Bio-oil has significantly less oxygen and higher calorific value due to pressure applied during pyrolysis | [90] |
| Sweet Sorghum Bagasse | Properties of bio-oil were found to vary across the fractional condensers. | [90] |
| Waste Tires | Contains aromatic, aliphatic, polar and hetero-atom fractions. | [92] |
Non-Lignocellulosic Wastes
3.1.2. Non-Biodegradable Wastes
3.2. Sample Preparation/Pretreatment
3.2.1. Physical/Mechanical Pretreatment
3.2.2. Chemical Pretreatment
3.2.3. Physio–Chemical Pretreatment
3.2.4. Biological Pretreatment
4. Different Types of Co-Pyrolysis
4.1. Non-Catalytic Co-Pyrolysis
4.2. Catalytic Co-Pyrolysis
| Feedstocks | Reactor and Operating Conditions | Catalyst | Remarks or Liquid Yield (wt.%) | References |
|---|---|---|---|---|
| Rice husk + PE | Parr reactor, T 350–430 °C | - | 72 | [124] |
| Empty fruit bunch (efb) of palm + solid tire waste | EFB to tire waste ratio of 25:75 at a temperature of 500 °C; tube reactor | - | 44.3 | [115] |
| Pine cone + LDPE | Semi-batch glass reactor, temp. 500 °C | - | 63.9 | [93] |
| Frying oil + plastic waste | FO/PW ratio of 1:1; fast heating rate (up to 50 °C/min) and a lower reaction time (25 min) | - | 81 | [116] |
| Pine cone + PP | Semi-batch glass reactor, T 500 °C | - | 64.1 | [93] |
| Pine cone + PS | Semi-batch glass reactor, T 500 °C | - | 69.7 | [93] |
| Almond shell + HDPE | Fixed-bed reactor, T 500 °C | - | 50.88 | [125] |
| Cellulose + PS | Pyrex reactor, T 450 °C | - | 80.10 | [126] |
| Cellulose + PVC | Fixed-bed reactor, T 450 °C | - | 45 | [127] |
| Sugarcane bagasse + HDPE | Fixed-bed reactor, T 400–700 °C | - | 60.2 | [128] |
| Pine sawdust + LDPE | Fixed-bed reactor; 450 °C | HZSM-5 | Improvement in the yield and selectivity of aromatics in obtained bio-oil. | [16] |
| Sugarcane bagasse + HDPE | Fixed-bed reactor; 400–700 °C | Mesoporous FAU | Increase in liquid yield and enhancement in the quality of bio-oil. | [66] |
| Sugarcane Bagasse + PET | Tandem μ-reactor coupled with GC 400–800 °C | HZSM-5/ Na2CO3/ γ-Al2O3 | Increase in the production of aromatic compounds such as benzene, toluene, xylenes and ethylbenzene (BTXE). | [17] |
| Grape seeds + waste tire | Auger reactor (pilot scale) | CaO | Deoxygenated bio-oil with improved physical properties, such as viscosity and density. | [67] |
| Sugarcane bagasse + PS | Fixed-bed reactor 500 °C | HZSM-5, MgO, CaO | Improvement in aromatic compound yields in obtained bio-oil. Increase in calorific value and density upgradation comparable to standard diesel fuel quality | [129] |
| Camellia shell + take-out solid waste | Pyro-probe pyrolyzer coupled with GC 700 °C | HZSM-5, CaO, MgO | Generation of aliphatic hydrocarbons in obtained bio-oil; inhibited acid formation | [130] |
| Rice straw + ulvaprolifera macroalgae | Fixed-bed reactor 700 °C | NiFe-LDO/AC catalysts | Improved acid sites, and thereby, advanced deoxygenation and aromatization | [18] |
| Seaweeds + cellulose | Fixed-bed reactor 500 °C | ZSM-5/MCM-41 | Improved cracking, dehydration, decarbonylation, decarboxylation, dealkylation, aromatization, oligomerization, and deamination | [131] |
4.2.1. Catalysts Used in Co-Pyrolysis
4.2.2. Zeolites
4.2.3. Metal Oxides
4.2.4. Dual-Catalyst Catalytic Method
4.2.5. Biochar as Catalyst
4.3. Condensation Stage
5. Factors Affecting Co-Pyrolysis Process
5.1. Effect of Temperature
5.2. Particle Size
5.3. Blending Ratio
5.4. Pressure
5.5. Heating Rate
6. Co-Pyrolysis Products and Applications
6.1. Bio-Oil
6.2. Biochar
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.; Kumar, G.; Yang, Y.-H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total Environ. 2020, 765, 144429. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Sharma, S. A Renewable Source of Hydrocarbons and High Value Co-Products from Algal Biomass. In Liquid Biofuel Production; Singh, L.K., Chaudhary, G., Eds.; Wiley Scrivener Publishing House: Beverly, MA, USA, 2019; pp. 35–72. [Google Scholar]
- Mobil, E. Outlook for Energy: A Perspective to 2040; Exxon Mobil: Irving, TX, USA, 2019. [Google Scholar]
- Bhatt, A.K.; Bhatia, R.K.; Thakur, S.; Rana, N.; Sharma, V.; Rathour, R.K. Fuel from Waste: A Review on Scientific Solution for Waste Management and Environment Conservation. In Prospects of Alternative Transportation Fuels. Energy, Environment, and Sustainability; Singh, A., Agarwal, R., Agarwal, A., Dhar, A., Shukla, M., Eds.; Springer: Singapore, 2018; pp. 205–233. [Google Scholar] [CrossRef]
- Walia, A.; Sharma, A.; Sharma, S.; Mehta, P. Microalgae based biofuels—production, improvement, processing and extraction. In Biofuels; Kumar, A., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 137–187. [Google Scholar]
- Bhatia, S.K.; Joo, H.-S.; Yang, Y.-H. Biowaste-to-bioenergy using biological methods—A mini-review. Energy Convers. Manag. 2018, 177, 640–660. [Google Scholar] [CrossRef]
- Malmgren, A.; Riley, G. Biomass Power Generation. In Comprehensive Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 27–53. [Google Scholar]
- Joshi, V.K.; Walia, A.; Rana, N. Production of Bioethanol from Food Industry Waste: Microbiology, Biochemistry and Technology. In Biomass Conversion: The Interface of Biotechnology, Chemistry and Materials Science; Chinnappan, B., Shikha, B., Dhillon, R.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 251–311. [Google Scholar] [CrossRef]
- Walia, A.; Putatunda, C.; Solanki, P.; Pathania, S.; Bhatia, R.K. Co-Conversion of Algal Biomass to Biofuel. In Liquid Biofuels: Fundamentals 2021, Characterization, and Applications; Shadangi, K.P., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 391–439. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Kim, S.-H.; Yoon, J.-J.; Yang, Y.-H. Current status and strategies for second generation biofuel production using microbial systems. Energy Convers. Manag. 2017, 148, 1142–1156. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X. The thermochemical conversion of biomass into biofuels. In Biomass, Biopolymer-Based Materials, and Bioenergy; Verma, D., Fortunati, E., Jain, S., Zhang, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–368. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J. Thermochemical conversion of microalgal biomass. In Second and Third Generation of FeEdstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–382. [Google Scholar] [CrossRef]
- Guran, S. Sustainable Waste-to-Energy Technologies: Gasification and Pyrolysis. In Sustainable Food Waste-To-Energy Systems; Trabold, T.A., Babbitt, C.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 141–158. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Ryu, H.W.; Kim, D.H.; Jae, J.; Lam, S.S.; Park, E.D.; Park, Y.-K. Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour. Technol. 2020, 310, 123473. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Ghorbannezhad, P.; Park, S.; Onwudili, J.A. Co-pyrolysis of biomass and plastic waste over zeolite- and sodium-based catalysts for enhanced yields of hydrocarbon products. Waste Manag. 2019, 102, 909–918. [Google Scholar] [CrossRef]
- Hao, J.; Qi, B.; Li, D.; Zeng, F. Catalytic co-pyrolysis of rice straw and ulva prolifera macroalgae: Effects of process parameter on bio-oil up-gradation. Renew. Energy 2020, 164, 460–471. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.M.A.W. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R.B. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; Samer, M., Ed.; InTechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Aysu, T.; Durak, H.; Güner, S.; Bengü, A.; Esim, N. Bio-oil production via catalytic pyrolysis of Anchusa azurea: Effects of operating conditions on product yields and chromatographic characterization. Bioresour. Technol. 2016, 205, 7–14. [Google Scholar] [CrossRef]
- Luque, L.; Oudenhoven, S.; Westerhof, R.; Van Rossum, G.; Berruti, F.; Kersten, S.; Rehmann, L. Comparison of ethanol production from corn cobs and switchgrass following a pyrolysis-based biorefinery approach. Biotechnol. Biofuels 2016, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Islam, Z.U.; Zhisheng, Y.; Hassan, E.B.; Dongdong, C.; Hongxun, Z. Microbial conversion of pyrolytic products to biofuels: A novel and sustainable approach toward second-generation biofuels. J. Ind. Microbiol. Biotechnol. 2015, 42, 1557–1579. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.-T.; Show, P.-L. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Choi, Y.-K.; Kim, H.J.; Song, H.-S.; Lee, S.M.; Park, S.L.; Lee, H.S.; Koh, J.; et al. Application of macroalgal biomass derived biochar and bioelectrochemical system with Shewanella for the adsorptive removal and biodegradation of toxic azo dye. Chemosphere 2020, 264, 128539. [Google Scholar] [CrossRef]
- Sheikh, M.I.; Kim, C.-H.; Park, H.-H.; Nam, H.-G.; Lee, G.S.; Jo, H.S.; Lee, J.-Y.; Kim, J.W. A synergistic effect of pretreatment on cell wall structural changes in barley straw (Hordeum vulgare L.) for efficient bioethanol production. J. Sci. Food Agric. 2014, 95, 843–850. [Google Scholar] [CrossRef]
- Song, H.J.; Gurav, R.; Bhatia, S.K.; Bin Lee, E.; Kim, H.J.; Yang, Y.-H.; Kan, E.; Kim, H.H.; Lee, S.H.; Choi, Y.-K. Treatment of microcystin-LR cyanotoxin contaminated water using Kentucky bluegrass-derived biochar. J. Water Process. Eng. 2021, 41, 102054. [Google Scholar] [CrossRef]
- Verma, M.; Godbout, S.; Brar, S.K.; Solomatnikova, O.; Lemay, S.P.; Larouche, J.P. Biofuels Production from Biomass by Thermochemical Conversion Technologies. Int. J. Chem. Eng. 2012, 2012, 542426. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, H.; Peng, L.; Zhang, Q.; Yao, S. Recent developments in the catalytic conversion of cellulose. Biotechnol. Biotechnol. Equip. 2014, 28, 981–988. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhou, G.; Feng, Y.; Wang, Y.; Yu, G.; Deng, S.; Huang, J.; Wang, B. Enhancing the production of renewable petrochemicals by co-feeding of biomass with plastics in catalytic fast pyrolysis with ZSM-5 zeolites. Appl. Catal. A Gen. 2014, 481, 173–182. [Google Scholar] [CrossRef]
- Martínez, J.D.; Veses, A.; Mastral, A.M.; Murillo, R.; Navarro, M.V.; Puy, N.; Artigues, A.; Bartrolí, J.; García, T. Co-pyrolysis of biomass with waste tyres: Upgrading of liquid bio-fuel. Fuel Process. Technol. 2013, 119, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Uzoejinwa, B.B.; He, X.; Wang, S.; Abomohra, A.E.-F.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Batalha, N.; Mahmudul, H.M.; Perkins, G.; Konarova, M. A review on advanced catalytic co-pyrolysis of biomass and hydrogen-rich feedstock: Insights into synergistic effect, catalyst development and reaction mechanism. Bioresour. Technol. 2020, 310, 123457. [Google Scholar] [CrossRef]
- Salvilla, J.N.V.; Ofrasio, B.I.G.; Rollon, A.P.; Manegdeg, F.G.; Abarca, R.R.M.; de Luna, M.D.G. Synergistic co-pyrolysıs of polyolefin plastics with wood and agricultural wastes for biofuel production. Appl. Energy 2020, 279, 115668. [Google Scholar] [CrossRef]
- Johansson, A.-C.; Sandström, L.; Öhrman, O.G.; Jilvero, H. Co-pyrolysis of woody biomass and plastic waste in both analytical and pilot scale. J. Anal. Appl. Pyrolysis 2018, 134, 102–113. [Google Scholar] [CrossRef]
- Wang, C.; Bi, H.; Lin, Q.; Jiang, X.; Jiang, C. Co-pyrolysis of sewage sludge and rice husk by TG–FTIR–MS: Pyrolysis behavior, kinetics, and condensable/non-condensable gases characteristics. Renew. Energy 2020, 160, 1048–1066. [Google Scholar] [CrossRef]
- Mushtaq, F.; Mat, R.; Ani, F.N. A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew. Sustain. Energy Rev. 2014, 39, 555–574. [Google Scholar] [CrossRef]
- Dwivedi, P.; Mishra, P.; Mondal, M.K.; Srivastava, N. Non-biodegradable polymeric waste pyrolysis for energy recovery. Heliyon 2019, 5, e02198. [Google Scholar] [CrossRef] [Green Version]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Ozbay, N.; Yargiç, A.S.; Şahin, R.Z.Y.; Yaman, E. Valorization of banana peel waste via in-situ catalytic pyrolysis using Al-Modified SBA-15. Renew. Energy 2019, 140, 633–646. [Google Scholar] [CrossRef]
- Aqsha, A.; Tijani, M.M.; Mahinpey, N. Catalytic pyrolysis of straw biomasses (wheat, flax, oat and barley straw) and the comparison of their product yields. In Energy Production and Management in the 21st Century; Brebbia, C.A., Magaril, E.R., Khodorovsky, M.Y., Eds.; WIT Press: Ashurst, UK, 2014; pp. 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Chouhan, A.P.S. A Slow Pyrolysis of Cotton Stalk (Gossypium arboretum) Waste for Bio-Oil Production. J. Pharm. Chem. Biol. Sci. 2015, 3, 143–149. [Google Scholar]
- Bensidhom, G.; Trabelsi, A.B.H.; Alper, K.; Sghairoun, M.; Zaafouri, K.; Trabelsi, I. Pyrolysis of Date palm waste in a fixed-bed reactor: Characterization of pyrolytic products. Bioresour. Technol. 2018, 247, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ogunsuyi, H.; Adejumobi, I. Pyrolysis of Groundnut Peels and Shells for BioBased Products (Bio-Oil and Bio-Char). Int. J. Res. Innov. Appl. Sci. 2020, 5, 75–78. [Google Scholar]
- Sharma, R.; Sheth, P.N.; Gujrathi, A.M. Kinetic modeling and simulation: Pyrolysis of Jatropha residue de-oiled cake. Renew. Energy 2016, 86, 554–562. [Google Scholar] [CrossRef]
- Richana, N.; Winarti, C.; Hidayat, T.; Prastowo, B. Hydrolysis of Empty Fruit Bunches of Palm Oil (Elaeis Guineensis Jacq.) by Chemical, Physical, and Enzymatic Methods for Bioethanol Production. Int. J. Chem. Eng. Appl. 2015, 6, 422–426. [Google Scholar] [CrossRef] [Green Version]
- Ukaew, S.; Schoenborn, J.; Klemetsrud, B.; Shonnard, D.R. Effects of torrefaction temperature and acid pretreatment on the yield and quality of fast pyrolysis bio-oil from rice straw. J. Anal. Appl. Pyrolysis 2018, 129, 112–122. [Google Scholar] [CrossRef]
- Savou, V.; Grause, G.; Kumagai, S.; Saito, Y.; Kameda, T.; Yoshioka, T. Pyrolysis of sugarcane bagasse pretreated with sulfuric acid. J. Energy Inst. 2019, 92, 1149–1157. [Google Scholar] [CrossRef]
- Guerrero, M.R.B.; Paula, M.M.D.S.; Zaragoza, M.M.; Gutiérrez, J.S.; Velderrain, V.G.; Ortiz, A.L.; Collins-Martínez, V. Thermogravimetric study on the pyrolysis kinetics of apple pomace as waste biomass. Int. J. Hydrogen Energy 2014, 39, 16619–16627. [Google Scholar] [CrossRef]
- Sánchez, J.D.; Ramírez, G.E.; Barajas, M.J.; Vargas, J.D.S.; Caballero, G.E.R.; Meneses, M.J.B. Comparative kinetic study of the pyrolysis of mandarin and pineapple peel. J. Anal. Appl. Pyrolysis 2016, 118, 192–201. [Google Scholar] [CrossRef]
- AlNouss, A.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; McKay, G. Pyrolysis Study of Different Fruit Wastes Using an Aspen Plus Model. Front. Sustain. Food Syst. 2021, 5, 604001. [Google Scholar] [CrossRef]
- Blinová, L.; Sirotiak, M.; Bartošová, A.; Soldán, M. Review: Utilization of Waste From Coffee Production. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2017, 25, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.; Srivastava, R.; Singh, A. Catalytic co-pyrolysis of paper biomass and plastic mixtures (HDPE (high density polyethylene), PP (polypropylene) and PET (polyethylene terephthalate)) and product analysis. Energy 2016, 103, 513–521. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J. A comparative investigation into the formation behaviors of char, liquids and gases during pyrolysis of pinewood and lignocellulosic components. Bioresour. Technol. 2014, 170, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Dornau, A.; Robson, J.F.; Thomas, G.H.; McQueen-Mason, S.J. Robust microorganisms for biofuel and chemical production from municipal solid waste. Microb. Cell Factories 2020, 19, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Du, G.; Li, J.; Fang, Y.; Hou, L.; Chen, G.; Ma, D. Supercritical water pyrolysis of sewage sludge. Waste Manag. 2017, 59, 371–378. [Google Scholar] [CrossRef]
- Hollis, S.; Keener, H.; Smith, M. Manure Processing Technologies-Pyrolysis. In Technical, Environmental and Economic Assessment of Manure Processing Technologies; The Ohio State University: Ohio, OH, USA, 2011. [Google Scholar]
- Li, D.-C.; Jiang, H. The thermochemical conversion of non-lignocellulosic biomass to form biochar: A review on characterizations and mechanism elucidation. Bioresour. Technol. 2017, 246, 57–68. [Google Scholar] [CrossRef]
- Bhandari, T.R.; Lamsal, B.; Adhikari, R. Pyrolyzed human hair: A review on synthesis, characterization and applications. BIBECHANA 2021, 18, 231–239. [Google Scholar] [CrossRef]
- Li, Z.; Reimer, C.; Picard, M.; Mohanty, A.K.; Misra, M. Characterization of Chicken Feather Biocarbon for Use in Sustainable Biocomposites. Front. Mater. 2020, 7, 3. [Google Scholar] [CrossRef]
- Hassan, H.; Lim, J.; Hameed, B. Catalytic co-pyrolysis of sugarcane bagasse and waste high-density polyethylene over faujasite-type zeolite. Bioresour. Technol. 2019, 284, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Sanahuja-Parejo, O.; Veses, A.; López, J.M.; Murillo, R.; Callén, M.S.; García, T. Ca-based Catalysts for the Production of High-Quality Bio-Oils from the Catalytic Co-Pyrolysis of Grape Seeds and Waste Tyres. Catalysts 2019, 9, 992. [Google Scholar] [CrossRef] [Green Version]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Mamaeva, A.; Tahmasebi, A.; Tian, L.; Yu, J. Microwave-assisted catalytic pyrolysis of lignocellulosic biomass for production of phenolic-rich bio-oil. Bioresour. Technol. 2016, 211, 382–389. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Biomass Explained. 2021. Available online: https://www.eia.gov/energyexplained/biomass/ (accessed on 23 June 2021).
- Sakhuja, D.; Ghai, H.; Rathour, R.K.; Kumar, P.; Bhatt, A.K.; Bhatia, R.K. Cost-effective production of biocatalysts using inexpensive plant biomass: A review. 3 Biotech. 2021, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech. 2014, 5, 337–353. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Bajpai, P. Wood and Fiber Fundamentals. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 19–74. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Han, Y.H.; Park, Y.-L.; Park, J.Y.; Jung, H.-R.; Yang, S.-Y.; Song, H.-S.; Kim, S.-H.; et al. Bioconversion of barley straw lignin into biodiesel using Rhodococcus sp. YHY01. Bioresour. Technol. 2019, 289, 121704. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Hassan, H.; Lim, J.; Hameed, B. Recent progress on biomass co-pyrolysis conversion into high-quality bio-oil. Bioresour. Technol. 2016, 221, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Zabeti, M.; Baltrusaitis, J.; Seshan, K. Chemical routes to hydrocarbons from pyrolysis of lignocellulose using Cs promoted amorphous silica alumina catalyst. Catal. Today 2016, 269, 156–165. [Google Scholar] [CrossRef]
- Garg, R.; Anand, N.; Kumar, D. Pyrolysis of babool seeds (Acacia nilotica) in a fixed bed reactor and bio-oil characterization. Renew. Energy 2016, 96, 167–171. [Google Scholar] [CrossRef]
- Krishna, B.B.; Biswas, B.; Ohri, P.; Kumar, J.; Singh, R.; Bhaskar, T. Pyrolysis of Cedrus deodara saw mill shavings in hydrogen and nitrogen atmosphere for the production of bio-oil. Renew. Energy 2016, 98, 238–244. [Google Scholar] [CrossRef]
- Krishna, B.B.; Biswas, B.; Kumar, J.; Singh, R.; Bhaskar, T. Role of Reaction Temperature on Pyrolysis of Cotton Residue. Waste Biomass-Valorization 2015, 7, 71–78. [Google Scholar] [CrossRef]
- Al-Salem, S. Thermal pyrolysis of high density polyethylene (HDPE) in a novel fixed bed reactor system for the production of high value gasoline range hydrocarbons (HC). Process. Saf. Environ. Prot. 2019, 127, 171–179. [Google Scholar] [CrossRef]
- Zattini, G.; Leonardi, C.; Mazzocchetti, L.; Cavazzoni, M.; Montanari, I.; Tosi, C.; Benelli, T.; Giorgini, L. Pyrolysis of Low-Density Polyethylene. In Sustainable Design and Manufacturing 2017. SDM 2017. Smart Innovation, Systems and Technologies 68; Campana, G., Howlett, R., Setchi, R., Cimatti, B., Eds.; Springer: Cham, Switzerland, 2017; pp. 480–490. [Google Scholar] [CrossRef]
- Rinaldi, N.; Simanungkalit, S.P. Bio-oil production from palm fronds by fast pyrolysis process in fluidized bed reactor. In Proceedings of the International Symposium on Applied Chemistry (ISAC), Jakarta, Indonesia, 23–25 October 2017. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Ben, H.; Luo, Y.; Wang, R. Catalytic Fast Pyrolysis of Poly (Ethylene Terephthalate) (PET) with Zeolite and Nickel Chloride. Polymers 2020, 12, 705. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Shao, S.; Jiang, Y.; Vitidsant, T.; Reubroycharoen, P.; Xiao, R. Improving hydrocarbon yield by two-step pyrolysis of pinewood in a fluidized-bed reactor. Fuel Process. Technol. 2017, 159, 19–26. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis Study of Polypropylene and Polyethylene Into Premium Oil Products. Int. J. Green Energy 2014, 12, 663–671. [Google Scholar] [CrossRef]
- Williams, P. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lei, H.; Chen, S.; Wu, J. Catalytic co-pyrolysis of lignocellulosic biomass with polymers: A critical review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Mullen, C.A.; Dorado, C.; Boateng, A.A. Co-pyrolysis of biomass and polyethylene over HZSM-5: Effects of plastic addition on coke formation and catalyst deactivation. In Proceedings of the TCS2016—Symposium on Thermal and Catalytic Sciences for Biofuels and Biobased Products, Chapel Hill, NC, USA, 1–4 November 2016; p. 26. [Google Scholar]
- Fermoso, J.; Coronado, J.; Serrano, D.; Pizarro, P. Pyrolysis of microalgae for fuel production. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 259–281. [Google Scholar] [CrossRef]
- Azizi, K.; Moraveji, M.K.; Najafabadi, H.A. Characteristics and kinetics study of simultaneous pyrolysis of microalgae Chlorella vulgaris, wood and polypropylene through TGA. Bioresour. Technol. 2017, 243, 481–491. [Google Scholar] [CrossRef]
- Bhatia, R.; Sakhuja, D.; Mundhe, S.; Walia, A. Renewable Energy Products through Bioremediation of Wastewater. Sustainability 2020, 12, 7501. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef]
- Bordoloi, N.; Narzari, R.; Sut, D.; Saikia, R.; Chutia, R.S.; Kataki, R. Characterization of bio-oil and its sub-fractions from pyrolysis of Scenedesmus dimorphus. Renew. Energy 2016, 98, 245–253. [Google Scholar] [CrossRef]
- Sakhuja, D.; Ghai, H.; Bhatia, R.K.; Bhatt, A.K. Management of e-Waste: Technological Challenges and Opportunities. In Handbook of Solid Waste Management; Springer: Singapore, 2021; pp. 1–35. [Google Scholar] [CrossRef]
- Singh, M.; Salaudeen, S.A.; Gilroyed, B.H.; Al-Salem, S.M.; Dutta, A. A review on co-pyrolysis of biomass with plastics and tires: Recent progress, catalyst development, and scaling up potential. Biomass Convers. Biorefinery 2021, 1–25. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Z.; Lu, X.; Ma, X. Catalytic co-pyrolysis of microwave pretreated chili straw and polypropylene to produce hydrocarbons-rich bio-oil. Bioresour. Technol. 2020, 319, 124191. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Bai, X. Synergistic enhancement of product quality through fast co-pyrolysis of acid pretreated biomass and waste plastic. Energy Convers. Manag. 2018, 164, 629–638. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, D.; Ren, X.; Zhang, Q.; Cai, H.; Yi, W.; Lei, H. Catalytic co-pyrolysis of waste corn stover and high-density polyethylene for hydrocarbon production: The coupling effect of potassium and HZSM-5 zeolite. J. Anal. Appl. Pyrolysis 2020, 150, 104895. [Google Scholar] [CrossRef]
- Khan, S.R.; Zeeshan, M.; Masood, A. Enhancement of hydrocarbons production through co-pyrolysis of acid-treated biomass and waste tire in a fixed bed reactor. Waste Manag. 2020, 106, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Krutof, A.; Hawboldt, K.A. Upgrading of biomass sourced pyrolysis oil review: Focus on co-pyrolysis and vapour upgrading during pyrolysis. Biomass Convers. Biorefinery 2018, 8, 775–787. [Google Scholar] [CrossRef]
- Lv, X.; Liu, H.; Huang, Y.; Yao, J.; Yuan, H.; Yin, X.; Wu, C. Synergistic effects on co-pyrolysis of low-temperature hydrothermally pretreated high-protein microalgae and polypropylene. Energy Convers. Manag. 2021, 229, 113772. [Google Scholar] [CrossRef]
- Bu, Q.; Chen, K.; Xie, W.; Liu, Y.; Cao, M.; Kong, X.; Chu, Q.; Mao, H. Hydrocarbon rich bio-oil production, thermal behavior analysis and kinetic study of microwave-assisted co-pyrolysis of microwave-torrefied lignin with low density polyethylene. Bioresour. Technol. 2019, 291, 121860. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Duan, D.; Zhao, Y.; Yu, Z.; Jiang, L.; Wu, Q. Bridging the relationship between hydrothermal pretreatment and co-pyrolysis: Effect of hydrothermal pretreatment on aromatic production. Energy Convers. Manag. 2018, 180, 36–43. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.W.; Sahu, J. Optimization and characterization studies on bio-oil production from palm shell by pyrolysis using response surface methodology. Biomass-Bioenergy 2011, 35, 3604–3616. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis processes-An overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.; Mishra, D. Thermolysis of waste plastics to liquid fuel: A suitable method for plastic waste management and manufacture of value added products—A world prospective. Renew. Sustain. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Sunarno, S.E.; Fermi, M.I.; Utama, P.S. Non-Catalytic Co-pyrolysis of Empty Fruit Bunch of Palm and Solid Tire Waste Into Upgrade Bio-oil. Int. J. Renew. Energy Res. 2020, 10, 687–692. [Google Scholar]
- Mahari, W.A.W.; Chong, C.T.; Cheng, C.K.; Lee, C.L.; Hendrata, K.; Yek, P.N.Y.; Ma, N.L.; Lam, S.S. Production of value-added liquid fuel via microwave co-pyrolysis of used frying oil and plastic waste. Energy 2018, 162, 309–317. [Google Scholar] [CrossRef]
- Kumar, K.P.; Srinivas, S. Catalytic Co-pyrolysis of Biomass and Plastics (Polypropylene and Polystyrene) Using Spent FCC Catalyst. Energy Fuels 2019, 34, 460–473. [Google Scholar] [CrossRef]
- Liu, J.; Hou, Q.; Ju, M.; Ji, P.; Sun, Q.; Li, W. Biomass Pyrolysis Technology by Catalytic Fast Pyrolysis, Catalytic Co-Pyrolysis and Microwave-Assisted Pyrolysis: A Review. Catalysts 2020, 10, 742. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Yu, Z.; Deng, T.; Chen, X.; Chen, L.; Dai, M. A study on catalytic co-pyrolysis of kitchen waste with tire waste over ZSM-5 using TG-FTIR and Py-GC/MS. Bioresour. Technol. 2019, 289, 121585. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Hameed, B. Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review. J. Clean. Prod. 2020, 265, 121762. [Google Scholar] [CrossRef]
- Yildiz, G.; Ronsse, F.; van Duren, R.; Prins, W. Challenges in the design and operation of processes for catalytic fast pyrolysis of woody biomass. Renew. Sustain. Energy Rev. 2016, 57, 1596–1610. [Google Scholar] [CrossRef]
- Gin, A.; Hassan, H.; Ahmad, M.; Hameed, B.; Din, A.M. Recent progress on catalytic co-pyrolysis of plastic waste and lignocellulosic biomass to liquid fuel: The influence of technical and reaction kinetic parameters. Arab. J. Chem. 2021, 14, 103035. [Google Scholar] [CrossRef]
- Pinto, F.; Miranda, M.; Costa, P. Production of liquid hydrocarbons from rice crop wastes mixtures by co-pyrolysis and co-hydropyrolysis. Fuel 2016, 174, 153–163. [Google Scholar] [CrossRef]
- Önal, E.; Uzun, B.B.; Pütün, A.E. Bio-oil production via co-pyrolysis of almond shell as biomass and high density polyethylene. Energy Convers. Manag. 2014, 78, 704–710. [Google Scholar] [CrossRef]
- Meylemans, H.A.; Quintana, R.L.; Goldsmith, B.R.; Harvey, B.G. Solvent-Free Conversion of Linalool to Methylcyclopentadiene Dimers: A Route To Renewable High-Density Fuels. ChemSusChem 2011, 4, 465–469. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Effect of interactions of PVC and biomass components on the formation of polycyclic aromatic hydrocarbons (PAH) during fast co-pyrolysis. RSC Adv. 2015, 5, 11371–11377. [Google Scholar] [CrossRef]
- Hassan, H.; Hameed, B.H.; Lim, J.K. Co-pyrolysis of sugarcane bagasse and waste high-density polyethylene: Synergistic effect and product distributions. Energy 2020, 191, 116545. [Google Scholar] [CrossRef]
- Iftikhar, H.; Zeeshan, M.; Iqbal, S.; Muneer, B.; Razzaq, M. Co-pyrolysis of sugarcane bagasse and polystyrene with ex-situ catalytic bed of metal oxides/HZSM-5 with focus on liquid yield. Bioresour. Technol. 2019, 289, 121647. [Google Scholar] [CrossRef]
- Deng, T.; Yu, Z.; Zhang, X.; Zhang, Y.; Chen, L.; Ma, X. Catalytic co-pyrolysis behaviors and kinetics of camellia shell and take-out solid waste using pyrolyzer—gas chromatography/mass spectrometry and thermogravimetric analyzer. Bioresour. Technol. 2019, 297, 122419. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Lakshmikandan, M.; Wang, S.; Wang, Q.; He, Z.; Abomohra, A.E.-F. Catalytic co-pyrolysis of seaweeds and cellulose using mixed ZSM-5 and MCM-41 for enhanced crude bio-oil production. J. Therm. Anal. 2020, 143, 827–842. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Z.; Xia, S.; Lu, Q.; Walters, K.B. Catalytic Pyrolysis of Biomass and Polymer Wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Ruan, R.; Li, J.; Ma, L.; Wang, C.; Zhou, W. Aromatics production from fast co-pyrolysis of lignin and waste cooking oil catalyzed by HZSM-5 zeolite. Appl. Energy 2020, 263, 114629. [Google Scholar] [CrossRef]
- Zheng, A.; Huang, Z.; Wei, G.; Zhao, K.; Jiang, L.; Zhao, Z.; Tian, Y.; Li, H. Controlling Deoxygenation Pathways in Catalytic Fast Pyrolysis of Biomass and Its Components by Using Metal-Oxide Nanocomposites. iScience 2019, 23, 100814. [Google Scholar] [CrossRef] [Green Version]
- Stefanidis, S.; Karakoulia, S.; Kalogiannis, K.; Iliopoulou, E.; Delimitis, A.; Yiannoulakis, H.; Zampetakis, T.; Lappas, A.; Triantafyllidis, K. Natural magnesium oxide (MgO) catalysts: A cost-effective sustainable alternative to acid zeolites for the in situ upgrading of biomass fast pyrolysis oil. Appl. Catal. B Environ. 2016, 196, 155–173. [Google Scholar] [CrossRef]
- Mochizuki, T.; Chen, S.-Y.; Toba, M.; Yoshimura, Y. Pyrolyzer–GC/MS system-based analysis of the effects of zeolite catalysts on the fast pyrolysis of Jatropha husk. Appl. Catal. A Gen. 2013, 456, 174–181. [Google Scholar] [CrossRef]
- Mante, O.D.; Rodriguez, J.A.; Babu, S.P. Selective defunctionalization by TiO2 of monomeric phenolics from lignin pyrolysis into simple phenols. Bioresour. Technol. 2013, 148, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Vinu, R. Peroxide-assisted microwave activation of pyrolysis char for adsorption of dyes from wastewater. Bioresour. Technol. 2016, 216, 511–519. [Google Scholar] [CrossRef]
- Che, Q.; Yang, M.; Wang, X.; Chen, X.; Chen, W.; Yang, Q.; Yang, H.; Chen, H. Aromatics production with metal oxides and ZSM-5 as catalysts in catalytic pyrolysis of wood sawdust. Fuel Process. Technol. 2019, 188, 146–152. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Wang, J.; Zhang, B.; Fan, L.; Liu, S.; Wang, Y.; Liu, Y.; Zhong, D.; Chen, P.; et al. Improving hydrocarbon yield from catalytic fast co-pyrolysis of hemicellulose and plastic in the dual-catalyst bed of CaO and HZSM-5. Bioresour. Technol. 2018, 261, 86–92. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.; Xia, M.; Yang, H.; Chen, Y.; Chen, X.; Che, Q.; Chen, H. Catalytic deoxygenation co-pyrolysis of bamboo wastes and microalgae with biochar catalyst. Energy 2018, 157, 472–482. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhai, Y.; Li, S.; Liu, X.; Liu, X.; Wang, B.; Liu, Y.; Li, C.; Hu, Y. Catalytic co-pyrolysis of sewage sludge and rice husk over biochar catalyst: Bio-oil upgrading and catalytic mechanism. Waste Manag. 2020, 114, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gouws, S.; Carrier, M.; Bunt, J.; Neomagus, H. Co-pyrolysis of coal and raw/torrefied biomass: A review on chemistry, kinetics and implementation. Renew. Sustain. Energy Rev. 2020, 135, 110189. [Google Scholar] [CrossRef]
- Wu, F.; Ben, H.; Yang, Y.; Jia, H.; Wang, R.; Han, G. Effects of Different Conditions on Co-Pyrolysis Behavior of Corn Stover and Polypropylene. Polymers 2020, 12, 973. [Google Scholar] [CrossRef]
- Huang, W.; Gong, F.; Fan, M.; Zhai, Q.; Hong, C.; Li, Q. Production of light olefins by catalytic conversion of lignocellulosic biomass with HZSM-5 zeolite impregnated with 6wt.% lanthanum. Bioresour. Technol. 2012, 121, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T.; He, J.; Kumar, R.; Lu, Q. Catalytic pyrolysis of lignocellulosic biomass: A review of variations in process factors and system structure. Renew. Sustain. Energy Rev. 2020, 134, 110305. [Google Scholar] [CrossRef]
- Xie, Q.; Peng, P.; Liu, S.; Min, M.; Cheng, Y.; Wan, Y.; Li, Y.; Lin, X.; Liu, Y.; Chen, P.; et al. Fast microwave-assisted catalytic pyrolysis of sewage sludge for bio-oil production. Bioresour. Technol. 2014, 172, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, J.T.; Itabiyi, E.A.; Okekunle, P.O. A Comprehensive Review of Biomass Pyrolysis as a Process of Renewable Energy Generation. J. Nat. Sci. Res. 2015, 5, 99–105. [Google Scholar]
- Ahmad, R.; Baharein, N.A.F.K.; Mohamed, A.R.; Kasim, N.N. Co-pyrolysis of lemongrass waste and residual cooking oil in a fixed bed reactor. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012014. [Google Scholar] [CrossRef]
- Quan, C.; Gao, N. Copyrolysis of Biomass and Coal: A Review of Effects of Copyrolysis Parameters, Product Properties, and Synergistic Mechanisms. BioMed Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Xu, S. Co-pyrolysis of coal and biomass. Coal Convers. 2022, 25, 7–12. [Google Scholar]
- Zullaikah, S.; Lenggono, A.S.; Nury, D.F.; Rachimoellah, M. Effect of blending ratio to the liquid product on co-pyrolysis of low rank coal and oil palm empty fruit bunch. MATEC Web Conf. 2018, 156, 03023. [Google Scholar] [CrossRef] [Green Version]
- Schubert, T.; Lehner, M.; Karner, T.; Hofer, W.; Lechleitner, A. Influence of reaction pressure on co-pyrolysis of LDPE and a heavy petroleum fraction. Fuel Process. Technol. 2019, 193, 204–211. [Google Scholar] [CrossRef]
- Collot, A.-G.; Zhuo, Y.; Dugwell, D.; Kandiyoti, R. Co-pyrolysis and co-gasification of coal and biomass in bench-scale fixed-bed and fluidised bed reactors. Fuel 1999, 78, 667–679. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, N.; Liu, Q.; Wang, W.; Ma, X. Co-pyrolysis of bituminous coal and biomass in a pressured fluidized bed. Chin. J. Chem. Eng. 2019, 27, 1666–1673. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Effect of heating rate on the pyrolysis yields of rapeseed. Renew. Energy 2006, 31, 803–810. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind. Crop. Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Mateo, W.; Lei, H.; Villota, E.; Qian, M.; Zhao, Y.; Huo, E.; Zhang, Q.; Lin, X.; Wang, C.; Huang, Z. Synthesis and characterization of sulfonated activated carbon as a catalyst for bio-jet fuel production from biomass and waste plastics. Bioresour. Technol. 2019, 297, 122411. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the applications of bio-oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Li, H.; Mahmood, N.; Ma, Z.; Zhu, M.; Wang, J.; Zheng, J.; Yuan, Z.; Wei, Q.; Xu, C. Preparation and characterization of bio-polyol and bio-based flexible polyurethane foams from fast pyrolysis of wheat straw. Ind. Crop. Prod. 2017, 103, 64–72. [Google Scholar] [CrossRef]
- Hakeem, I.G.; Halder, P.; Marzbali, M.H.; Patel, S.; Kundu, S.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K. Research progress on levoglucosan production via pyrolysis of lignocellulosic biomass and its effective recovery from bio-oil. J. Environ. Chem. Eng. 2021, 9, 105614. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, L.; Liu, K.; Wang, J.; Zhu, H.; Song, Q.; Shu, X. Co-pyrolysis of sewage sludge and cotton stalks. Waste Manag. 2019, 89, 430–438. [Google Scholar] [CrossRef]
- Huang, H.-J.; Yang, T.; Lai, F.-Y.; Wu, G.-Q. Co-pyrolysis of sewage sludge and sawdust/rice straw for the production of biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- Meng, J.; Tao, M.; Wang, L.; Liu, X.; Xu, J. Changes in heavy metal bioavailability and speciation from a Pb-Zn mining soil amended with biochars from co-pyrolysis of rice straw and swine manure. Sci. Total Environ. 2018, 633, 300–307. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Jung, H.; Woo, S.H. Synergistic dye adsorption by biochar from co-pyrolysis of spent mushroom substrate and Saccharina japonica. Bioresour. Technol. 2017, 244, 1142–1149. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, X.; Zeng, F.; Li, H.; Chen, X. The hierarchical porous structure bio-char assessments produced by co-pyrolysis of municipal sewage sludge and hazelnut shell and Cu(II) adsorption kinetics. Environ. Sci. Pollut. Res. 2018, 25, 19423–19435. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, Z.; Li, J.; Xie, W.; Jiang, Q.; Bi, M.; Zhang, Y. A comparison of the characteristics and atrazine adsorption capacity of co-pyrolysed and mixed biochars generated from corn straw and sawdust. Environ. Res. 2019, 172, 561–568. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghai, H.; Sakhuja, D.; Yadav, S.; Solanki, P.; Putatunda, C.; Bhatia, R.K.; Bhatt, A.K.; Varjani, S.; Yang, Y.-H.; Bhatia, S.K.; et al. An Overview on Co-Pyrolysis of Biodegradable and Non-Biodegradable Wastes. Energies 2022, 15, 4168. https://doi.org/10.3390/en15114168
Ghai H, Sakhuja D, Yadav S, Solanki P, Putatunda C, Bhatia RK, Bhatt AK, Varjani S, Yang Y-H, Bhatia SK, et al. An Overview on Co-Pyrolysis of Biodegradable and Non-Biodegradable Wastes. Energies. 2022; 15(11):4168. https://doi.org/10.3390/en15114168
Chicago/Turabian StyleGhai, Hemant, Deepak Sakhuja, Shikha Yadav, Preeti Solanki, Chayanika Putatunda, Ravi Kant Bhatia, Arvind Kumar Bhatt, Sunita Varjani, Yung-Hun Yang, Shashi Kant Bhatia, and et al. 2022. "An Overview on Co-Pyrolysis of Biodegradable and Non-Biodegradable Wastes" Energies 15, no. 11: 4168. https://doi.org/10.3390/en15114168