Probing the Coalescence Mechanism of Oil Droplets in Fluids Produced by Oil Wells and the Microscopic Interaction between Molecules in Oil Films
Abstract
:1. Introduction
2. Experiments and Models
2.1. Theoretical Part
2.1.1. Fluid Flow Equation in the Process of Coalescence of Oil Droplets
- (1)
- The system was not affected by gravity during the coalescence of oil droplets.
- (2)
- During the coalescence of oil droplet, the water phase in the system was stable, and flow phenomenon did not occur.
- (3)
- The variation of viscosity with temperature during the coalescence of oil droplet was ignored.
- (4)
- The influence of the components of crude oil on coalescence was ignored.
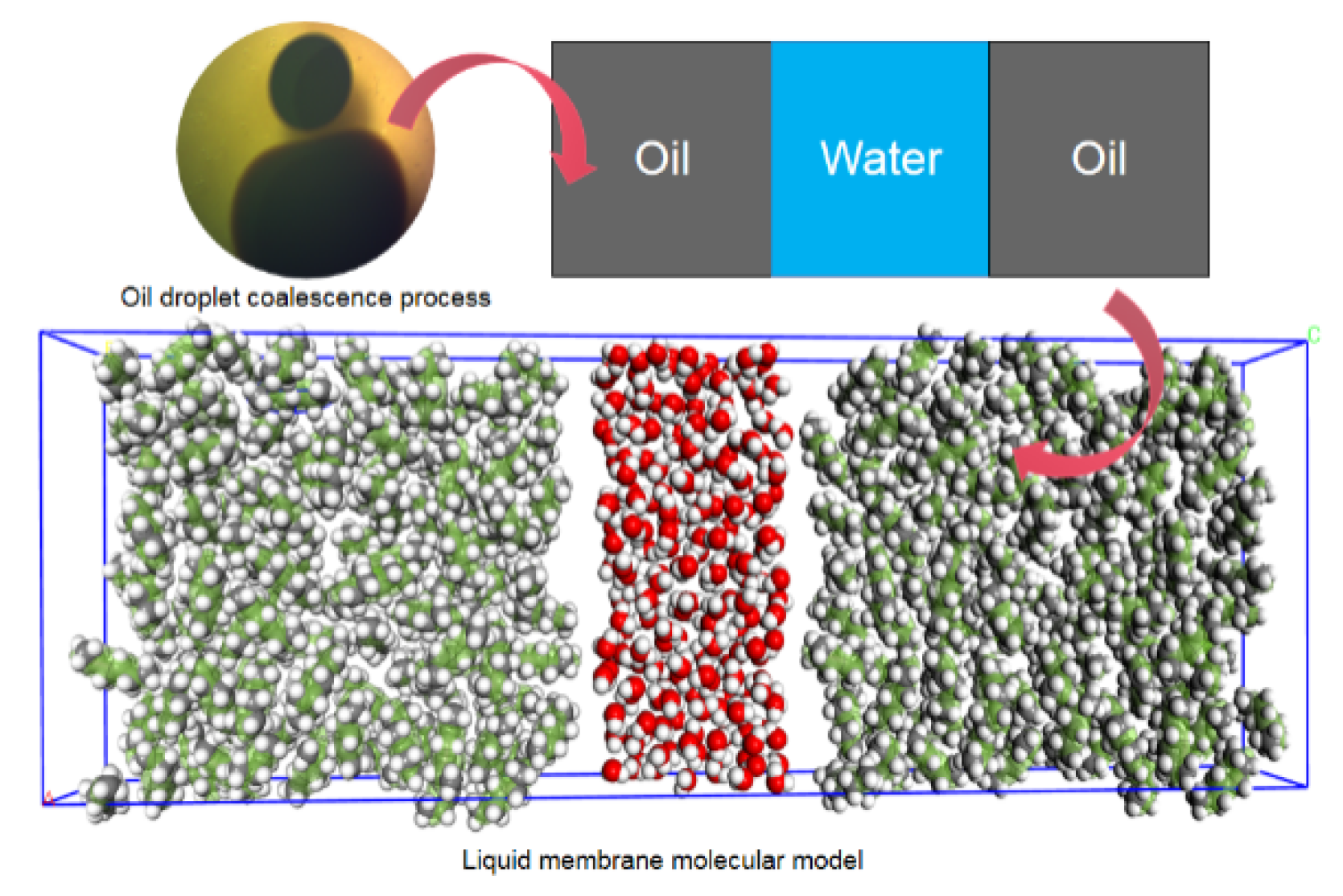
2.1.2. Molecular Simulation Model of Oil Droplet Coalescence
2.2. Experimental PART
2.2.1. Experimental Materials
2.2.2. Experiment on Composition Measurement of Simulated Oil Group
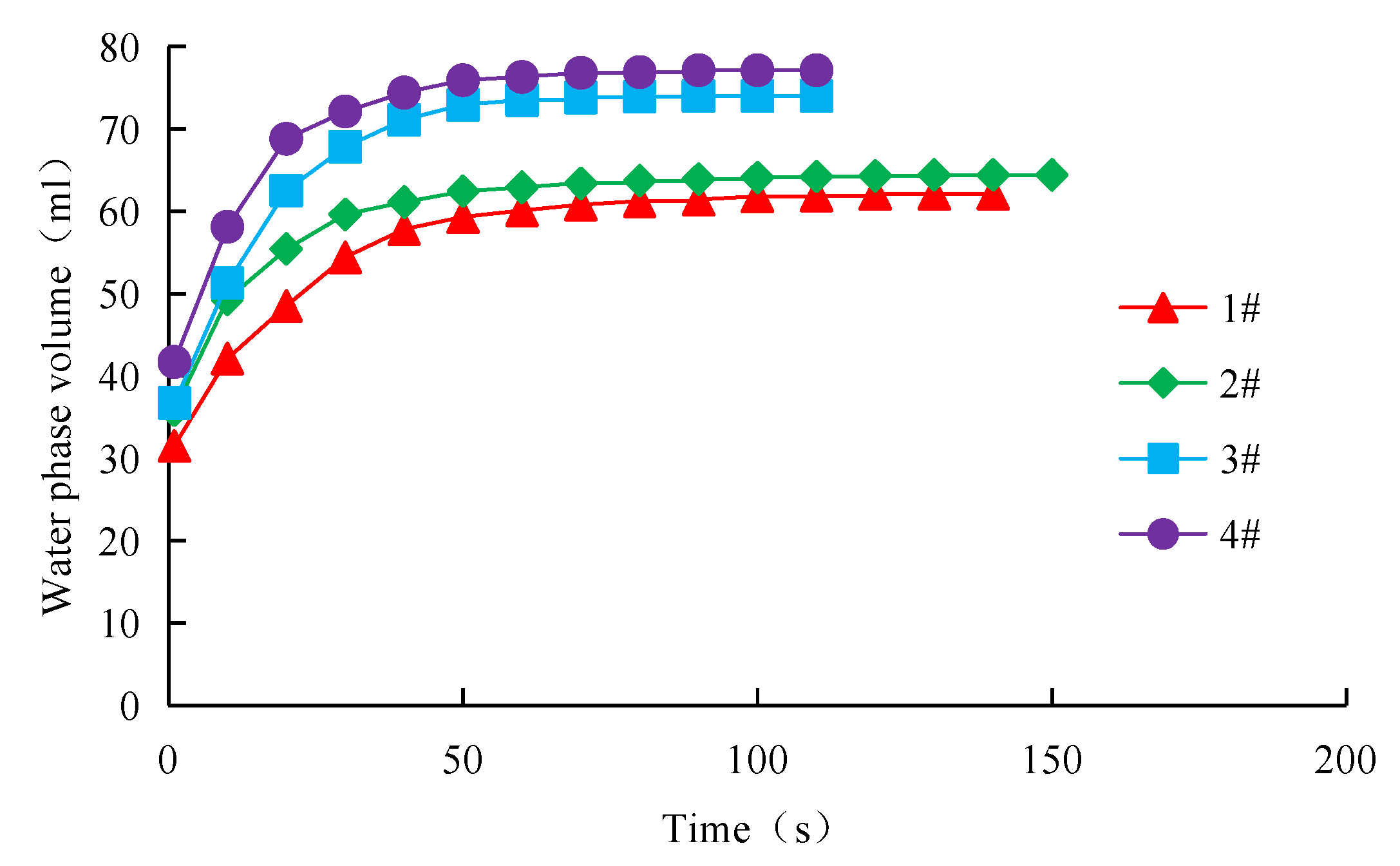
2.2.3. Experiment of Emulsion Stability Evaluation
3. Result Analysis
3.1. Microscopic Characteristics of Oil Droplets Coalescence Process
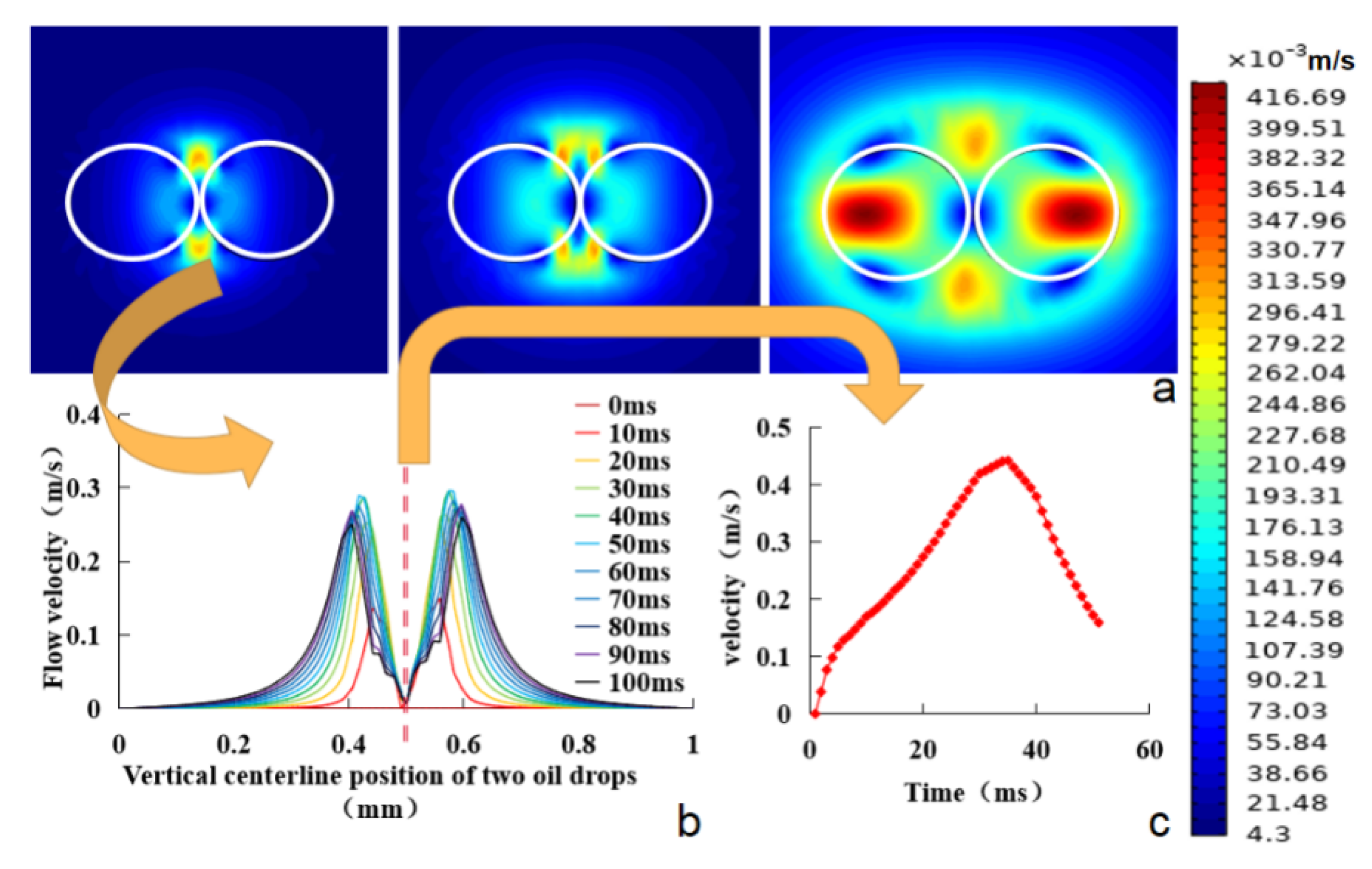
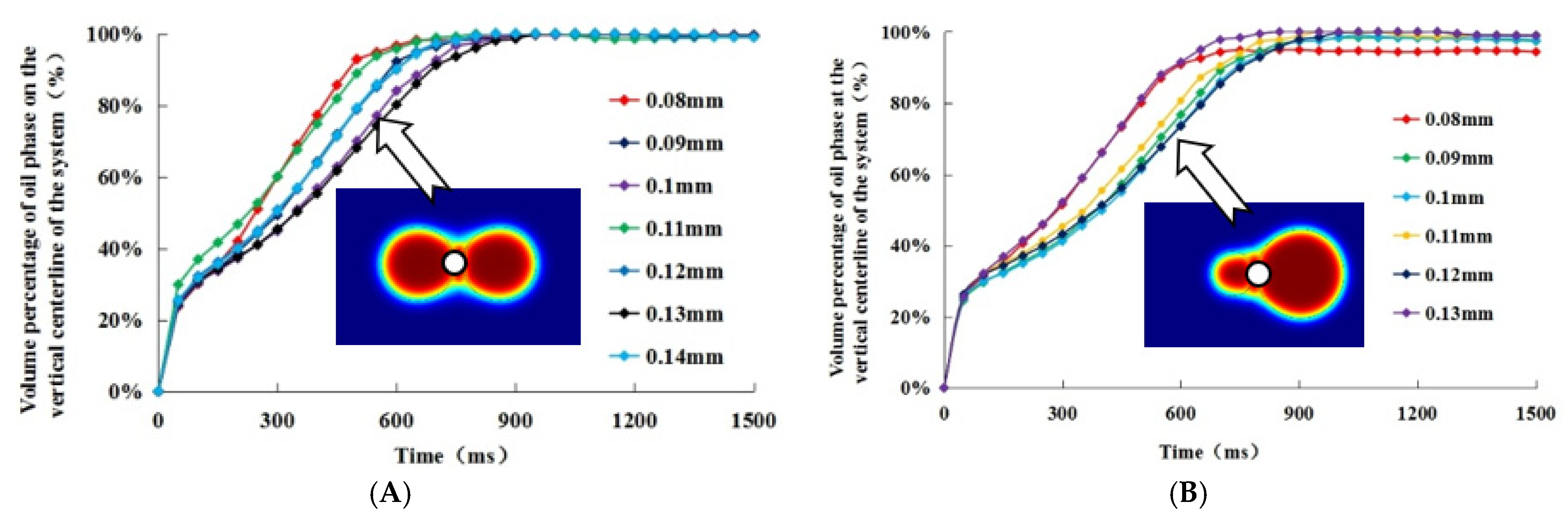
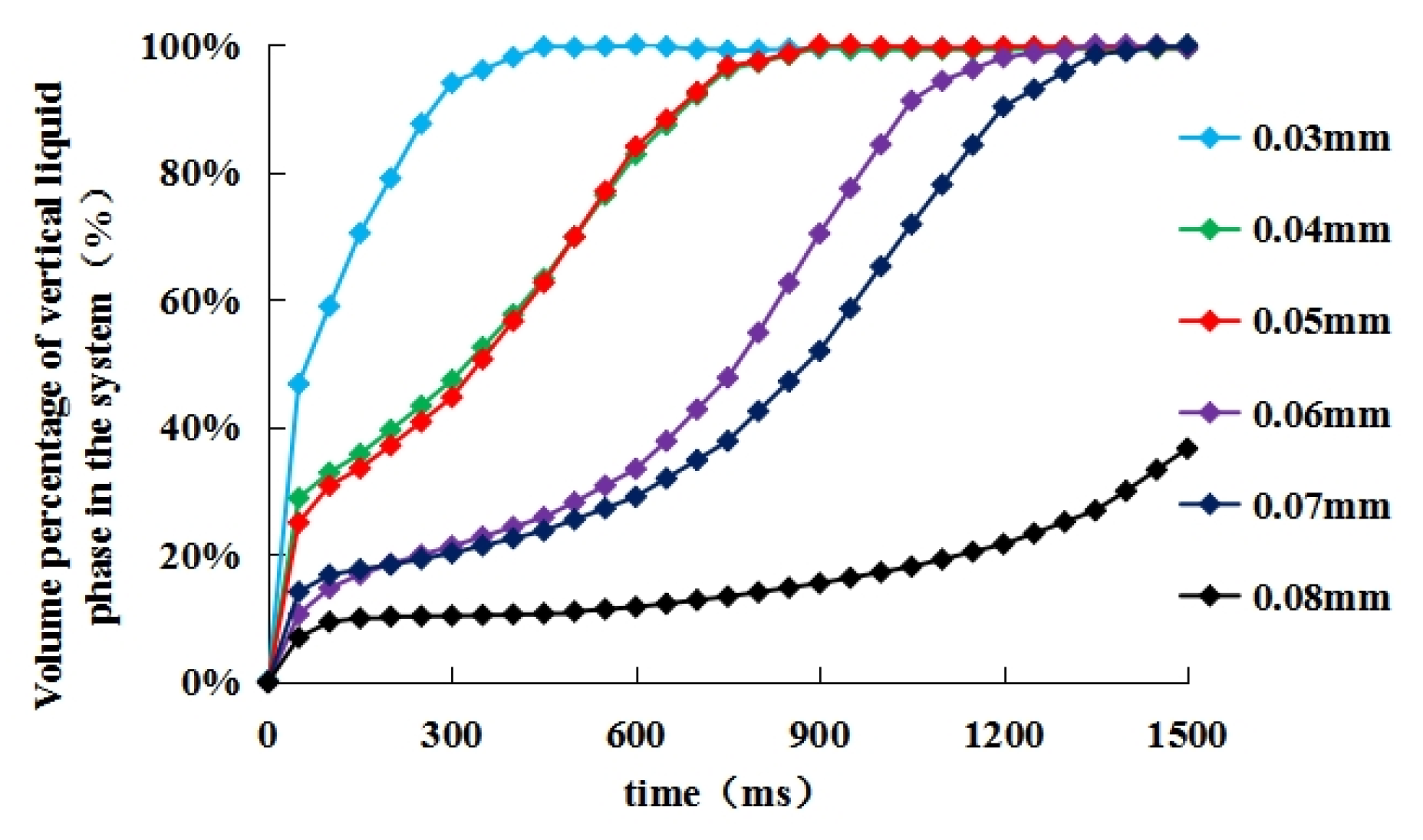
3.2. Effects of Droplet Size and Distance between Two Droplets on the Efficiency of Oil Droplet Coalescence
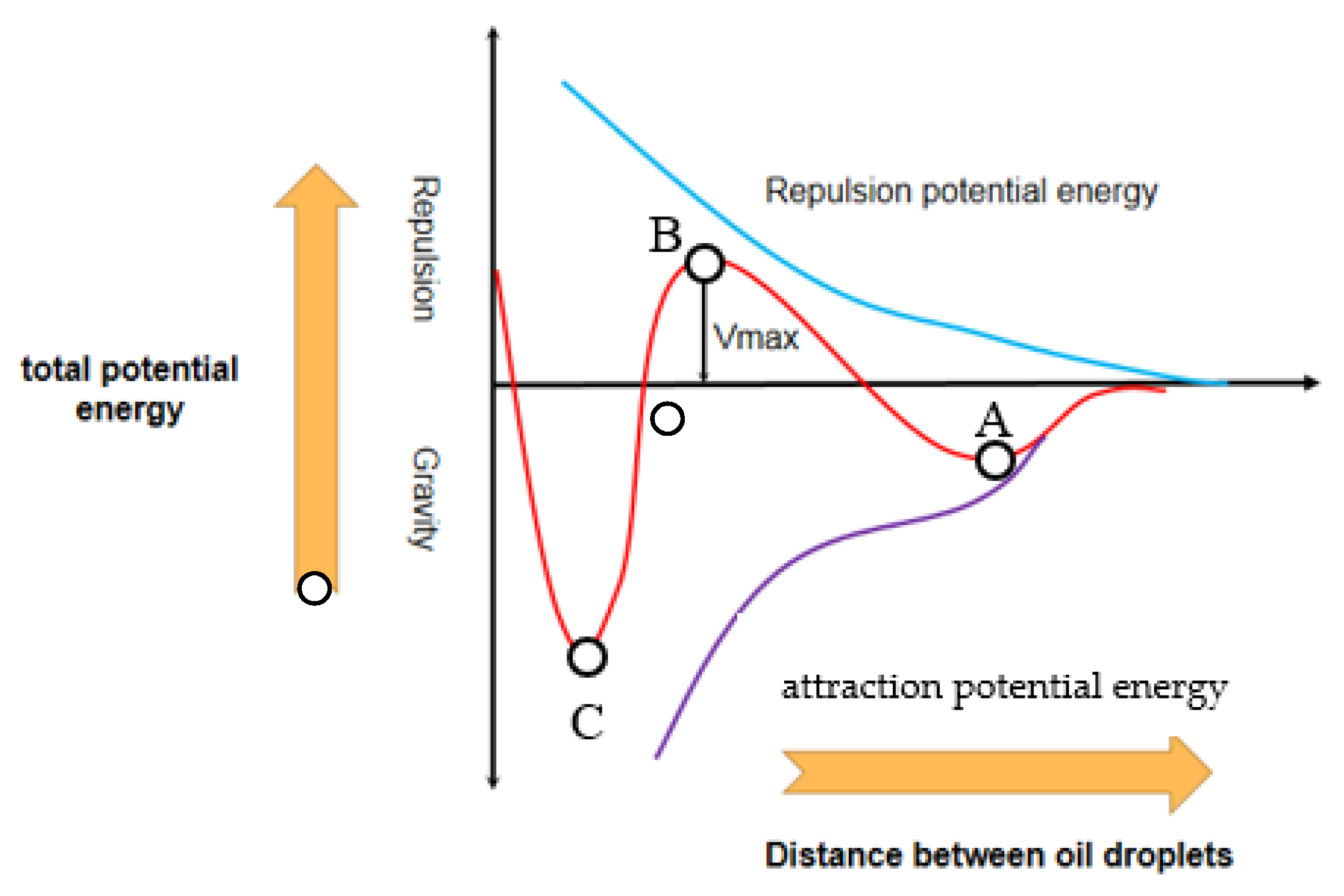
3.3. Micro-Motion Characteristics of Oil Film Position Molecules during the Coalescence of Oil Droplet
4. Conclusions
- (1)
- In the coalescence of oil droplets, the fluid velocity at the vertical position between the two emulsified oil droplets showed a gradually increasing trend. The fluid velocity at the position where two oil droplets were close to each other was relatively higher than other positions, and a relative movement trend was generated at the same time. Finally, the coalescence was completed at the position of the maximum fluid velocity. When the coalescence was completed, the fluid velocity in the system decreased gradually and finally reached a stable state.
- (2)
- There was no obvious correspondence between different oil droplet sizes and coalescence time due to the combined effect of the attraction potential energy and the repulsive potential energy under different distance between the center of the two oil droplets. However, this does not mean the droplet size had no effect on the coalescence time. When the radius of two oil droplets was same, the two oil droplets moved towards the middle at the same time and gathered together at the vertical position to form a new large oil droplet. When the radius of the two oil droplets was different, the coalescence of two droplets did not occur at the middle position between the two oil droplets. It was obvious that there was a significant migration from the relatively small oil droplets to the larger ones. The coalescence of the two oil droplets could be seen as large oil droplets attracted small oil droplets to move towards themselves, so as to coalescence into larger oil droplets.
- (3)
- At the initial time, the molecules in the position of oil film were relatively stable, and there was a clear boundary between the oil film and the water phase. During the coalescence of oil droplets, the oil film pulled, oil film in the middle moved towards each other, and the oil film on the upper and lower sides moved outwards, and the boundary between the oil film and the water phase was gradually blurred. With an increase in the carbon chain length of oil droplets, the emulsion system was more stable.
- (4)
- Under the condition of the same number of carbon atoms, a more complex molecular structure of carbon chain increased the space occupied by water molecules between the oil films. This means that a more complex molecular structure of crude oil in the emulsion system increased the strength of the liquid film between oil droplets, and increased the stability in the emulsion system. Among the following four crude oil molecules with the same number of carbon atoms, chain-like saturated hydrocarbons were the most stable, followed by chain-like carbon–carbon double bonds in component crude oil. Crude oils containing chain-like carbon–carbon triple bonds were the third most stable. Cyclanes were the least stable. An increase in the asphaltene content was an important reason for the enhancement of emulsifying ability and stability in the emulsion system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Y.; Cao, G.; Nan, X.; Li, S.; An, H.; Wang, X.; Wang, Z. Effect of sodium fluosilicate particles in acidification flowback fluid on emulsification stability of crude oil. J. Pet. Sci. Eng. 2021, 202, 108484. [Google Scholar] [CrossRef]
- Khurana, E.; DeVane, R.H.; Kohlmeyer, A.; Klein, M.L. Probing peptide nanotube self-assembly at a liquid−liquid interface with coarse-grained molecular dynamics. Nano Lett. 2008, 8, 3626–3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarts, D.G.; Lekkerkerker, H.N.; Guo, H.; Wegdam, G.H.; Bonn, D. Hydrodynamics of droplet coalescence. Phys. Rev. Lett. 2005, 95, 164503. [Google Scholar] [CrossRef]
- Chen, D.; Cardinaels, R.; Moldenaers, P. Effect of confinement on droplet coalescence in shear flow. Langmuir 2009, 25, 12885–12893. [Google Scholar] [CrossRef] [PubMed]
- Rayhani, M.; Simjoo, M.; Chahardowli, M. Effect of water chemistry on the stability of water-in-crude oil emulsion: Role of aqueous ions and underlying mechanisms. J. Pet. Sci. Eng. 2022, 211, 110123. [Google Scholar] [CrossRef]
- Shardt, O.; Derksen, J.J.; Mitra, S.K. Simulations of droplet coalescence in simple shear flow. Langmuir 2013, 29, 6201–6212. [Google Scholar] [CrossRef]
- Zhao, L.; Torlapati, J.; Boufadel, M.C.; King, T.; Robinson, B.; Lee, K. VDROP: A comprehensive model for droplet formation of oils and gases in liquids—Incorporation of the interfacial tension and droplet viscosity. Chem. Eng. J. 2014, 253, 93–106. [Google Scholar] [CrossRef]
- Angardi, V.; Ettehadi, A.; Yücel, Ö. Critical review of emulsion stability and characterization techniques in oil processing. J. Energy Resour. Technol. 2021, 144, 040801. [Google Scholar] [CrossRef]
- Pope, K.; Duan, X. Droplet Coalescence in Liquid/Liquid Separation. J. Fluids Eng. 2020, 142, 111402. [Google Scholar]
- Cui, H.; Cao, G.; Zhu, S.; Mu, J.; Chou, X. Study on the preparation and formation factors of frother emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128155. [Google Scholar] [CrossRef]
- Laben, A.B.; Kayiem, H.H.A.; Alameen, M.A.; Khan, J.A.; Belhaj, A.F.; Elraies, K.A. Experimental study on the performance of emulsions produced during ASP flooding. J. Pet. Explor. Prod. Technol. 2021, 179, 841–854. [Google Scholar] [CrossRef]
- Kizling, J.; Kronberg, B. On the formation of concentrated stable w/o emulsions. Adv. Colloid Interface Sci. 2001, 89–90, 395–399. [Google Scholar] [CrossRef]
- Zhapbasbayev, U.K.; Kudaibergenov, S.E.; Mankhanova, A.E.; Sadykov, R.M. Experimental study of alkaline-surfactant-polymer compositions for ASP-flooding of cores from highly viscous oil reservoirs. Thermophys. Aeromech. 2018, 25, 909–916. [Google Scholar] [CrossRef]
- Kirkpatrick, R.; Lockett, M. The influence of approach velocity on bubble coalescence. Chem. Eng. Sci. 1974, 29, 2363–2373. [Google Scholar] [CrossRef]
- Bai, Y.; Cao, G.; An, H.; Zhang, H. Generation laws and distribution characteristics of carbon dioxide hydrate in a reaction kettle. Exp. Therm. Fluid Sci. 2020, 116, 110125. [Google Scholar] [CrossRef]
- Xu, B.; Kang, W.; Wang, X.; Meng, L. Influence of Water Content and Temperature on Stability of W/O Crude Oil Emulsion. Pet. Sci. Technol. 2013, 31, 1099–1108. [Google Scholar] [CrossRef]
- Huang, B.; Nan, X.; Fu, C.; Guo, T. Study of the bubble collapse mechanism and its influencing factors on stability under ultra-low surface tension. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126440. [Google Scholar] [CrossRef]
- Gao, S.; Chen, S.; Pu, H.; Gong, L.; Ma, S.; Luo, Q.; Wang, X.; Gao, A.; Zhang, B. Fine characterization of large composite channel sandbody architecture and its control on remaining oil distribution: A case study of alkaline—surfactant—polymer (ASP) flooding test area in Xingshugang oilfield, China. J. Pet. Sci. Eng. 2019, 175, 363–374. [Google Scholar] [CrossRef]
- An, H.; Cao, G.; Bai, Y.; Wang, D.; Meng, F. Study on the stability of emulsion based on molecular dynamics. J. Dispers. Sci. Technol. 2021, 42, 1723–1732. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Zhou, F.; Song, Z.; Liang, X. Study on reservoir damage during acidizing for high-temperature and ultra-deep tight sandstone. J. Pet. Sci. Eng. 2020, 191, 107231. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, G.; Zuo, J.; Bai, Y. Rheological properties and plugging behavior of active crude oil. Pet. Sci. Technol. 2020, 38, 131–145. [Google Scholar] [CrossRef]
- Kong, X.; Wang, J.; Liang, Z.; Zhang, L.; Zhao, J. Design, Synthesis and Characterization of Bitumen Emulsifiers Based on Molecular Simulation. Kem. Ind. Časopis Kemičara Kem. Inženjera Hrvat. 2019, 68, 1–6. [Google Scholar] [CrossRef]
- Cao, G.; Cheng, Q.; Liu, Y.; Bu, R.; Zhang, N.; Wang, P. Influencing Factors of Surfactant Stripping Crude Oil and Spontaneous Imbibition Mechanism of Surfactants in a Tight Reservoir. ACS Omega 2022, 7, 19010–19020. [Google Scholar] [CrossRef]
- Boyle, E.J.; Scriven, L.; Davis, H.T. The generalized van der Waals theory applied to the electrical double layer. J. Chem. Phys. 1987, 86, 2309–2318. [Google Scholar] [CrossRef]
- Fingas, M.; Fieldhouse, B. Studies on crude oil and petroleum product emulsions: Water resolution and rheology. Colloids Surf. A Physicochem. Eng. Asp. 2009, 333, 67–81. [Google Scholar] [CrossRef]
- Neto, D.M.C.; Sad, C.M.S.; Silva, M.; Santos, F.D.; Pereira, L.B.; Corona, R.R.B.; Silva, S.R.C.; Bassane, J.F.P.; Castro, E.V.R.; Filgueiras, P.R.; et al. Rheological study of the behavior of water-in-oil emulsions of heavy oils. J. Pet. Sci. Eng. 2019, 173, 1323–1331. [Google Scholar] [CrossRef]






| Number | Project | Parameter | Number | Project | Parameter |
|---|---|---|---|---|---|
| 1 | Oil drop radius | 0.08–0.14 mm | 7 | Oil droplet spacing | 0.03–0.08 mm |
| 2 | Oil drop density | 0.8 × 103 kg/m3 | 8 | Oil phase temperature | 293.15–353.15 K |
| 3 | Water phase density | 103 kg/m3 | 9 | Water phase temperature | 358.15–453.15 K |
| 4 | Interfacial tension | 7 × 10−2 mN/m | 10 | System size | 1 mm × 1 mm |
| 5 | Oil phase viscosity | 1–1000 mPa·s | 11 | Simulation time | 1500 ms |
| 6 | Water phase viscosity | 0.5–500 mPa·s | 12 | Time Step | 1 ms |
| Sample Number | Kerosene | White Oil | Crude Oil from Liaohe Oilfield | Crude Oil from Daqing Oilfield | Crude Oil from Xinjiang Oilfield |
|---|---|---|---|---|---|
| 1# | 1 | 1 | 0 | 1 | 0 |
| 2# | 2 | 1 | 0 | 1 | 0 |
| 3# | 3 | 1 | 0 | 1 | 0 |
| 4# | 4 | 1 | 0 | 1 | 0 |
| 5# | 1 | 2 | 0 | 1 | 0 |
| 6# | 1 | 3 | 0 | 1 | 0 |
| 7# | 1 | 4 | 0 | 1 | 0 |
| 8# | 1 | 1 | 1 | 0 | 0 |
| 9# | 1 | 1 | 0 | 0 | 1 |
| Sample Number | Saturated Hydrocarbon (%) | Aromatic Hydrocarbon (%) | Resin (%) | Asphaltene (%) |
|---|---|---|---|---|
| 1# | 53.1 | 25.8 | 8.6 | 12.5 |
| 2# | 68.8 | 16.7 | 6.2 | 8.3 |
| 3# | 74.2 | 14.3 | 5.4 | 6.1 |
| 4# | 78.4 | 12.7 | 3.8 | 5.1 |
| 5# | 58.4 | 27.1 | 6.1 | 8.4 |
| 6# | 58.7 | 29.9 | 5.2 | 6.2 |
| 7# | 59 | 32.2 | 3.7 | 5.1 |
| 8# | 44.7 | 26.4 | 8.7 | 20.2 |
| 9# | 49.6 | 27.8 | 15.8 | 6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Nan, X.; Fu, C.; Liu, W.; Guo, W.; Wang, S.; Zhang, L. Probing the Coalescence Mechanism of Oil Droplets in Fluids Produced by Oil Wells and the Microscopic Interaction between Molecules in Oil Films. Energies 2022, 15, 4274. https://doi.org/10.3390/en15124274
Huang B, Nan X, Fu C, Liu W, Guo W, Wang S, Zhang L. Probing the Coalescence Mechanism of Oil Droplets in Fluids Produced by Oil Wells and the Microscopic Interaction between Molecules in Oil Films. Energies. 2022; 15(12):4274. https://doi.org/10.3390/en15124274
Chicago/Turabian StyleHuang, Bin, Xiaohan Nan, Cheng Fu, Weibo Liu, Wei Guo, Siqi Wang, and Lu Zhang. 2022. "Probing the Coalescence Mechanism of Oil Droplets in Fluids Produced by Oil Wells and the Microscopic Interaction between Molecules in Oil Films" Energies 15, no. 12: 4274. https://doi.org/10.3390/en15124274





