Corrosion of Iron Covered with Iron Oxide Film by Chlorine and Hydrogen Chloride Gases: A Molecular Dynamics Simulation Study Using the ReaxFF
Abstract
:1. Introduction
2. Simulation Methods and Details
2.1. ReaxFF-MD Simulation
2.2. Simulation Details
3. Results and Discussion
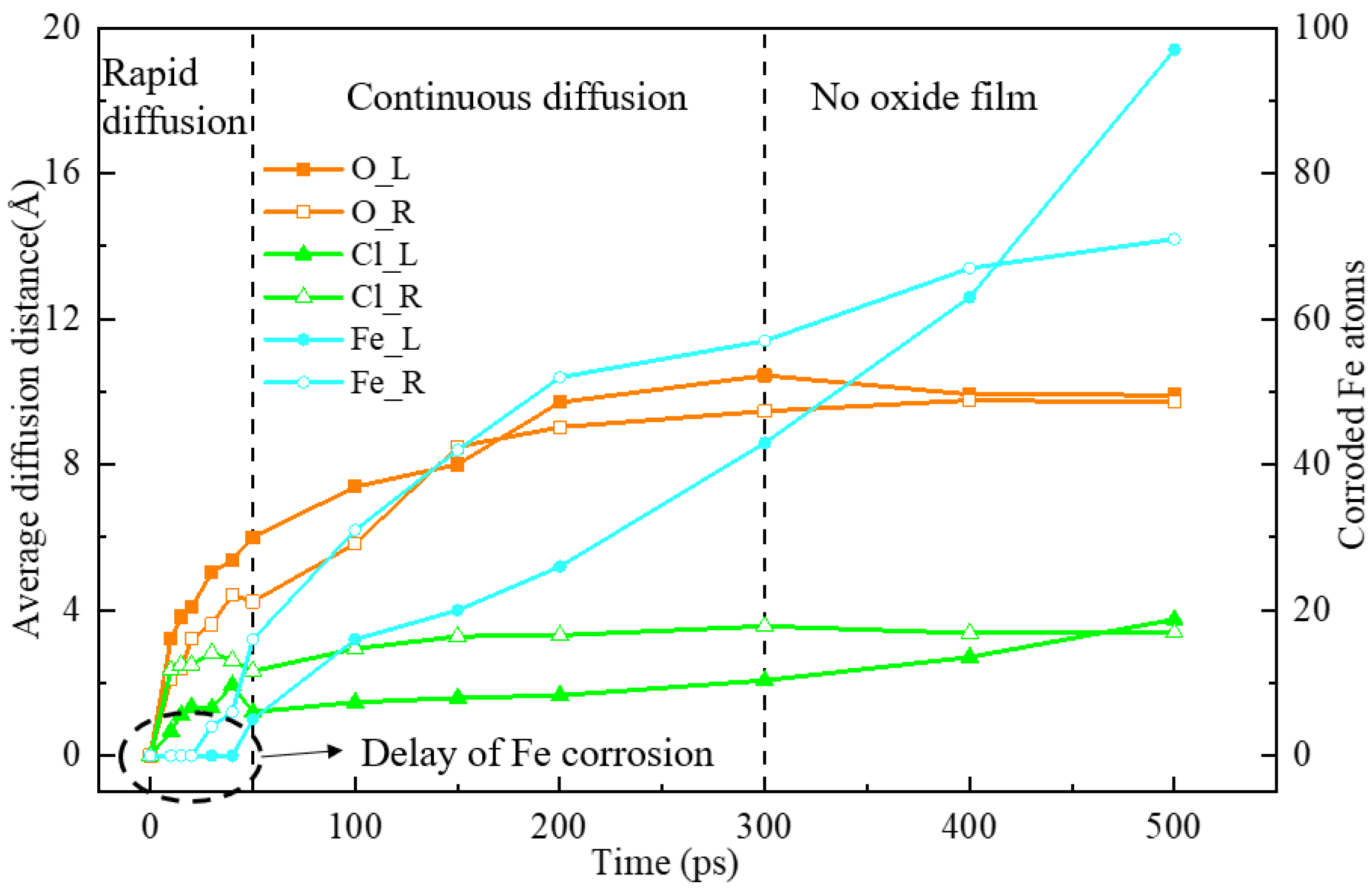
3.1. Anti-Corrosion Verification of Iron Oxide
3.2. Role of Intact/Damaged Oxide Film in Chlorine Corrosion
3.3. Role of Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antunes, R.A.; de Oliveira, M.C.L. Corrosion in biomass combustion: A materials selection analysis and its interaction with corrosion mechanisms and mitigation strategies. Corros. Sci. 2013, 76, 6–26. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Yue, M.; Li, Y. Reaction Kinetics of Chlorine Corrosion to Heating Surfaces during Coal and Biomass Cofiring. J. Chem. 2020, 2020, 2175795. [Google Scholar] [CrossRef]
- Cantatore, V.; Ogaz, M.A.O.; Liske, J.; Jonsson, T.; Svensson, J.-E.; Johansson, L.-G.; Panas, I. Oxidation Driven Permeation of Iron Oxide Scales by Chloride from Experiment Guided First-Principles Modeling. J. Phys. Chem. C 2019, 123, 25957–25966. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Wu, X.; Zhang, X. Chlorine-Induced High-Temperature Corrosion of Boiler Steels Combusting Sha Erhu Coal Compared to Biomass. Energy Fuels 2018, 32, 4237–4247. [Google Scholar] [CrossRef]
- Chen, T.C.; Xiang, J.H.; Jiang, L.F.; Xiong, J.; Bai, L.Y.; Xu, X.H.; Xu, X.C. High-temperature Corrosion Behavior of Q235 Steel in Oxidizing Atmosphere Containing Chlorine. J. Chin. Soc. Corros. Prot. 2021, 41, 560–564. [Google Scholar] [CrossRef]
- Maurice, V.; Marcus, P. Progress in corrosion science at atomic and nanometric scales. Prog. Mater. Sci. 2018, 95, 132–171. [Google Scholar] [CrossRef]
- Zi, M.; Chen, D.; Wu, G. Molecular dynamics simulation of methane hydrate formation on metal surface with oil. Chem. Eng. Sci. 2018, 191, 253–261. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Messali, M.; Aljuhani, A.; Aouad, M.; Hammouti, B.; Belghiti, M.; Chauhan, D.S.; Quraishi, M. Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: Electrochemical and molecular dynamics simulation studies. J. Mol. Liq. 2018, 249, 997–1008. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; van Duin, A.C.T. Isotope Effects in Water: Differences of Structure, Dynamics, Spectrum, and Proton Transport between Heavy and Light Water from ReaxFF Reactive Force Field Simulations. J. Phys. Chem. Lett. 2018, 9, 5445–5452. [Google Scholar] [CrossRef]
- Batuer, A.; Chen, D.; He, X.; Huang, Z. Simulation methods of cotton pyrolysis based on ReaxFF and the influence of volatile removal ratio on volatile evolution and char formation. Chem. Eng. J. 2020, 405, 126633. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, Z.; Tian, S.; Chen, X. Effect of oxidation on crack propagation of Si nanofilm: A ReaxFF molecular dynamics simulation study. Appl. Surf. Sci. 2019, 480, 1100–1108. [Google Scholar] [CrossRef]
- Dongol, R.; Wang, L.; Cormack, A.; Sundaram, S. Molecular dynamics simulation of sodium aluminosilicate glass structures and glass surface-water reactions using the reactive force field (ReaxFF). Appl. Surf. Sci. 2018, 439, 1103–1110. [Google Scholar] [CrossRef]
- Sim, H.S.; Yetter, R.A.; Hong, S.; van Duin, A.C.; Dabbs, D.M.; Aksay, I.A. Functionalized graphene sheet as a dispersible fuel additive for catalytic decomposition of methylcyclohexane. Combust. Flame 2020, 217, 212–221. [Google Scholar] [CrossRef]
- Jin, Y.; Duan, F.; Mu, X. Functionalization enhancement on interfacial shear strength between graphene and polyethylene. Appl. Surf. Sci. 2016, 387, 1100–1109. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Ma, F.; Lu, Y.; Zhao, T. Pyrolysis mechanisms of graphene oxide revealed by ReaxFF molecular dynamics simulation. Appl. Surf. Sci. 2020, 509, 145247. [Google Scholar] [CrossRef]
- Jeon, B.; Van Overmeere, Q.; van Duin, A.C.T.; Ramanathan, S. Nanoscale oxidation and complex oxide growth on single crystal iron surfaces and external electric field effects. Phys. Chem. Chem. Phys. 2012, 15, 1821–1830. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, C.-J.; Lu, W.-Q. Molecular dynamics simulation on the corrosion characteristics of iron in liquid lead. Ann. Nucl. Energy 2018, 116, 31–41. [Google Scholar] [CrossRef]
- Li, W.; Mi, Z.; Qin, S.; Gao, L.; He, J.; Guo, L.; Qiao, L. CS-AFM study on Pb-induced degradation of passive film on nickel-based alloy in high temperature and high pressure water. Corros. Sci. 2018, 144, 249–257. [Google Scholar] [CrossRef]
- DorMohammadi, H.; Pang, Q.; Árnadóttir, L.; Isgor, O.B. Atomistic simulation of initial stages of iron corrosion in pure water using reactive molecular dynamics. Comput. Mater. Sci. 2018, 145, 126–133. [Google Scholar] [CrossRef]
- Farzi, N.; Hydarifar, M.-H.; Izadi, M.E. The investigation of surface corrosion of Fe3C in H2SO4 solution and the role of thiophene as an inhibitor by ReaxFF molecular dynamics. Mater. Chem. Phys. 2022, 283, 125984. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef] [Green Version]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W.A. ReaxFF Reactive Force Field for Molecular Dynamics Simulations of Hydrocarbon Oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manyele, S.V. Analysis of Medical Waste Incinerator Performance Based on Fuel Consumption and Cycle Times. Engineering 2012, 4, 625–635. [Google Scholar] [CrossRef]
- Król, D.; Motyl, P.; Poskrobko, S. Chlorine Corrosion in a Low-Power Boiler Fired with Agricultural Biomass. Energies 2022, 15, 382. [Google Scholar] [CrossRef]









| Model | N(Fe) | N(Fe2O3) | N(Cl2) | N(HCl) | N(H2O) | Size of the Simulated Box |
|---|---|---|---|---|---|---|
| A | - | 40 + 40 + 40 | 64 | 64 | - | 25.37 Å × 9.82 Å × 66.42 Å |
| B | 756 + 756 | 40 + 24 | 128 | 128 | - | 25.37 Å × 9.82 Å × 147.48 Å |
| C | 756 + 756 | 40 + 40 | 128 | 128 | 128 | 25.37 Å × 9.82 Å × 168.94 Å |
| Model | Factor | Corroded Iron Atoms (Fe Layer) | Proportion |
|---|---|---|---|
| A | – | – | – |
| B | Oxide film | 17 | 2.25% |
| Cracked oxide film | 23 | 3.04% | |
| C | Water | 71 | 4.63% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Yang, Y.; Yang, N.; Tong, L.; Yin, S.; Yu, L.; Wang, L. Corrosion of Iron Covered with Iron Oxide Film by Chlorine and Hydrogen Chloride Gases: A Molecular Dynamics Simulation Study Using the ReaxFF. Energies 2022, 15, 4237. https://doi.org/10.3390/en15124237
Qiu Y, Yang Y, Yang N, Tong L, Yin S, Yu L, Wang L. Corrosion of Iron Covered with Iron Oxide Film by Chlorine and Hydrogen Chloride Gases: A Molecular Dynamics Simulation Study Using the ReaxFF. Energies. 2022; 15(12):4237. https://doi.org/10.3390/en15124237
Chicago/Turabian StyleQiu, Yinan, Yan Yang, Na Yang, Lige Tong, Shaowu Yin, Lang Yu, and Li Wang. 2022. "Corrosion of Iron Covered with Iron Oxide Film by Chlorine and Hydrogen Chloride Gases: A Molecular Dynamics Simulation Study Using the ReaxFF" Energies 15, no. 12: 4237. https://doi.org/10.3390/en15124237
APA StyleQiu, Y., Yang, Y., Yang, N., Tong, L., Yin, S., Yu, L., & Wang, L. (2022). Corrosion of Iron Covered with Iron Oxide Film by Chlorine and Hydrogen Chloride Gases: A Molecular Dynamics Simulation Study Using the ReaxFF. Energies, 15(12), 4237. https://doi.org/10.3390/en15124237







