Film Boiling Conjugate Heat Transfer during Immersion Quenching
Abstract
:1. Introduction
2. Methodology
2.1. Governing Equations
2.1.1. Fluid Region
2.1.2. Solid Region
2.2. Interfacial Terms
2.2.1. Momentum
2.2.2. Heat Transfer
2.2.3. Interfacial Phase Change
2.3. Numerical Solvers and Schemes
3. Solid–Fluid Interface
3.1. Wall Boiling
3.2. Solid–Fluid Interface Temperature
4. Application, Validation and Numerical Stability
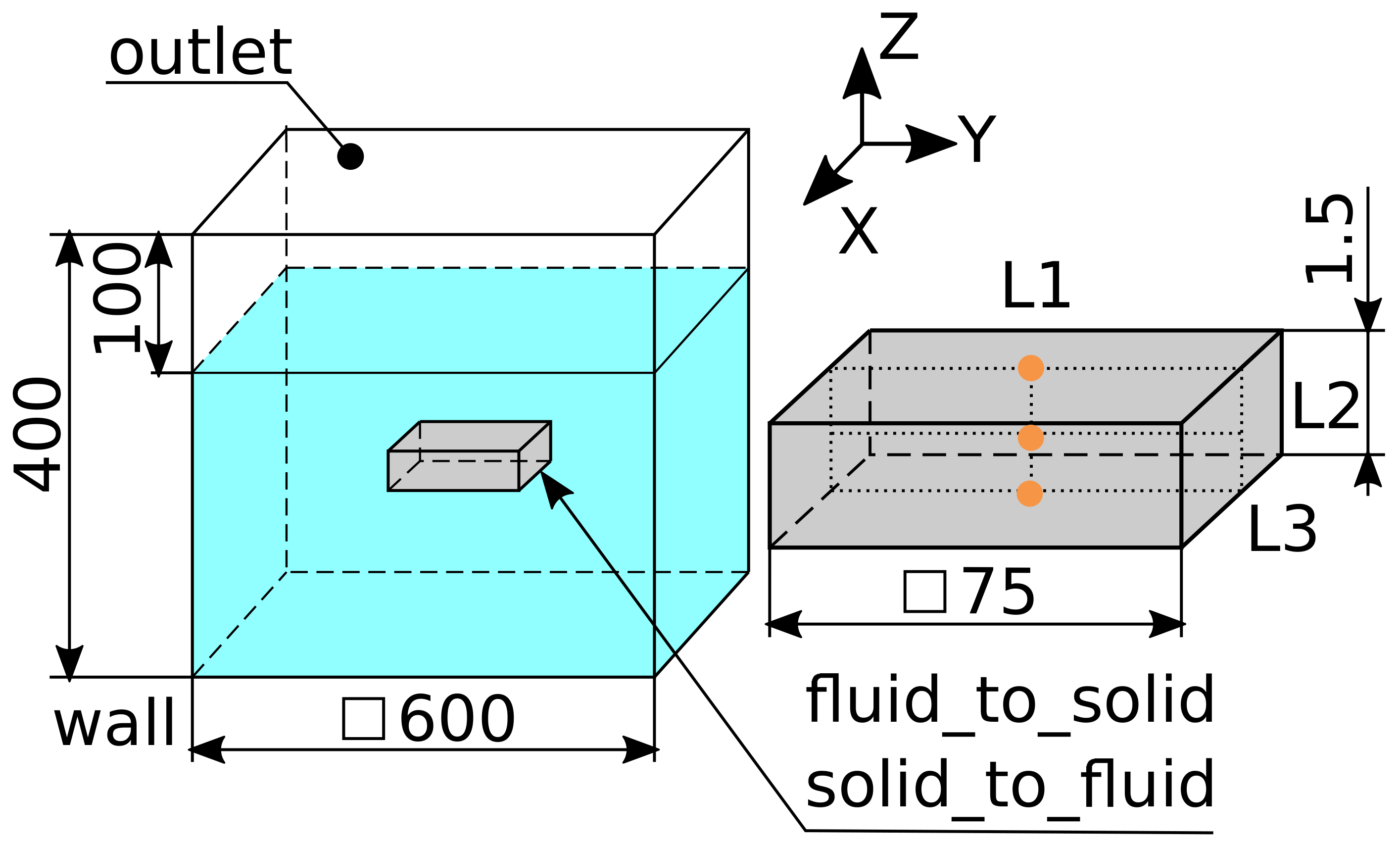
4.1. Geometry, Initial and Boundary Conditions
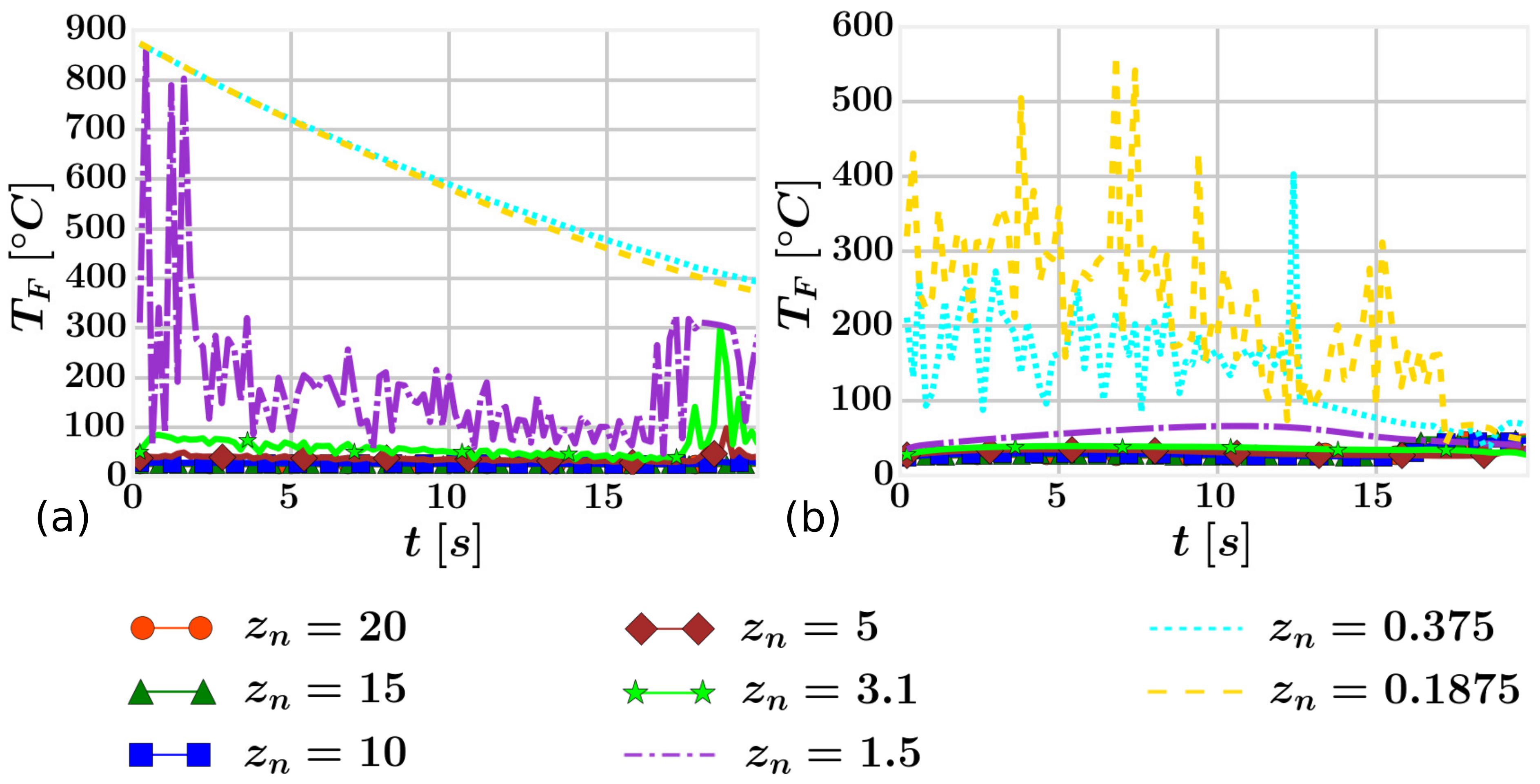
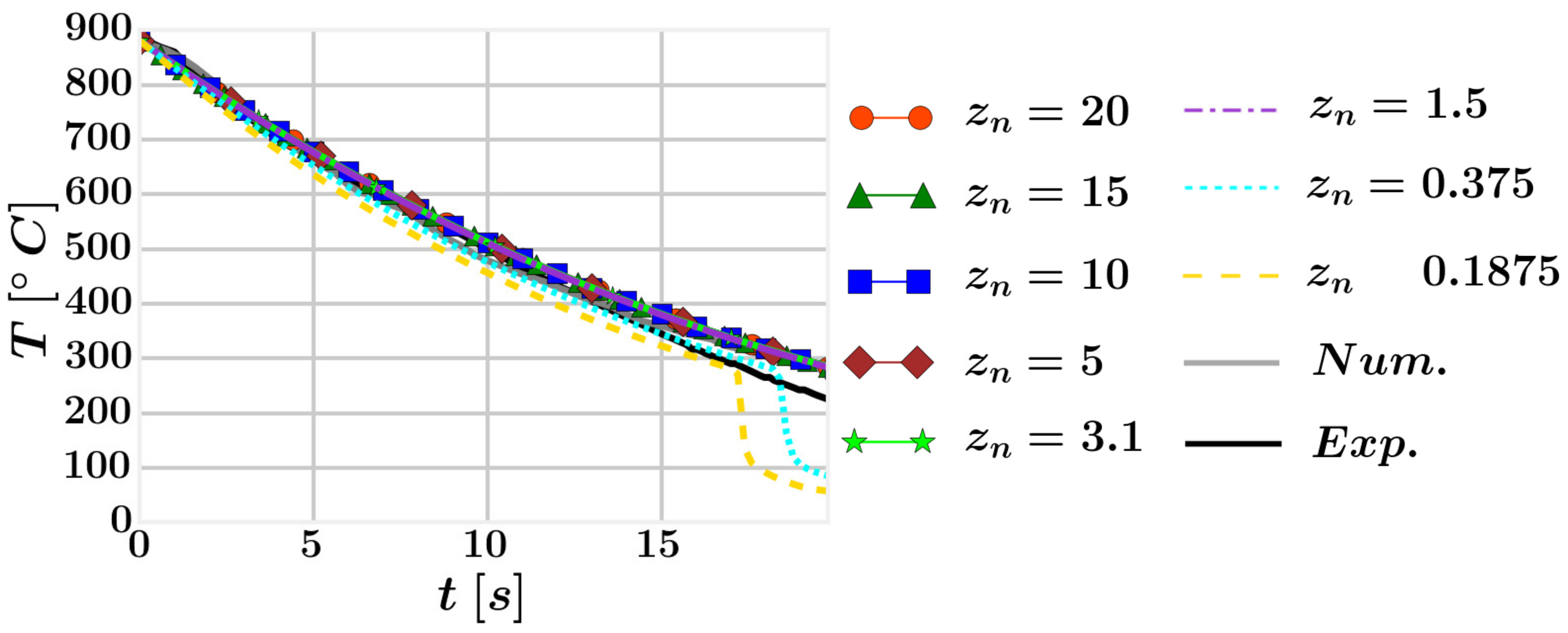
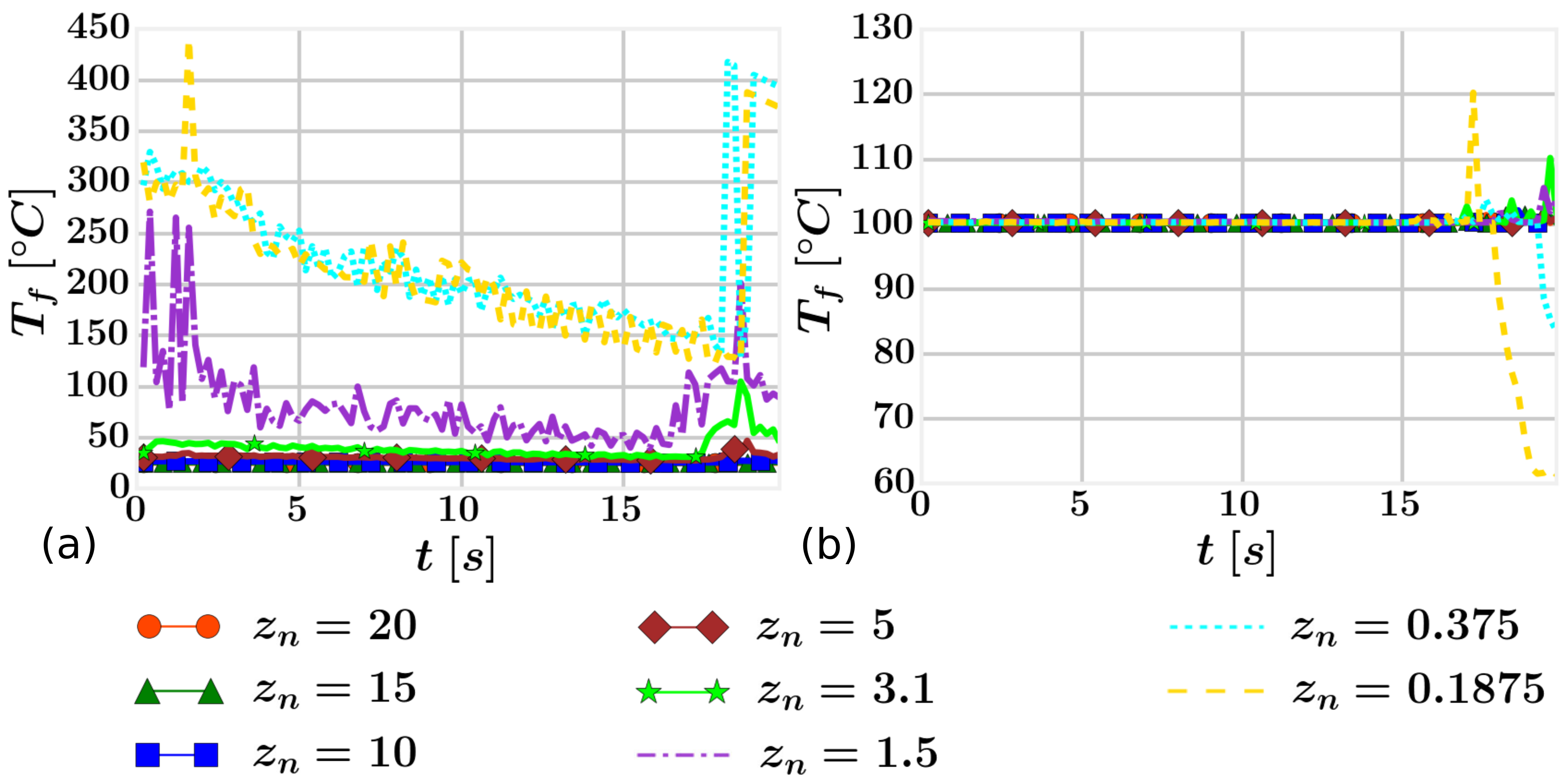
4.2. Mesh Definition
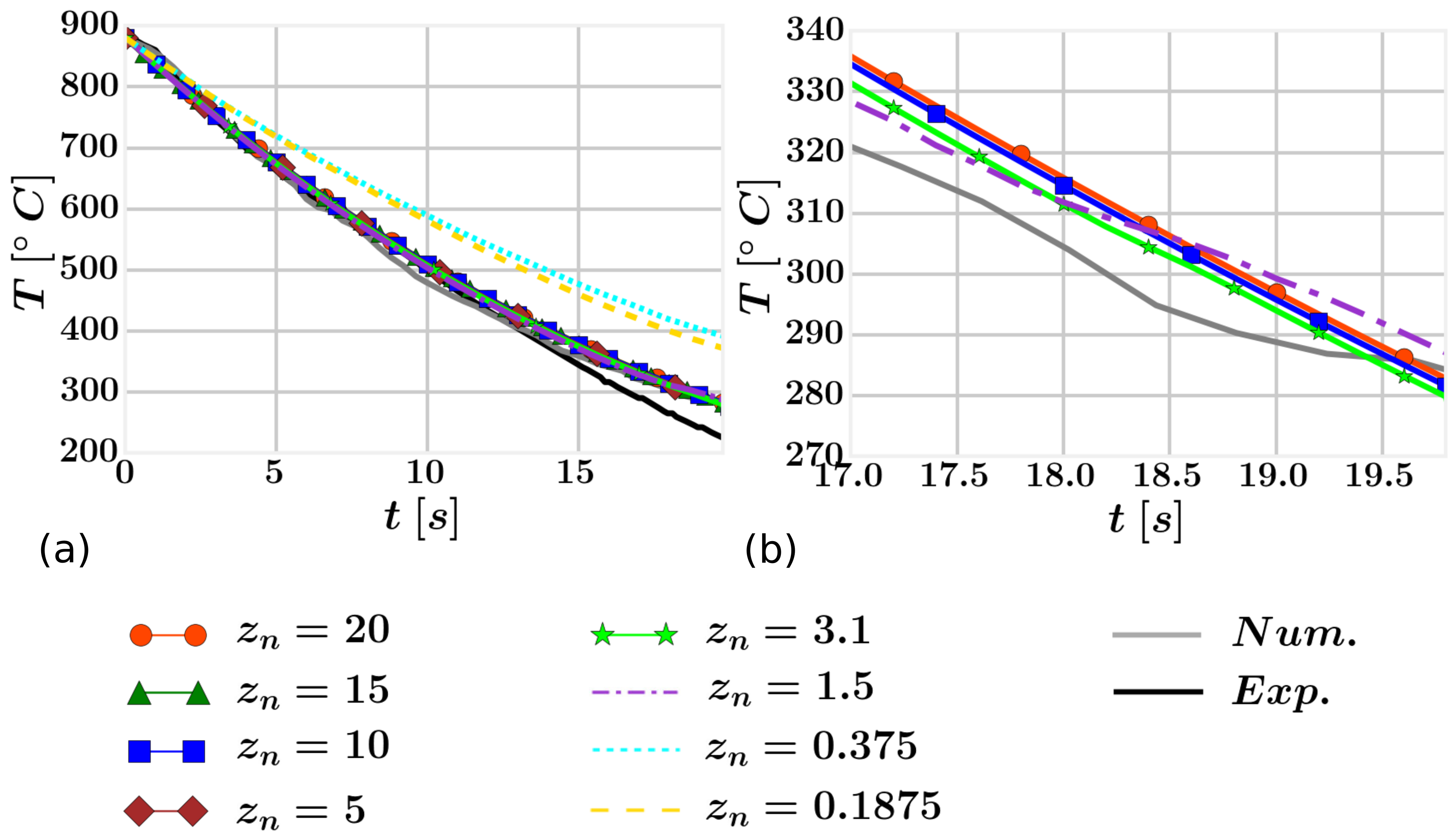
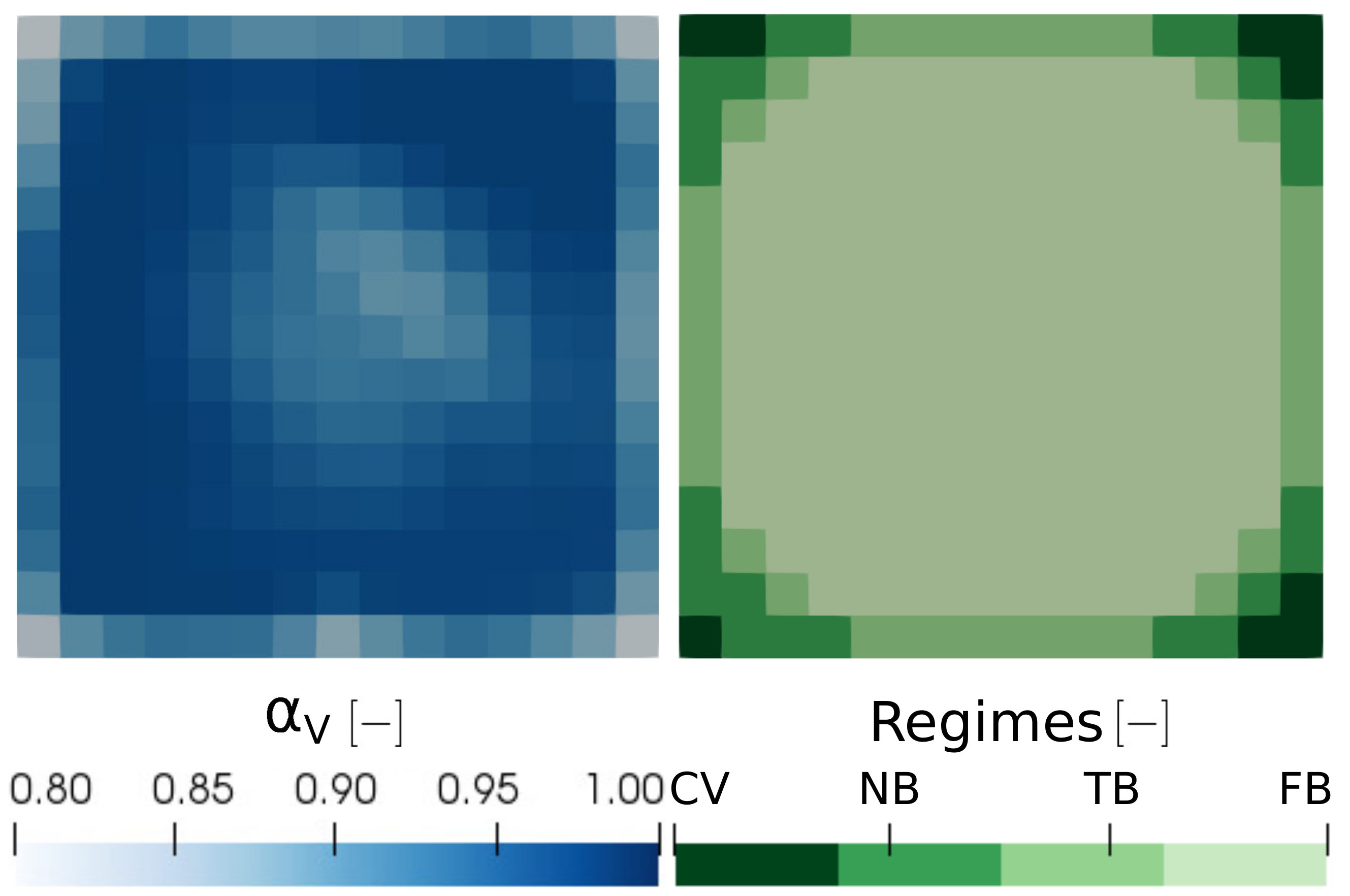
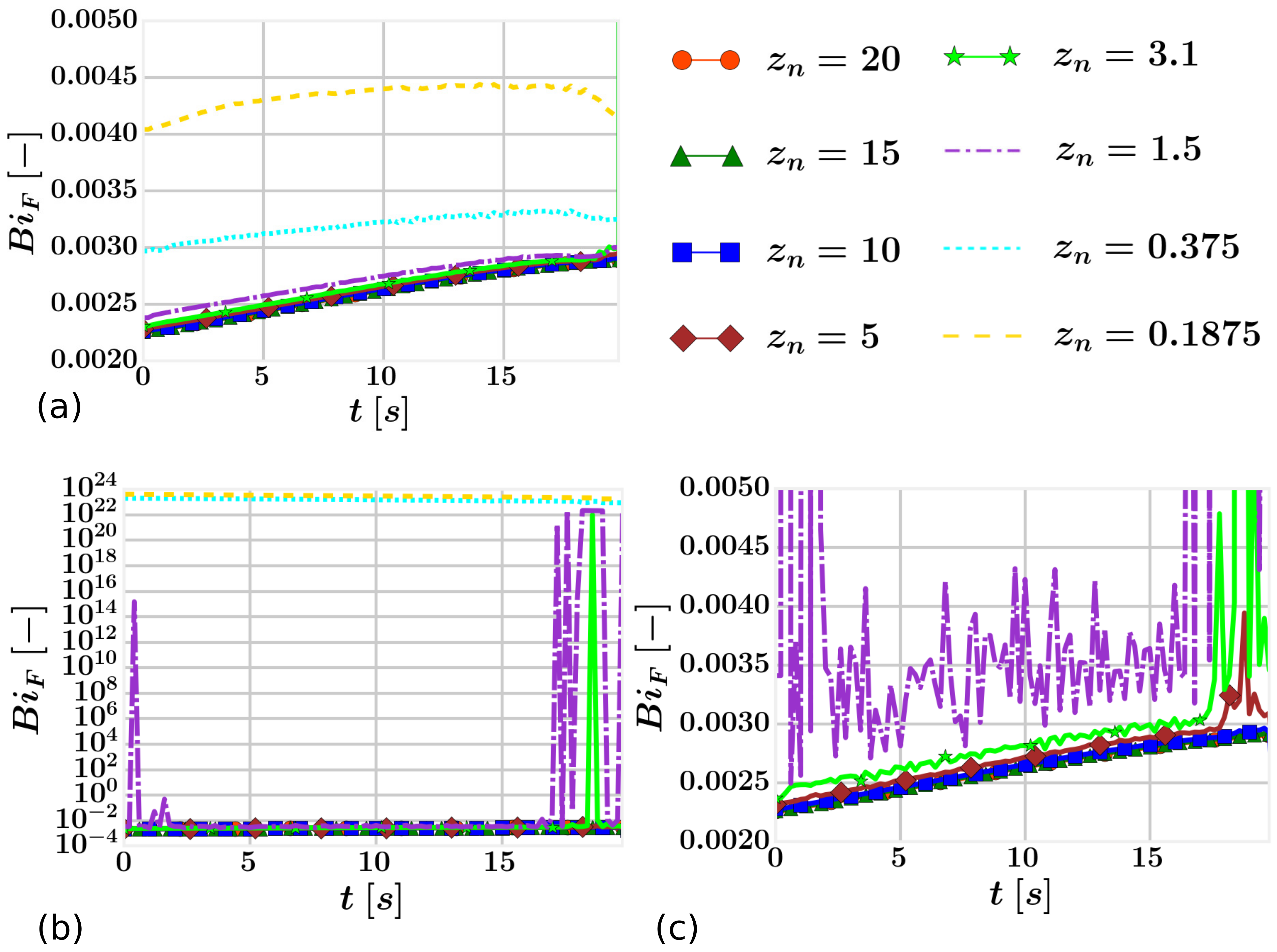
4.3. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandez, R.; Folsom, C.P.; Woolstenhulme, N.E.; Jensen, C.B.; Bess, J.D.; Gorton, J.P.; Brown, N.R. Review of pool boiling critical heat flux (CHF) and heater rod design for CHF experiments in TREAT. Prog. Nucl. Energy 2020, 123, 103303. [Google Scholar] [CrossRef]
- Kamenický, R.; Domalapally, P.K.; Arnoult, X. Thermo-mechanical stress analysis of the water cooled DEMO First Wall mock-up components. Fusion Eng. Des. 2018, 136, 1618–1623. [Google Scholar] [CrossRef]
- Kalani, A.; Kandlikar, G.S. Enhanced Pool Boiling with Ethanol at Subatmospheric Pressures for Electronics Cooling. J. Heat Transf. 2013, 135, 111002. [Google Scholar] [CrossRef] [Green Version]
- Liscic, B.; Tensi, H.; Canale, L.; Totten, G. Quenching Theory and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Kulacki, F.A. (Ed.) Handbook of Thermal Science and Engineering; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Wang, D.M.; Alajbegovic, A.; Su, X.; Jan, J. Numerical modelling of Quench Cooling Using Eulerian Two-Fluid Method. In Proceedings of the ASME 2002 International Mechanical Engineering Congress and Exposition, New Orleans, LA, USA, 17–22 November 2002; pp. 179–185. [Google Scholar]
- Wang, D.M.; Alajbegovic, A.; Su, X.; Jan, J. Numerical Simulation of Water Quenching Process of an Engine Cylinder Head. In Proceedings of the ASME/JSME 2003 4th Joint Fluids Summer Engineering Conference, Honolulu, HI, USA, 6–10 July 2003; pp. 1571–1578. [Google Scholar]
- Srinivasan, V.; Moon, K.; Greif, D.; Wang, D.; Kim, M. Numerical simulation of immersion quench cooling process using an Eulerian multi-fluid approach. Appl. Therm. Eng. 2010, 30, 499–509. [Google Scholar] [CrossRef]
- Srinivasan, V.; Moon, K.; Greif, D.; Wang, D.M.; Kim, M. Numerical simulation of immersion quenching process of an engine cylinder head. Appl. Math. Model. 2010, 34, 2111–2128. [Google Scholar] [CrossRef] [Green Version]
- Greif, D.; Kovacic, Z.; Srinivasan, V.; Wang, D.M.; Suffa, M. Coupled Numerical Analysis of Quenching Process of Internal Combustion Engine Cylinder Head. BHM Berg- Hüttenmännische Mon. 2009, 154, 509. [Google Scholar] [CrossRef]
- Jan, J.; Prabhu, E.; Lasecki, J.; Weiss, U.; Kaynar, A.; Eroglu, S. Development and Validation of CFD Methodology to Simulate Water Quenching Process. In Proceedings of the ASME Conference: International Manufacturing Science and Engineering Conference, Detroit, MI, USA, 9–13 June 2014. [Google Scholar]
- Kopun, R.; Škerget, L.; Hriberšek, M.; Zhang, D.; Stauder, B.; Greif, D. Numerical simulation of immersion quenching process for cast aluminium part at different pool temperatures. Appl. Therm. Eng. 2014, 65, 74–84. [Google Scholar] [CrossRef]
- Kopun, R.; Škerget, L.; Hriberšek, M.; Zhang, D.; Edelbauer, W. Numerical Investigations of Quenching Cooling Processes for Different Cast Aluminum Parts. Stroj. Vestn. 2014, 60, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.S.; Kopun, R.; Kosir, N.; Edelbauer, W. Advances in the numerical investigation of the immersion quenching process. IOP Conf. Ser. Mater. Sci. Eng. 2017, 164, 012004. [Google Scholar] [CrossRef] [Green Version]
- Jan, J.; Mackenzie, D.S. On the Characterization of Heat Transfer Rate in Various Boiling Regimes Using Quenchometers and Its Application for Quenching Process Simulations. In Proceedings of the Thermal Processing in Motion 2018, Spartanburg, SC, USA, 5–7 June 2018; pp. 112–123. [Google Scholar]
- Jan, J.; MacKenzie, D.S. On the Development of Parametrical Water Quenching Heat Transfer Model Using Cooling Curves by ASTM D6200 Quenchometer. J. Mater. Eng. Perform. 2020, 29, 3612–3625. [Google Scholar] [CrossRef]
- Krause, F.; Schüttenberg, S.; Fritsching, U. Modelling and simulation of flow boiling heat transfer. Int. J. Numer. Methods Heat Fluid Flow 2010, 20, 312–331. [Google Scholar] [CrossRef]
- Khalloufi, M.; Valette, R.; Hachem, E. Adaptive Eulerian framework for boiling and evaporation. J. Comput. Phys. 2019, 401, 109030. [Google Scholar] [CrossRef]
- AVL FIRE. Available online: https://www.avl.com/fire (accessed on 8 February 2022).
- Smith, M. ABAQUS/Standard User’s Manual, Version 6.9; Dassault Systèmes Simulia Corp: Johnston, RI, USA, 2009. [Google Scholar]
- Verstraete, T.; Scholl, S. Stability analysis of partitioned methods for predicting conjugate heat transfer. Int. J. Heat Mass Transf. 2016, 101, 852–869. [Google Scholar] [CrossRef]
- Weller, H.G.; Tabor, G.; Jasak, H.; Fureby, C. A tensorial approach to computational continuum mechanics using object-oriented techniques. Comput. Phys. 1998, 12, 620–631. [Google Scholar] [CrossRef]
- Kurul, N.; Podowski, M. Multidimensional effects in forced convection subcooled boiling. In Proceedings of the Ninth International Heat Transfer Conference, Jerusalem, Israel, 19–24 August 1990; Volume 2, pp. 21–26. [Google Scholar]
- Peltola, J.; Pättikangas, T.J.H. Development and validation of a boiling model for OpenFOAM multiphase solver. In Proceedings of the CFD4NRS-4 Conference Proceedings, Daejeon, Korea, 10–12 September 2012. [Google Scholar]
- Jayatilleke, C.L.V. The Influence of Prandtl Number and Surface Roughness on the Resistance of the Laminar Sub-Layer to Momentum and Heat Transfer. Ph.D. Thesis, Imperial College of Science and Technology, London, UK, 1966. [Google Scholar]
- Lahey, R.T. The simulation of multidimensional multiphase flows. Nucl. Eng. Des. 2005, 235, 1043–1060. [Google Scholar] [CrossRef]
- Behzadi, A.; Issa, R.; Rusche, H. Modelling of dispersed bubble and droplet flow at high phase fractions. Chem. Eng. Sci. 2004, 59, 759–770. [Google Scholar] [CrossRef]
- Yeoh, G.H. Computational Techniques for Multi-Phase Flows: Basics and Applications, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2010. [Google Scholar]
- Launder, B.E.; Spalding, D.B. The numerical computation of turbulent flows. Comput. Methods Appl. Mech. Eng. 1974, 3, 269–289. [Google Scholar] [CrossRef]
- Weller, H.G. Derivation, Modelling and Solution of the Conditionally Averaged Two-Phase Flow Equations; Technical Report; OpenCFD Limited: Bracknell, UK, 2002. [Google Scholar]
- Rusche, H. Computational Fluid Dynamics of Dispersed TwoPhase Flows at High Phase Fractions. Ph.D. Thesis, Imperial College London, London, UK, 2002. [Google Scholar]
- Alali, A.; Schöffel, P.J.; Herb, J.; Macian, R. Numerical investigations on the coupling of the one-group interfacial area transport equation and subcooled boiling models for nuclear safety applications. Ann. Nucl. Energy 2018, 120, 155–168. [Google Scholar] [CrossRef]
- Baglietto, E.; Demarly, E.; Kommajosyula, R.; Lubchenko, N.; Magolan, B.; Sugrue, R. A Second Generation Multiphase-CFD Framework Toward Predictive modelling of DNB. Nucl. Technol. 2019, 205, 1–22. [Google Scholar] [CrossRef]
- Tu, J.; Yeoh, G. On numerical modelling of low-pressure subcooled boiling flows. Int. J. Heat Mass Transf. 2002, 45, 1197–1209. [Google Scholar] [CrossRef]
- Mamoru, I.; Seungjin, K.; Joseph, K. Development of Interfacial Area Transport Equation. Nucl. Eng. Technol. 2005, 37, 525–536. [Google Scholar]
- Ishii, M.; Zuber, N. Drag coefficient and relative velocity in bubbly, droplet or particulate flows. AIChE J. 1979, 25, 843–855. [Google Scholar] [CrossRef]
- Tomiyama, A.; Tamai, H.; Zun, I.; Hosokawa, S. Transverse migration of single bubbles in simple shear flows. Chem. Eng. Sci. 2002, 57, 1849–1858. [Google Scholar] [CrossRef]
- Antal, S.; Lahey, R.; Flaherty, J. Analysis of phase distribution in fully developed laminar bubbly two-phase flow. Int. J. Multiph. Flow 1991, 17, 635–652. [Google Scholar] [CrossRef]
- Burns, A.; Frank, T.; Hamill, I.; Shi, J. The Favre Averaged Drag Model for Turbulent Dispersion in Eulerian Multi-Phase Flows. In Proceedings of the 5th International Conference on Multiphase Flow, ICMF’04, Yokohama, Japan, 30 May–4 June 2004; Volume 392, pp. 1–17. [Google Scholar]
- Otromke, M. Implementation and Comparison of Correlations for interfacial Forces in a Gas-Liquid System within an Euler-Euler Framework Computational Fluid Dynamics of Dispersed Two-Phase Flows at High Phase Fractions. Ph.D. Thesis, Mannheim University of Applied Sciences, Mannheim, Germany, 2013. [Google Scholar]
- Ranz, W.E.; Marshall, W.R. Evaporation from Drops, Parts I and IId. Chem. Eng. Prog. 1952, 48, 141–146. [Google Scholar]
- Van der Vorst, H.A. Bi-CGSTAB: A Fast and Smoothly Converging Variant of Bi-CG for the Solution of Nonsymmetric Linear Systems. SIAM J. Sci. Stat. Comput. 1992, 13, 631–644. [Google Scholar] [CrossRef]
- Barrett, R.; Berry, M.; Chan, T.F.; Demmel, J.; Donato, J.; Dongarra, J.; Eijkhout, V.; Pozo, R.; Romine, C.; der Vorst, H.V. Templates for the Solution of Linear Systems: Building Blocks for Iterative Methods, 2nd ed.; SIAM: Philadelphia, PA, USA, 1994. [Google Scholar]
- Behrens, T. OpenFOAM’s Basic Solvers for Linear Systems of Equations Solvers, Preconditioners, Smoothers; Technical Report; Technical University of Denmark: Kgs. Lyngby, Denmark, 2002. [Google Scholar]
- Márquez Damián, S. An Extended Mixture Model for the Simultaneous Treatment of Short and Long Scale Interfaces. Ph.D. Thesis, Universidad Nacional Del Litoral, Santa Fe, Argentina, 2013. [Google Scholar] [CrossRef]
- van Leer, B. Towards the ultimate conservative difference scheme. II. Monotonicity and conservation combined in a second-order scheme. J. Comput. Phys. 1974, 14, 361–370. [Google Scholar] [CrossRef]
- Končar, B.; Krepper, E.; Egorov, Y. CFD modelling of subcooled flow boiling for nuclear engineering applications. In Proceedings of the International Conference Nuclear Energy for New Europe 2005, Bled, Slovenia, 5–8 September 2005. [Google Scholar]
- Schroeder-Richter, D.; Bartsch, G. Analytical calculation of DNB-superheating by a postulated thermo-mechanical effect of nucleate boiling. Int. J. Multiph. Flow 1994, 20, 1143–1167. [Google Scholar] [CrossRef]
- Bromley, L.A. Heat Transfer in Stable Film Boiling; Technical Report; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 1949.
- Zuber, N. On the Stability of Boiling Heat Transfer; Technical Report; United States Atomic Energy Commission: Washington, DC, USA, 1958.
- Hua, T.; Xu, J. Quenching boiling in subcooled liquid nitrogen for solidification of aqueous materials. Mater. Sci. Eng. A 2000, 292, 169–172. [Google Scholar] [CrossRef]
- Tolubinsky, V.I.; Kostanchuk, D.M. Vapour bubbles growth rate and heat transfer intensity at subcooled water boiling. In Proceedings of the International Heat Transfer Conference 4, Paris-Versailles, France, 31 August–5 September 1970. [Google Scholar]
- Lemmert, M.; Chawla, J.M. Influence of flow velocity on surface boiling heat transfer coefficient. Heat Transf. Boil. 1977, 237, 111–116. [Google Scholar]
- Egorov, Y.; Menter, F. Experimental Implementation of the RPI Wall Boiling Model in CFX-5.6; Technical Report; ANSYS Gmbh: Darmstadt, Germany, 2004. [Google Scholar]
- Bowring, R.W. Physical Model, Based on Bubble Detachment, and Calculation of Steam Voidage in the Sub-Cooled Region of a Heated Channel; Technical Report; Institutt for Atomenergi: Kjeller, Norway, 1962. [Google Scholar]
- Cole, R. A photographic study of pool boiling in the region of the critical heat flux. AIChE J. 1960, 6, 533–538. [Google Scholar] [CrossRef]
- Michaelides, E.E.; Crowe, C.T.; Schwarzkopf, J.D. Multiphase Flow Handbook; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Schlichting, H.; Gersten, K. Boundary-Layer Theory, 9th ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]




| Dispersed Vapour | Continuous Vapour | |
|---|---|---|
| Drag | Ishii & Zuber [36] | Ishii & Zuber [36] |
| Lift | Tomiyama [37] | - |
| Wall Lubrication | Antal [38] | - |
| Virtual Mass | Constant | Constant |
| Turbulent Disp. | Burns [39], Otromke [40] | - |
| Nu (vapour side) | Spherical | Ranz & Marshall [41] |
| Nu (liquid side) | Ranz & Marshall [41] | Spherical |
| Variable | Numerical Solver | |
|---|---|---|
| Solid | h | PCG, DIC |
| Fluid | , k, | PBiCGStab, DILU GAMG, GaussSiedel MULES |
| Term | Numerical Schemes | |
|---|---|---|
| Solid | ddt grad div laplacian | Euler linear linear corrected |
| Fluid | ddt grad div div , h, k, , K laplacian | Euler linear vanLeer upwind linear corrected |
| Vapour | Convective h. t. | Convective h. t. | Convective h. t. | Film b. |
| Liquid | Convective h. t. | Nucleate b. | Transition b. | Film b. |
| [W/(mK)] | [J/(kgK)] | [kg/m] | [Pa s] | |
|---|---|---|---|---|
| solid | 11.4 | 435 | 8000 | - |
| liquid | 0.6 | 4185 | 1000 | 0.001 |
| vapour | 0.025 | 2010 | 0.000012 |
| [C] | [C] | [C] | ||
|---|---|---|---|---|
| wall | noSlip | 25 | 25 | - |
| outlet | - | |||
| outlet | 25 | 25 | - | |
| fluid_to_solid | noSlip | Section 3.2 | - | |
| solid_to_fluid | - | - | - | Section 3.2 |
| No el. | No el. x-y-z | [mm] | |
|---|---|---|---|
| Fluid | 27,950 40,842 57,094 123,800 219,475 278,625 367,350 422,275 | 35-35-23 39-39-27 43-43-31 55-55-41 65-65-52 65-65-66 65-65-87 65-65-100 | 20.0
15.0 10.0 5.0 3.1 1.5 0.375 0.1875 |
| Solid | 900 | 15-15-4 | 0.375 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamenicky, R.; Frank, M.; Drikakis, D.; Ritos, K. Film Boiling Conjugate Heat Transfer during Immersion Quenching. Energies 2022, 15, 4258. https://doi.org/10.3390/en15124258
Kamenicky R, Frank M, Drikakis D, Ritos K. Film Boiling Conjugate Heat Transfer during Immersion Quenching. Energies. 2022; 15(12):4258. https://doi.org/10.3390/en15124258
Chicago/Turabian StyleKamenicky, Robin, Michael Frank, Dimitris Drikakis, and Konstantinos Ritos. 2022. "Film Boiling Conjugate Heat Transfer during Immersion Quenching" Energies 15, no. 12: 4258. https://doi.org/10.3390/en15124258
APA StyleKamenicky, R., Frank, M., Drikakis, D., & Ritos, K. (2022). Film Boiling Conjugate Heat Transfer during Immersion Quenching. Energies, 15(12), 4258. https://doi.org/10.3390/en15124258








