Investigation of Oil and Facility Characteristics of Plastic Waste Pyrolysis for the Advanced Waste Recycling Policy
Abstract
:1. Introduction
2. Experimental Section
2.1. Pyrolysis Facilities Using Plastic Waste
2.2. Sample Preparation and Analysis
2.2.1. Feedstock and Pyrolysis Residue
2.2.2. Pyrolysis Oil
2.2.3. Pyrolysis Gas and Air Pollutants
3. Results
3.1. Characteristics of Feedstock and Pyrolysis Products
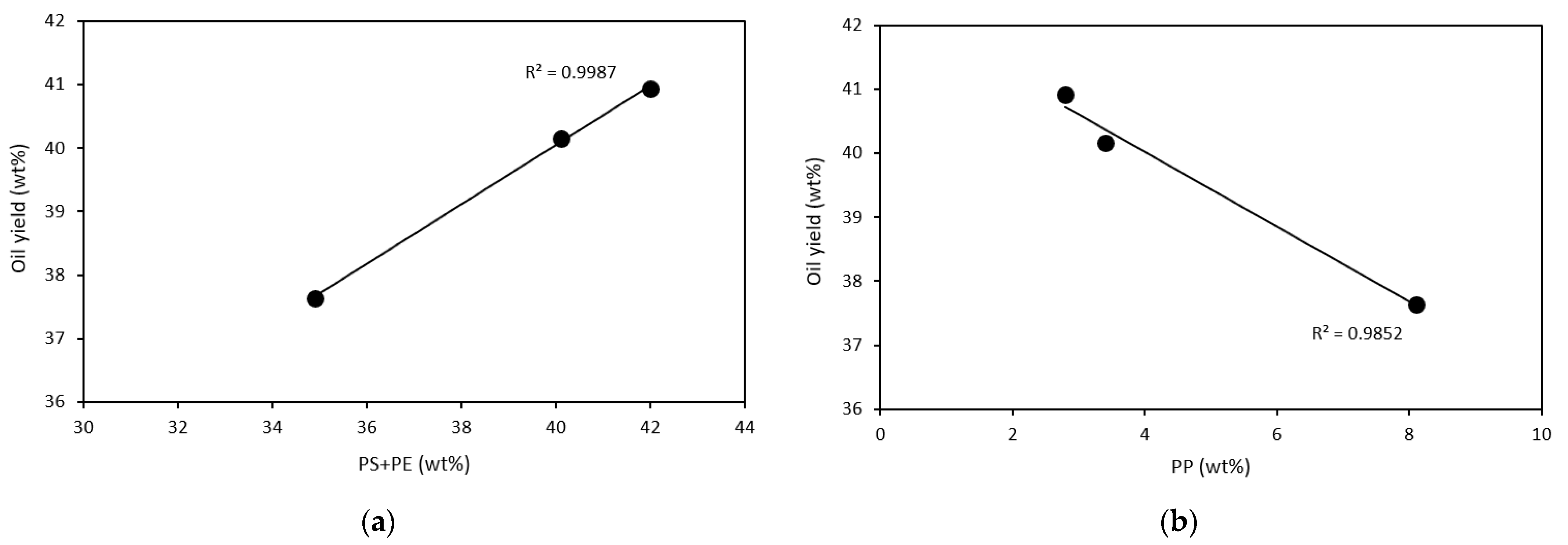
3.2. Material Balance from Pyrolysis Process
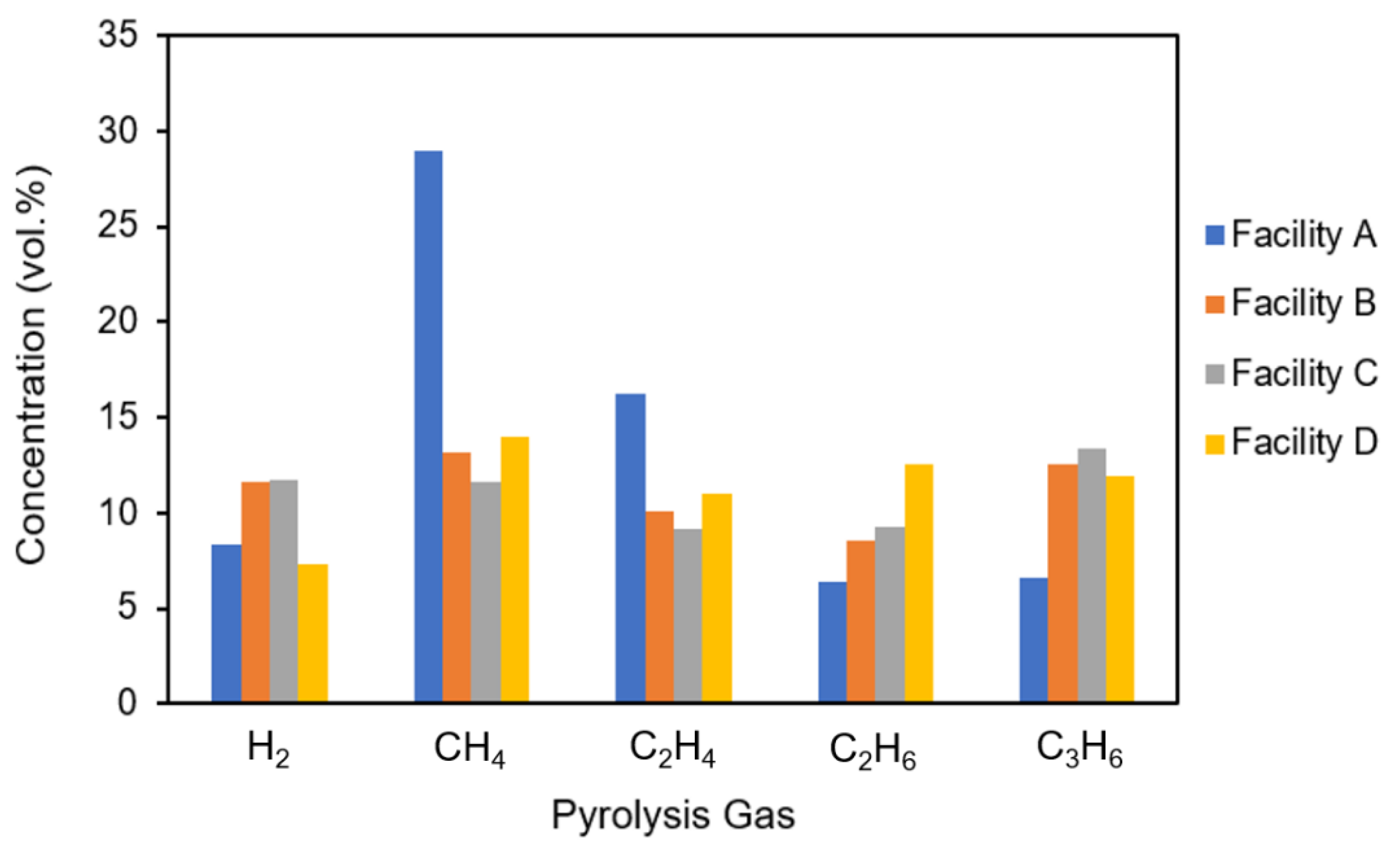
3.3. Air Pollutant Emissions from Pyrolysis Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiseo, I. Global Plastic Production 1950–2020. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 20 April 2022).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SYSTEMIQ. Reshaping Plastics: Pathways to a Circular, Climate Neutral Plastics System in Europe. Available online: https://plasticseurope.org/wp-content/uploads/2022/04/SYSTEMIQ-ReShapingPlastics-April2022.pdf (accessed on 3 May 2022).
- Yoon, J.S. Plastic Waste Recycling Rate South Korea 2008–2019. Available online: https://www.statista.com/statistics/1074985/south-korea-plastic-waste-recycling-rate/ (accessed on 3 May 2022).
- Al-Salem, S.M.; Yang, Y.; Wang, J.; Leeke, G.A. Pyro-oil and wax recovery from reclaimed plastic waste in a continuous auger pyrolysis reactor. Energies 2020, 13, 2040. [Google Scholar] [CrossRef] [Green Version]
- OECD. Improving Plastics Management: Trends, Policy Responses, and the Role of International Co-Operation and Trade. Available online: https://www.oecd.org/environment/waste/policy-highlights-improving-plastics-management.pdf (accessed on 3 May 2022).
- Han, T.U.; Kim, Y.-M.; Watanabe, A.; Teramae, N.; Park, Y.-K.; Kim, S. Pyrolysis kinetic analysis of poly (methyl methacrylate) using evolved gas analysis-mass spectrometry. Korean J. Chem. Eng. 2017, 34, 1214–1221. [Google Scholar] [CrossRef]
- Li, D.; Lei, S.; Wang, P.; Zhong, L.; Ma, W.; Chen, G. Study on the pyrolysis behaviors of mixed waste plastics. Renew. Energy 2021, 173, 662–674. [Google Scholar] [CrossRef]
- Kılıç, M.; Pütün, E.; Pütün, A.E. Optimization of Euphorbia rigida fast pyrolysis conditions by using response surface methodology. J. Anal. Appl. Pyrolysis 2014, 110, 163–171. [Google Scholar] [CrossRef]
- Erdogan, S. Recycling of Waste Plastics into Pyrolytic Fuels and Their Use in IC Engines. Available online: https://www.intechopen.com/chapters/70583 (accessed on 4 May 2022).
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Joung, H.T.; Seo, Y.C.; Kim, K.H.; Hong, J.H.; Yoo, T.W. Distribution and characteristics of pyrolysis products from automobile shredder residue using an experimental semi-batch reactor. Korean J. Chem. Eng. 2007, 24, 996–1002. [Google Scholar] [CrossRef]
- Onay, O.; Kockar, O.M. Slow, fast and flash pyrolysis of rapeseed. Renew. Energy 2003, 28, 2417–2433. [Google Scholar] [CrossRef]
- Palos, R.; Gutiérrez, A.; Vela, F.J.; Olazar, M.; Arandes, J.M.; Bilbao, J. Waste Refinery: The valorization of waste plastics and end-of-life tires in refinery units. A review. Energy Fuels 2021, 35, 3529–3557. [Google Scholar] [CrossRef] [PubMed]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic conversion of plastic waste to value-added products and fuels: A review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic pyrolysis of plastic waste: Moving toward pyrolysis based biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Fulgencio-Medrano, L.; García-Fernández, S.; Asueta, A.; Lopez-Urionabarrenechea, A.; Perez-Martinez, B.B.; Arandes, J.M. Oil production by pyrolysis of real plastic waste. Polymers 2022, 14, 553. [Google Scholar] [CrossRef] [PubMed]
- Olalo, J.A. Pyrolytic oil yield from waste plastic in quezon city, Philippines: Optimization using response surface methodology. Int. J. Renew. Energy Dev. 2022, 11, 325–332. [Google Scholar] [CrossRef]
- Kiran, N.; Ekinci, E.; Snape, C.E. Recycling of plastic wastes via pyrolysis. Resour. Conserv. Recycl. 2000, 29, 273–283. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.M.I.; Nizami, A.S. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Almohamadi, H.; Alamoudi, M.; Ahmed, U.; Shamsuddin, R.; Smith, K. Producing hydrocarbon fuel from the plastic waste: Techno-economic analysis. Korean J. Chem. Eng. 2021, 38, 2208–2216. [Google Scholar] [CrossRef]
- Son, J.-I.; Lee, S.-J.; Park, S.-I.; Kwon, E.-H.; Namkung, H.; Kang, J.-G.; Lee, W. Emission characteristics of polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDD/DFs) in commercial bio-SRF and SRF incineration plants. Energies 2022, 15, 2787. [Google Scholar] [CrossRef]

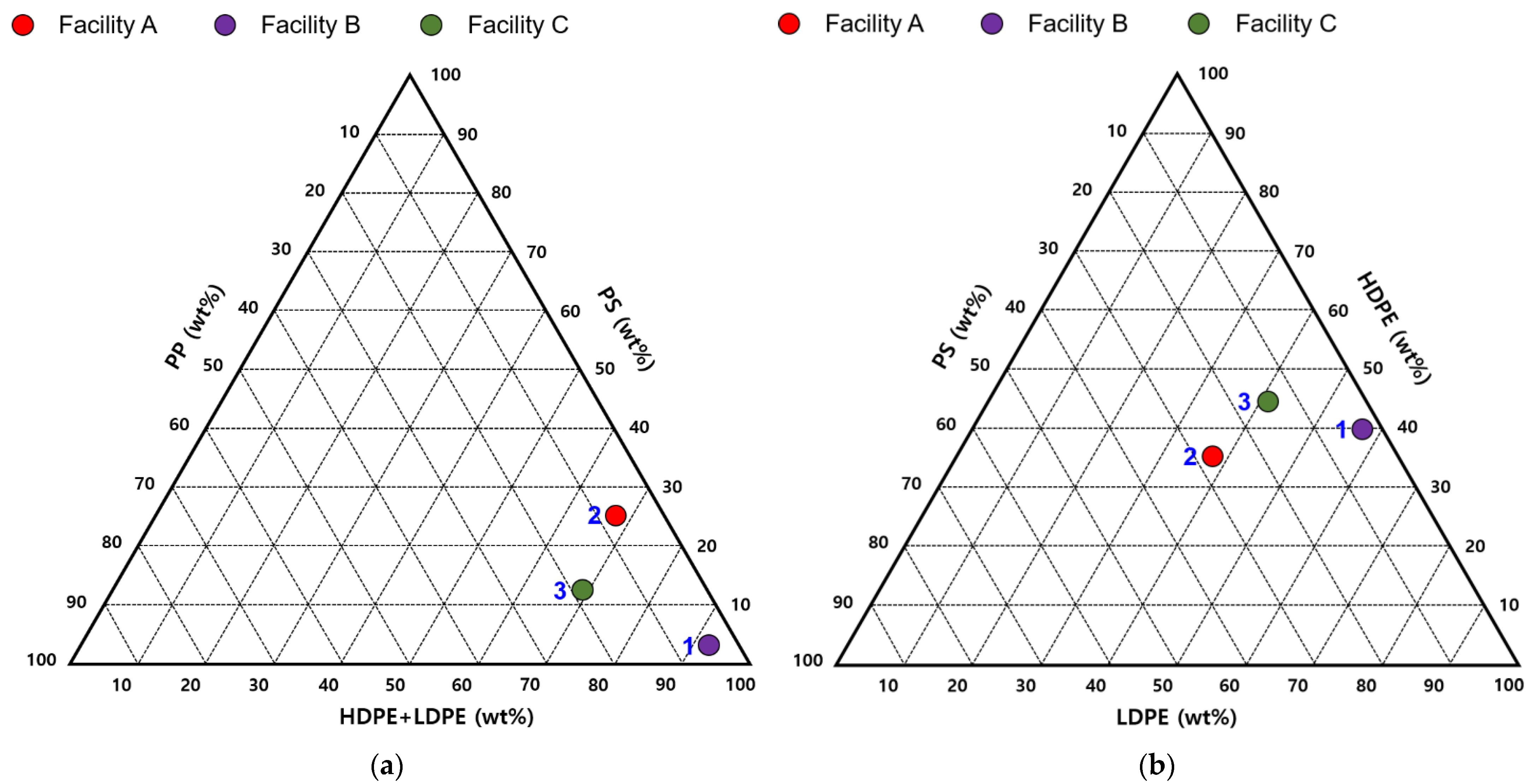
| Facility | Plastic Type | Other Materials 1 | |||||
|---|---|---|---|---|---|---|---|
| PP | PS | HDPE | LDPE | OTHER | Unknown | ||
| A | 3.4 | 11.1 | 14.1 | 14.9 | 8.2 | 24.2 | 24.1 |
| B | 2.8 | 1.3 | 16.9 | 23.8 | 27.0 | 25.2 | 3.0 |
| C | 8.1 | 5.1 | 15.5 | 14.3 | 27.4 | 13.4 | 16.2 |
| D | - | - | - | 99.6 | - | - | 0.4 |
| Facility A | Facility B | Facility C | Facility D | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Feedstock | Residue | Feedstock | Residue | Feedstock | Residue | Feedstock | Residue | ||
| Three components (Air-dry basis, wt%) | Moisture | 6.3 | 0.5 | 1.7 | 9.6 1 | 9.2 | 7.3 2 | 6.0 | 0.6 |
| Combustible | 86.5 | 42.6 | 95.5 | 40.7 | 88.0 | 49.1 | 92.9 | 55.8 | |
| Ash | 7.2 | 56.9 | 2.8 | 49.7 | 2.8 | 43.6 | 1.1 | 43.6 | |
| Proximate analysis (Dry basis, wt%) | Volatile matter | 90.0 | 35.1 | 96.1 | 24.4 | 94.3 | 35.7 | 97.3 | 30.0 |
| Fixed carbon | 2.9 | 9.1 | 2.4 | 19.1 | 2.5 | 15.7 | 1.5 | 24.1 | |
| Ash | 7.1 | 55.8 | 1.5 | 56.5 | 3.2 | 48.6 | 1.2 | 45.9 | |
| Ultimate analysis (Dry basis, wt%) | C | 70.92 | 50.82 | 80.55 | 21.54 | 75.85 | 31.14 | 84.93 | 43.84 |
| H | 11.39 | 1.63 | 13.16 | 1.40 | 11.70 | 2.46 | 13.45 | 1.14 | |
| N | 0.20 | 0.71 | 0.16 | 0.31 | 0.21 | 0.36 | 0.08 | 0.36 | |
| S | 0.26 | 0.29 | 0.05 | 0.14 | 0.04 | 0.14 | 0.08 | 0.45 | |
| Metal (mg/L) | Cd | N.D. | 0.002 | 0.004 | 0.007 | N.D. | 0.020 | N.D. | 0.001 |
| Pb | 0.011 | 0.186 | 0.009 | 0.042 | 0.076 | 0.288 | 0.083 | 0.215 | |
| As | 0.006 | N.D. | 0.006 | N.D. | N.D. | N.D. | 0.016 | N.D. | |
| Cu | 0.163 | 0.527 | 0.135 | 0.261 | 0.141 | 0.275 | 0.196 | 0.736 | |
| Higher heating value (kcal/kg) | 8678 | 3454 | 9848 | 1316 | 8956 | 2324 | 10156 | 4018 | |
| Ignition temperature (°C) | 422.4 | 421.9 | 395.1 | 397.5 | 467.0 | 543.3 | 398.6 | 470.6 | |
| Facility | Oil Type | Ash (wt%) | Moisture and Sediment (vol.%) | Sulfur (wt%) | Residual Carbon (wt%) | Metal (mg/L) | |||
|---|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Cr | As | ||||||
| A | Mixed oil (Light + Heavy) | 0.03 | N.D. | 0.09 | 0.03 | N.D. | 0.010 | 0.002 | 0.010 |
| B | Heavy oil | N.D. | N.D. | 0.05 | N.D. | 0.001 | 0.003 | 0.001 | N.D. |
| C | Heavy oil | N.D. | N.D. | 0.06 | 0.03 | 0.001 | 0.001 | 0.002 | N.D. |
| D | 1st light oil | N.D. | N.D. | 0.09 | N.D. | 0.001 | 0.007 | 0.002 | N.D. |
| 2nd light oil | N.D. | N.D. | 0.08 | N.D. | 0.001 | 0.003 | 0.001 | N.D. | |
| Heavy oil | N.D. | 0.30 | 0.09 | 0.01 | N.D. | N.D. | 0.001 | N.D. | |
| Facility | Residue | Oil | Gas |
|---|---|---|---|
| A | 11.38 | 40.16 | 48.46 |
| B | 17.58 | 40.93 | 41.49 |
| C | 21.88 | 37.64 | 40.48 |
| D | 14.50 | 84.90 | 0.60 |
| Facility | CO | NOx | SOx | H2S |
|---|---|---|---|---|
| A | 2.2 | 107.9 | N.D. | N.D. |
| B | 90.2 | 68.5 | 0.4 | 0.3 |
| C | 99.2 | 72.4 | 3.8 | 0.2 |
| D 1 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namkung, H.; Park, S.-I.; Lee, Y.; Han, T.U.; Son, J.-I.; Kang, J.-G. Investigation of Oil and Facility Characteristics of Plastic Waste Pyrolysis for the Advanced Waste Recycling Policy. Energies 2022, 15, 4317. https://doi.org/10.3390/en15124317
Namkung H, Park S-I, Lee Y, Han TU, Son J-I, Kang J-G. Investigation of Oil and Facility Characteristics of Plastic Waste Pyrolysis for the Advanced Waste Recycling Policy. Energies. 2022; 15(12):4317. https://doi.org/10.3390/en15124317
Chicago/Turabian StyleNamkung, Hueon, Se-In Park, Yoomin Lee, Tae Uk Han, Jun-Ik Son, and Jun-Gu Kang. 2022. "Investigation of Oil and Facility Characteristics of Plastic Waste Pyrolysis for the Advanced Waste Recycling Policy" Energies 15, no. 12: 4317. https://doi.org/10.3390/en15124317
APA StyleNamkung, H., Park, S.-I., Lee, Y., Han, T. U., Son, J.-I., & Kang, J.-G. (2022). Investigation of Oil and Facility Characteristics of Plastic Waste Pyrolysis for the Advanced Waste Recycling Policy. Energies, 15(12), 4317. https://doi.org/10.3390/en15124317








