Enhanced Photoelectrocatalytic Activity of TiO2 Nanowire Arrays via Copolymerized G-C3N4 Hybridization
Abstract
:1. Introduction
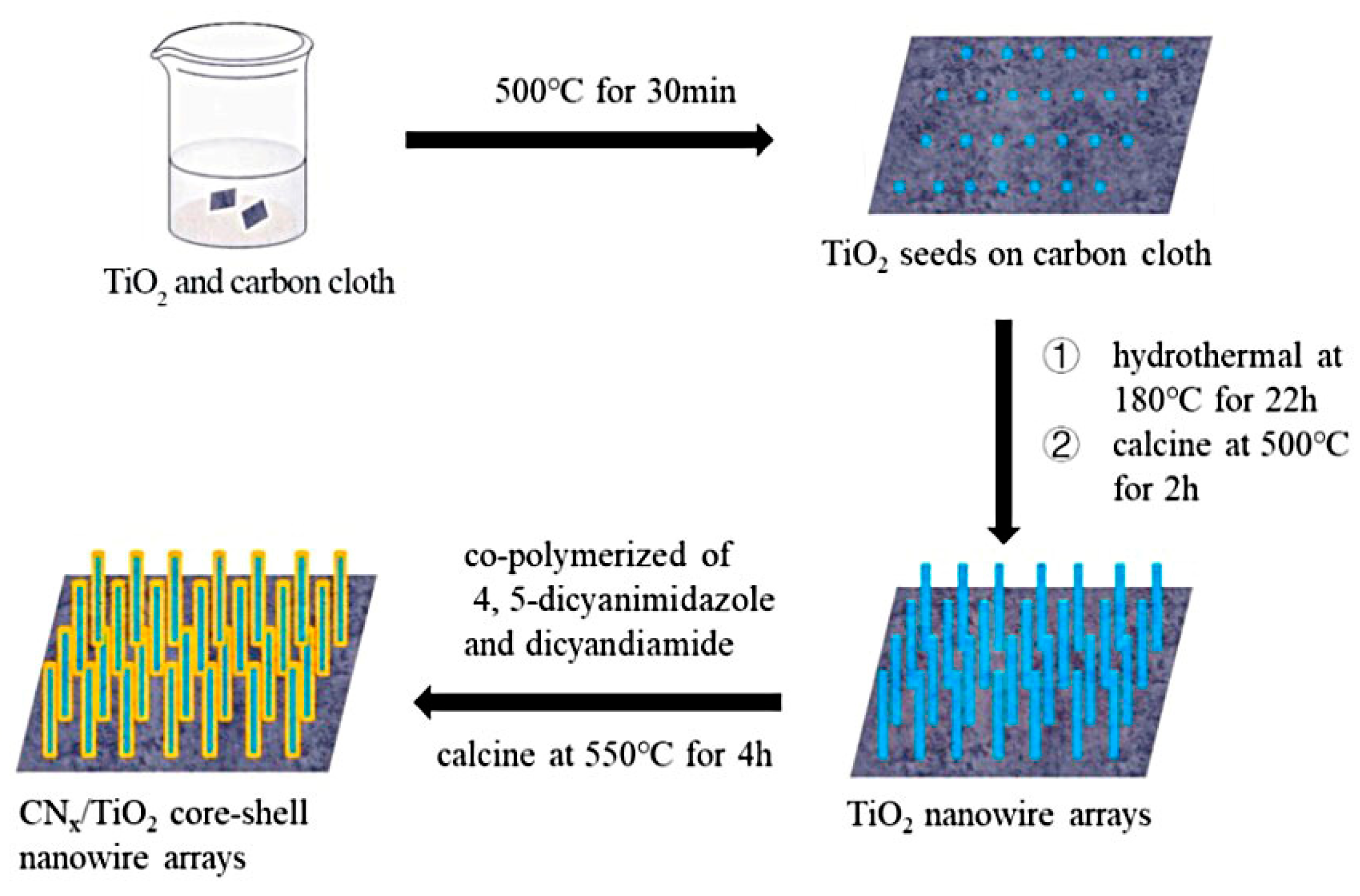
2. Experimental Section
2.1. Characterization
2.2. Photoelectrocatalysis Experiments
3. Results and Discussion
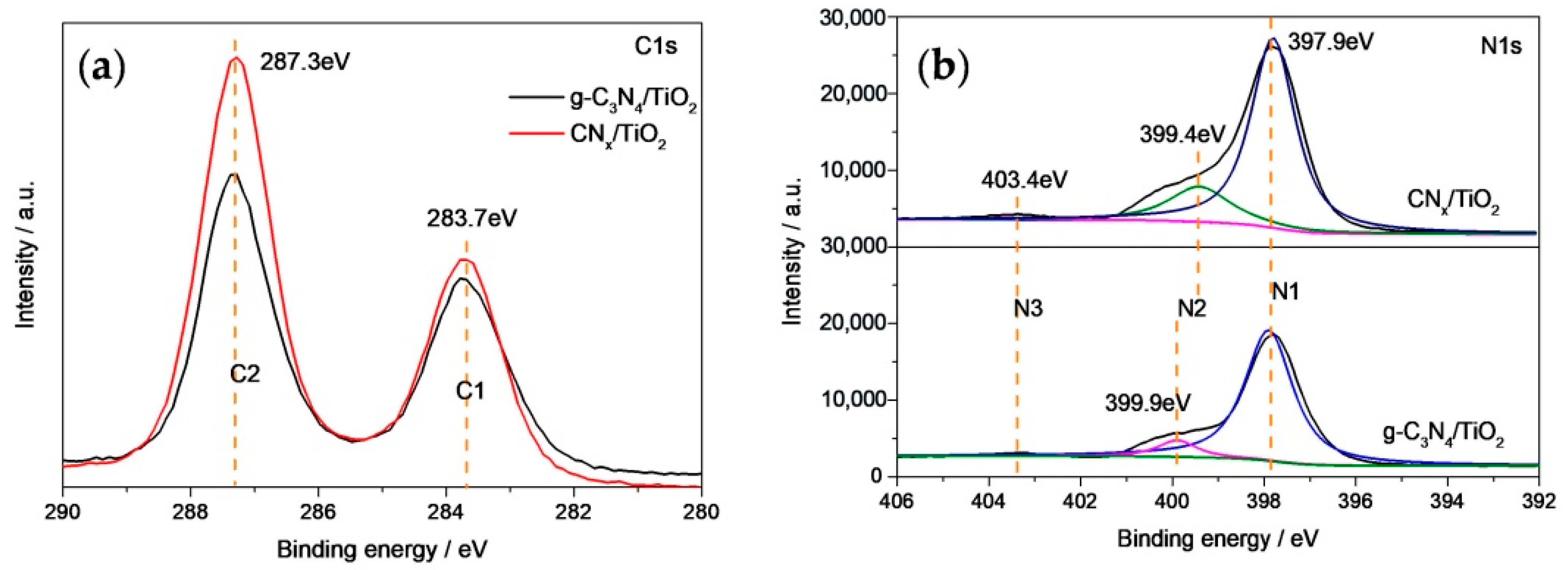
3.1. Catalyst Characterization
3.2. Enhancement of PC and PEC Activity
3.3. Mechanism of Enhancement of PEC Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Liu, Y.; Yao, W.; Liu, D.; Zong, R.; Zhang, M.; Ma, X.; Zhu, Y. Enhancement of visible light mineralization ability and photocatalytic activity of BiPO4/BiOI. Appl. Catal. B Environ. 2015, 163, 547–553. [Google Scholar] [CrossRef]
- Sun, J.X.; Yuan, Y.P.; Qiu, L.G.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Fabrication of composite photocatalyst g-C3N4-ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baruah, A.; Tonda, S.; Kumar, B.; Shanker, V.; Sreedhar, B. Cost-effective and eco-friendly synthesis of novel and stable N-doped ZnO/g-C3N4 core-shell nanoplates with excellent visible-light responsive photocatalysis. Nanoscale 2014, 6, 4830–4842. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, M.; Yang, J.; Li, X.; Hu, T.; Wang, J.; Sui, Y.; Wu, X.; Kong, L. Synergistic effect of efficient adsorption g-C3N4/ZnO composite for photocatalytic property. J. Phys. Chem. Solids 2014, 75, 441–446. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S.; Fu, X. Significantly enhanced visible-light photocatalytic activity of g-C3N4 via ZnO modification and the mechanism study. J. Mol. Catal. A Chem. 2013, 368–369, 9–15. [Google Scholar] [CrossRef]
- Wang, X.-J.; Yang, W.-Y.; Li, F.-T.; Xue, Y.-B.; Liu, R.-H.; Hao, Y.-J. In Situ Microwave-Assisted Synthesis of Porous N-TiO2/g-C3N4 Heterojunctions with Enhanced Visible-Light Photocatalytic Properties. Ind. Eng. Chem. Res. 2013, 52, 17140–17150. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, Q.; Liu, Y.; Wang, H.; Zhu, Y. Photocatalytic H2 evolution on MoS2-TiO2 catalysts synthesized via mechanochemistry. Phys. Chem. Chem. Phys. 2015, 17, 933–940. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, W.; Sun, Y.; Dong, F.; Zhou, Y.; Ho, W.K. Water-assisted production of honeycomb-like g-C3N4 with ultralong carrier lifetime and outstanding photocatalytic activity. Nanoscale 2015, 7, 2471–2479. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Jin, R.; Yang, Y.; Fang, X.; Xing, Y.; Song, S. Preparation and enhanced visible light photocatalytic activity of novel g-C3N4 nanosheets loaded with Ag2CO3 nanoparticles. Nanoscale 2015, 7, 758–764. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Gao, X.; Yao, W.; Wei, W.; Chen, X.; Zong, R.; Zhu, Y. Core-shell g-C3N4@ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities. Appl. Catal. B Environ. 2017, 217, 169–180. [Google Scholar] [CrossRef]
- Liang, F.; Zhu, Y. Enhancement of mineralization ability for phenol via synergetic effect of photoelectrocatalysis of g-C3N4 film. Appl. Catal. B Environ. 2016, 180, 324–329. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Wei, C.; Wang, F.; Wang, H.; Bian, Z. Interface engineering of Z-scheme α-Fe2O3/g-C3N4 photoanode: Simultaneous enhancement of charge separation and hole transportation for photoelectrocatalytic organic pollutant degradation. Chem. Eng. J. 2022, 435, 134873. [Google Scholar] [CrossRef]
- Dai, Z.; Lian, J.; Sun, Y.; Li, L.; Zhang, H.; Hu, N.; Ding, D. Fabrication of g-C3N4/Sn3O4/Ni electrode for highly efficient photoelectrocatalytic reduction of U(VI). Chem. Eng. J. 2022, 433, 133766. [Google Scholar] [CrossRef]
- Tao, W.; Wang, M.; Ali, R.; Nie, S.; Zeng, Q.; Yang, R.; Lau, W.-M.; He, L.; Tang, H.; Jian, X. Multi-layered porous hierarchical TiO2/g-C3N4 hybrid coating for enhanced visible light photocatalysis. Appl. Surf. Sci. 2019, 495, 143435. [Google Scholar] [CrossRef]
- Cheng, D.; Li, Y.; Yang, L.; Luo, S.; Yang, L.; Luo, X.; Luo, Y.; Li, T.; Gao, J.; Dionysiou, D.D. One-step reductive synthesis of Ti3+ self-doped elongated anatase TiO2 nanowires combined with reduced graphene oxide for adsorbing and degrading waste engine oil. J. Hazard. Mater. 2019, 378, 120752. [Google Scholar] [CrossRef] [PubMed]
- Janbandhu, S.Y.; Joshi, A.; Munishwar, S.R.; Gedam, R.S. CdS/TiO2 heterojunction in glass matrix: Synthesis, characterization, and application as an improved photocatalyst. Appl. Surf. Sci. 2019, 497, 143758. [Google Scholar] [CrossRef]
- Zhao, B.; Xiao, M.; Zhou, Y.N. Synaptic learning behavior of a TiO2 nanowire memristor. Nanotechnology 2019, 30, 425202. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Li, Y.; Liu, L.; Geng, Z.; Li, Y.; Chen, J.; Bai, W.; Jiang, G.; Zhao, Z. Controlled fabrication of TiO2/C3N4 core–shell nanowire arrays: A visible-light-responsive and environmental-friendly electrode for photoelectrocatalytic degradation of bisphenol A. J. Mater. Sci. 2018, 53, 11015–11026. [Google Scholar] [CrossRef]
- Weon, S.; Choi, W. TiO2 Nanotubes with Open Channels as Deactivation-Resistant Photocatalyst for the Degradation of Volatile Organic Compounds. Environ. Sci. Technol. 2016, 50, 2556–2563. [Google Scholar] [CrossRef]
- Teng, W.; Li, X.; Zhao, Q.; Chen, G. Fabrication of Ag/Ag3PO4/TiO2 heterostructure photoelectrodes for efficient decomposition of 2-chlorophenol under visible light irradiation. J. Mater. Chem. A 2013, 1, 9060–9068. [Google Scholar] [CrossRef]
- Pu, Y.C.; Wang, G.; Chang, K.D.; Ling, Y.; Lin, Y.K.; Fitzmorris, B.C.; Liu, C.M.; Lu, X.; Tong, Y.; Zhang, J.Z.; et al. Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting. Nano Lett. 2013, 13, 3817–3823. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Da, P.; Wu, H.; Zhao, D.; Zheng, G. Controlled Sn-doping in TiO2 nanowire photoanodes with enhanced photoelectrochemical conversion. Nano Lett. 2012, 12, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Deng, Z.; Chen, F.; Zhang, J.; Chen, H.; Anpo, M.; Huang, J.; Zhang, L. Hydrothermal doping method for preparation of Cr3+-TiO2 photocatalysts with concentration gradient distribution of Cr3+. Appl. Catal. B Environ. 2006, 62, 329–335. [Google Scholar] [CrossRef]
- Sheng, W.; Shi, J.-L.; Hao, H.; Li, X.; Lang, X. Polyimide-TiO2 hybrid photocatalysis: Visible light-promoted selective aerobic oxidation of amines. Chem. Eng. J. 2020, 379, 122399. [Google Scholar] [CrossRef]
- Bonan, R.F.; Mota, M.F.; da Costa Farias, R.M.; da Silva, S.D.; Bonan, P.R.F.; Diesel, L.; Menezes, R.R.; da Cruz Perez, D.E. In vitro antimicrobial and anticancer properties of TiO2 blow-spun nanofibers containing silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109876. [Google Scholar] [CrossRef]
- Low, J.; Dai, B.; Tong, T.; Jiang, C.; Yu, J. In Situ Irradiated X-Ray Photoelectron Spectroscopy Investigation on a Direct Z-Scheme TiO2 /CdS Composite Film Photocatalyst. Adv. Mater. 2019, 31, e1802981. [Google Scholar] [CrossRef]
- Mehdipour, H.; Akimov, A.V.; Jankowska, J.; Rezakhanai, A.T.; Tafreshi, S.S.; de Leeuw, N.H.; Moshfegh, A.Z.; Prezhdo, O.V. Persistent Quantum Coherence and Strong Coupling Enable Fast Electron Transfer across the CdS/TiO2 Interface: A Time-Domain ab Initio Simulation. J. Phys. Chem. C 2018, 122, 25606–25616. [Google Scholar] [CrossRef] [Green Version]
- Laatar, F.; Moussa, H.; Alem, H.; Balan, L.; Girot, E.; Medjahdi, G.; Ezzaouia, H.; Schneider, R. CdSe nanorod/TiO2 nanoparticle heterojunctions with enhanced solar- and visible-light photocatalytic activity. Beilstein J. Nanotechnol. 2017, 8, 2741–2752. [Google Scholar] [CrossRef] [Green Version]
- Hua, J.; Wang, M.; Jiao, Y.; Li, H.; Yang, Y. Strongly coupled CdX (X S, Se and Te) quantum dots/TiO2 nanocomposites for photocatalytic degradation of benzene under visible light irradiation. Optik 2018, 171, 95–106. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, T.; Zhang, M.; Li, Q.; Yang, J. Interfacial Construction of Zero-Dimensional/One-Dimensional g-C3N4 Nanoparticles/TiO2 Nanotube Arrays with Z-Scheme Heterostructure for Improved Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2018, 7, 2483–2491. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, W.; Wang, H.; Jiang, Y.; Han, S.; Sun, H.; Li, Y.; Jiang, G.; Zhao, Z.; Huan, Q. Promoted photoelectrocatalytic hydrogen evolution of a type II structure via an Al2O3 recombination barrier layer deposited using atomic layer deposition. Dalton Trans. 2017, 46, 10734–10741. [Google Scholar] [CrossRef] [PubMed]
- Agopcan, B.; Akyüz, D.; Karaca, F.; Sarıoğlu, C.; Koca, A. A new sulfur source for the preparation of efficient Cd(1−x)ZnxS photocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 8206–8220. [Google Scholar] [CrossRef]
- Yue, D.; Qian, X.; Zhang, Z.; Kan, M.; Ren, M.; Zhao, Y. CdTe/CdS Core/Shell Quantum Dots Cocatalyzed by Sulfur Tolerant [Mo3S13]2– Nanoclusters for Efficient Visible-Light-Driven Hydrogen Evolution. ACS Sustain. Chem. Eng. 2016, 4, 6653–6658. [Google Scholar] [CrossRef]
- Zhong, W.; Huang, X.; Xu, Y.; Yu, H. One-step facile synthesis and high H2-evolution activity of suspensible CdxZn1-xS nanocrystal photocatalysts in a S2−/SO32− system. Nanoscale 2018, 10, 19418–19426. [Google Scholar] [CrossRef]
- Tian, H.; Liu, X.; Liang, Z.; Qiu, P.; Qian, X.; Cui, H.; Tian, J. Gold nanorods/g-C3N4 heterostructures for plasmon-enhanced photocatalytic H2 evolution in visible and near-infrared light. J. Colloid Interface Sci. 2019, 557, 700–708. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, L.; Zhu, Q.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y. Hierarchical macro-mesoporous g-C3N4 with an inverse opal structure and vacancies for high-efficiency solar energy conversion and environmental remediation. Nanoscale 2019, 11, 20638–20647. [Google Scholar] [CrossRef]
- Fang, X.-X.; Ma, L.-B.; Liang, K.; Zhao, S.-J.; Jiang, Y.-F.; Ling, C.; Zhao, T.; Cheang, T.-Y.; Xu, A.-W. The doping of phosphorus atoms into graphitic carbon nitride for highly enhanced photocatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 11506–11512. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Lu, C.; Xiao, D.; Wu, S.; Liu, Y. Mechanical, thermal, and ablative properties between graphene oxide and graphitic carbon nitride based carbon/phenolic composites: A comparative study. Polym. Compos. 2018, 39, E1928–E1938. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. Engl. 2010, 49, 441–444. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X. A facile synthesis of covalent carbon nitride photocatalysts by Co-polymerization of urea and phenylurea for hydrogen evolution. J. Catal. 2013, 307, 246–253. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, S.; Yu, H.; Quan, X. g-C3N4/TiO2 hybrid photocatalyst with wide absorption wavelength range and effective photogenerated charge separation. Sep. Purif. Technol. 2012, 99, 50–54. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, M.; Zhu, Y. Photocatalytic enhancement of hybrid C3N4/TiO2 prepared via ball milling method. Phys. Chem. Chem. Phys. 2015, 17, 3647–3652. [Google Scholar] [CrossRef]
- Yang, N.; Li, G.; Wang, W.; Yang, X.; Zhang, W.F. Photophysical and enhanced daylight photocatalytic properties of N-doped TiO2/g-C3N4 composites. J. Phys. Chem. Solids 2011, 72, 1319–1324. [Google Scholar] [CrossRef]
- Chaudhary, D.; Kumar, S.; Khare, N. Boosting the visible-light photoelectrochemical performance of C3N4 by coupling with TiO2 and carbon nanotubes: An organic/inorganic hybrid photocatalyst nanocomposite for photoelectrochemical water spitting. Int. J. Hydrogen Energy 2020, 45, 30091–30100. [Google Scholar] [CrossRef]
- Ho, W.; Zhang, Z.; Lin, W.; Huang, S.; Zhang, X.; Wang, X.; Huang, Y. Copolymerization with 2,4,6-triaminopyrimidine for the rolling-up the layer structure, tunable electronic properties, and photocatalysis of g-C3N4. ACS Appl. Mater. Interfaces 2015, 7, 5497–5505. [Google Scholar] [CrossRef]
- Che, H.; Liu, C.; Che, G.; Liao, G.; Dong, H.; Li, C.; Song, N.; Li, C. Facile construction of porous intramolecular g-C3N4-based donor-acceptor conjugated copolymers as highly efficient photocatalysts for superior H2 evolution. Nano Energy 2020, 67, 104273. [Google Scholar] [CrossRef]
- Diamantopoulou, A.; Sakellis, E.; Romanos, G.E.; Gardelis, S.; Ioannidis, N.; Boukos, N.; Falaras, P.; Likodimos, V. Titania photonic crystal photocatalysts functionalized by graphene oxide nanocolloids. Appl. Catal. B Environ. 2019, 240, 277–290. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, R.; Wu, Q.; Yang, Z.; Fan, F.; Li, Y.; Jiang, G. Enhanced Photoelectrocatalytic Activity of TiO2 Nanowire Arrays via Copolymerized G-C3N4 Hybridization. Energies 2022, 15, 4180. https://doi.org/10.3390/en15124180
Wang Y, Li R, Wu Q, Yang Z, Fan F, Li Y, Jiang G. Enhanced Photoelectrocatalytic Activity of TiO2 Nanowire Arrays via Copolymerized G-C3N4 Hybridization. Energies. 2022; 15(12):4180. https://doi.org/10.3390/en15124180
Chicago/Turabian StyleWang, Yajun, Runhua Li, Qiaohuan Wu, Zhuang Yang, Fan Fan, Yuming Li, and Guiyuan Jiang. 2022. "Enhanced Photoelectrocatalytic Activity of TiO2 Nanowire Arrays via Copolymerized G-C3N4 Hybridization" Energies 15, no. 12: 4180. https://doi.org/10.3390/en15124180




