Use of Hydrothermal Carbonization and Cold Atmospheric Plasma for Surface Modification of Brewer’s Spent Grain and Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Brewer’s Spent Grain Sample
2.2. Hydrothermal Carbonization
2.3. Cold Atmospheric Plasma Jet Treatment
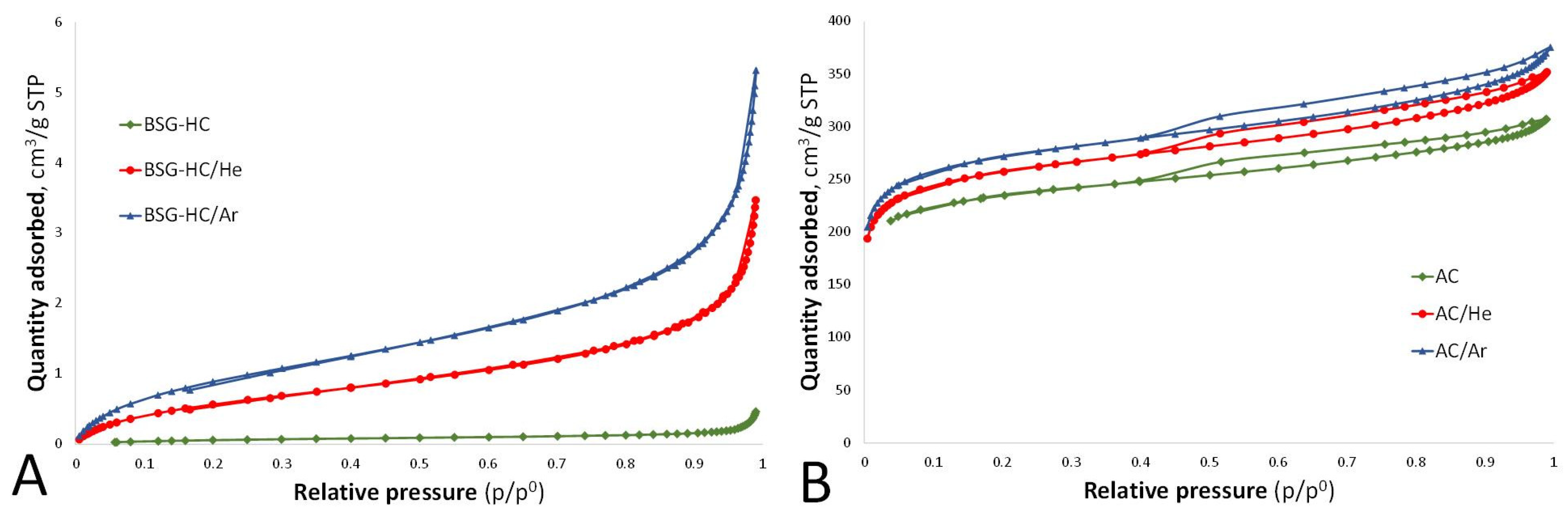
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beer Production Worldwide from 1998 to 2020 (in Billion Hectoliters). Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 1 June 2022).
- Bentzen, J.; Smith, V. Structural Changes in the Consumption of Beer, Wine and Spirits in OECD Countries from 1961 to 2014. Beverages 2018, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Jackowski, M.; Niedzwiecki, L.; Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s Spent Grains—Valuable Beer Industry by-Product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef] [PubMed]
- Enweremadu, C.; Waheed, M.A.; Adekunle, A.A.; Adeala, A. The Energy Potential of Brewer’s Spent Grain for Breweries in Nigeria. Eng. Appl. Sci. 2008, 3, 175–177. [Google Scholar]
- Jackowski, M.; Niedzwiecki, L.; Lech, M.; Wnukowski, M.; Arora, A.; Tkaczuk-Serafin, M.; Baranowski, M.; Krochmalny, K.; Veetil, V.K.; Seruga, P.; et al. HTC of Wet Residues of the Brewing Process: Comprehensive Characterization of Produced Beer, Spent Grain and Valorized Residues. Energies 2020, 13, 2058. [Google Scholar] [CrossRef] [Green Version]
- Jackowski, M.; Semba, D.; Trusek, A.; Wnukowski, M.; Niedzwiecki, L.; Baranowski, M.; Krochmalny, K.; Pawlak-Kruczek, H. Hydrothermal Carbonization of Brewery’s Spent Grains for the Production of Solid Biofuels. Beverages 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Mokrzycki, J.; Lorenc-Grabowska, E.; Kordek-Khalil, K.; Rutkowski, P. Hydrothermal and Pyrolytic Biochars from Waste Milk Thistle (Silybum Marianum) Extrudates as Precursors for Production of Effective Isoproturon Adsorbents. J. Water Process Eng. 2020, 37, 101459. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Sieradzka, M.; Magdziarz, A. Thermal Upgrading of Hydrochar from Anaerobic Digestion of Municipal Solid Waste Organic Fraction. Fuel 2022, 324, 124435. [Google Scholar] [CrossRef]
- Numviyimana, C.; Warchoł, J.; Khalaf, N.; Leahy, J.J.; Chojnacka, K. Phosphorus Recovery as Struvite from Hydrothermal Carbonization Liquor of Chemically Produced Dairy Sludge by Extraction and Precipitation. J. Environ. Chem. Eng. 2022, 10, 106947. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Ross, A.B.; Camargo-Valero, M.A. Mass and Energy Integration Study of Hydrothermal Carbonization with Anaerobic Digestion of Sewage Sludge. Renew. Energy 2021, 167, 473–483. [Google Scholar] [CrossRef]
- Wilk, M.; Śliz, M.; Gajek, M. The Effects of Hydrothermal Carbonization Operating Parameters on High-Value Hydrochar Derived from Beet Pulp. Renew. Energy 2021, 177, 216–228. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal Carbonization: Fate of Inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Moscicki, K.J.; Niedzwiecki, L.; Owczarek, P.; Wnukowski, M. Commoditization of Wet and High Ash Biomass: Wet Torrefaction—A Review. J. Power Technol. 2017, 97, 354–369. [Google Scholar]
- Funke, A.; Ziegler, F. Hydrothermal Carbonisation of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vásquez, V.R. Reaction Kinetics of Hydrothermal Carbonization of Loblolly Pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef]
- Tungal, R.; Shende, R.V. Hydrothermal Liquefaction of Pinewood (Pinus Ponderosa) for H2, Biocrude and Bio-Oil Generation. Appl. Energy 2014, 134, 401–412. [Google Scholar] [CrossRef]
- Nan, W.; Shende, A.R.; Shannon, J.; Shende, R.V. Insight into Catalytic Hydrothermal Liquefaction of Cardboard for Biofuels Production. Energy Fuels 2016, 30, 4933–4944. [Google Scholar] [CrossRef]
- Shende, R.; Tungal, R. Subcritical Aqueous Phase Reforming of Wastepaper for Biocrude and H2 Generation. Energy Fuels 2013, 27, 3194–3203. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Heat of Reaction Measurements for Hydrothermal Carbonization of Biomass. Bioresour. Technol. 2011, 102, 7595–7598. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of Thermal Pretreatment on Equilibrium Moisture Content of Lignocellulosic Biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef]
- Ahmed, M.; Andreottola, G.; Elagroudy, S.; Negm, M.S.; Fiori, L. Coupling Hydrothermal Carbonization and Anaerobic Digestion for Sewage Digestate Management: Influence of Hydrothermal Treatment Time on Dewaterability and Bio-Methane Production. J. Environ. Manag. 2021, 281, 111910. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A New Method Combining Hydrothermal Carbonization and Mechanical Compression In-Situ for Sewage Sludge Dewatering: Bench-Scale Verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M.; Aragon-Briceño, C.; Wnukowski, M.; Pożarlik, A.; Niedzwiecki, L.; Baranowski, M.; Czerep, M.; Seruga, P.; Pawlak-Kruczek, H.; et al. Cascade Membrane System for Separation of Water and Organics from Liquid By-Products of HTC of the Agricultural Digestate—Evaluation of Performance. Energies 2021, 14, 4752. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M.; Wnukowski, M.; Seruga, P.; Baranowski, M.; Pawlak-Kruczek, H.; Serafin-Tkaczuk, M.; Krochmalny, K.; Niedzwiecki, L. Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation. Energies 2020, 13, 262. [Google Scholar] [CrossRef] [Green Version]
- Aragón-Briceño, C.; Ross, A.B.B.; Camargo-Valero, M.A.A. Evaluation and Comparison of Product Yields and Bio-Methane Potential in Sewage Digestate Following Hydrothermal Treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Ferrentino, R.; Merzari, F.; Fiori, L.; Andreottola, G. Coupling Hydrothermal Carbonization with Anaerobic Digestion for Sewage Sludge Treatment: Influence of HTC Liquor and Hydrochar on Biomethane Production. Energies 2020, 13, 6262. [Google Scholar] [CrossRef]
- Sobek, S.; Tran, Q.K.; Junga, R.; Werle, S. Hydrothermal Carbonization of the Waste Straw: A Study of the Biomass Transient Heating Behavior and Solid Products Combustion Kinetics. Fuel 2022, 314, 122725. [Google Scholar] [CrossRef]
- Wnukowski, M.; Owczarek, P.; Niedźwiecki, Ł. Wet Torrefaction of Miscanthus—Characterization of Hydrochars in View of Handling, Storage and Combustion Properties. J. Ecol. Eng. 2015, 16, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.B.; Dubey, B.K. Binderless Fuel Pellets from Hydrothermal Carbonization of Municipal Yard Waste: Effect of Severity Factor on the Hydrochar Pellets Properties. J. Clean. Prod. 2020, 277, 124295. [Google Scholar] [CrossRef]
- Surup, G.R.; Leahy, J.J.; Timko, M.T.; Trubetskaya, A. Hydrothermal Carbonization of Olive Wastes to Produce Renewable, Binder-Free Pellets for Use as Metallurgical Reducing Agents. Renew. Energy 2020, 155, 347–357. [Google Scholar] [CrossRef]
- Murillo, H.A.; Díaz-Robles, L.A.; Santander, R.E.; Cubillos, F.A. Conversion of Residual Biomass into Valuable Biofuels by Co-Hydrothermal Carbonization for Utilization in Household Pellet Stoves. Biomass Bioenergy 2021, 151, 106153. [Google Scholar] [CrossRef]
- Tang, H.; Duan, Y.; Zhu, C.; Li, C.; She, M.; Zhou, Q.; Cai, L. Characteristics of a Biomass-Based Sorbent Trap and Its Application to Coal-Fired Flue Gas Mercury Emission Monitoring. Int. J. Coal Geol. 2017, 170, 19–27. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Biomass/Biochar Carbon Materials for CO2 Capture and Sequestration by Cyclic Adsorption Processes: A Review and Prospects for Future Directions. J. CO2 Util. 2022, 57, 101890. [Google Scholar] [CrossRef]
- Pajdak, A.; Skoczylas, N.; Szymanek, A.; Lutyński, M.; Sakiewicz, P. Sorption of CO2 and CH4 on Raw and Calcined Halloysite—Structural and Pore Characterization Study. Materials 2020, 13, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Liang, X.; Chen, H. A Novel Effective Approach for Synergistic NO Reduction during the Carbonation Process by Biomass-Modified Calcium-Based Sorbents. Chem. Eng. J. 2022, 439, 135795. [Google Scholar] [CrossRef]
- Pajdak, A.; Walawska, B.; Szymanek, A. The Effect of Structure Modification of Sodium Compounds on the SO2 and HCl Removal Efficiency from Fumes in the Conditions of Circulating Fluidised Bed. Chem. Biochem. Eng. Q. 2017, 31, 261–273. [Google Scholar] [CrossRef]
- Pajdak, A. Porównanie Kinetyki Sorpcji Wodoru w Stopie Metalicznym LaNi5 i Wielościennych Nanorurkach Węglowych. Przem. Chem. 2018, 1, 145–148. [Google Scholar] [CrossRef]
- Pajdak, A.; Skoczylas, N.; Dębski, A.; Grzegorek, J.; Maziarz, W.; Kudasik, M. CO2 and CH4 Sorption on Carbon Nanomaterials and Coals—Comparative Characteristics. J. Nat. Gas Sci. Eng. 2019, 72, 103003. [Google Scholar] [CrossRef]
- Skiba, M.; Młynarczuk, M. Estimation of Coal’s Sorption Parameters Using Artificial Neural Networks. Materials 2020, 13, 5422. [Google Scholar] [CrossRef]
- Ng, C.; Marshall, W.E.; Rao, R.M.; Bansode, R.R.; Losso, J.N. Activated Carbon from Pecan Shell: Process Description and Economic Analysis. Ind. Crops Prod. 2003, 17, 209–217. [Google Scholar] [CrossRef]
- Konno, K.; Oike, Y.; Ohba, Y.; Sasaki, O.; Takiguchi, Y.; Onoe, K.; Yamaguchi, T. Short-Time Preparation of NaOH-Activated Carbon from Sugar Cane Bagasse Using Microwave Plasma Heating. Green Sustain. Chem. 2017, 7, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Ma, Y.; Li, Y.; Li, G.; Nghiem, L.; Luo, W. Comparison between Cold Plasma, Ultrasonication, and Alkaline Hydrogen Peroxide Pretreatments of Garden Waste to Enhance Humification in Subsequent Composting with Kitchen Waste: Performance and Mechanisms. SSRN Electron. J. 2022, 354, 127228. [Google Scholar] [CrossRef]
- Benoit, M.; Rodrigues, A.; De Oliveira Vigier, K.; Fourré, E.; Barrault, J.; Tatibouët, J.-M.; Jérôme, F. Combination of Ball-Milling and Non-Thermal Atmospheric Plasma as Physical Treatments for the Saccharification of Microcrystalline Cellulose. Green Chem. 2012, 14, 2212. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Messineo, A. Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges. Energies 2021, 14, 2962. [Google Scholar] [CrossRef]






| Symbol | BSG-HC | BSG-HC/He | BSG-HC/Ar | AC | AC/He | AC/Ar |
|---|---|---|---|---|---|---|
| 0.46 | 3.47 | 5.32 | 307.2 | 351.9 | 375.5 | |
| 0.25 | 2.38 | 3.70 | 731.3 | 808.1 | 939.7 | |
| 0.06 | 0.55 | 0.85 | - | - | - | |
| 0.61 | 7.14 | 10.57 | 1077.5 | 1311.3 | 1417.7 | |
| - | - | - | 247.4 | 301.2 | 325.7 | |
| 0.65 | 5.01 | 7.59 | 173.2 | 224.3 | 235.7 | |
| 11.7 | 9.0 | 8.7 | 4.6 | 4.6 | 4.6 | |
| 0 | 0 | 0 | 353.68 | 383.45 | 405.56 | |
| 0.15 | 2.49 | 3.87 | 421.56 | 481.52 | 508.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krochmalny, K.; Pawlak-Kruczek, H.; Skoczylas, N.; Kudasik, M.; Gajda, A.; Gnatowska, R.; Serafin-Tkaczuk, M.; Czapka, T.; Jaiswal, A.K.; Vishwajeet; et al. Use of Hydrothermal Carbonization and Cold Atmospheric Plasma for Surface Modification of Brewer’s Spent Grain and Activated Carbon. Energies 2022, 15, 4396. https://doi.org/10.3390/en15124396
Krochmalny K, Pawlak-Kruczek H, Skoczylas N, Kudasik M, Gajda A, Gnatowska R, Serafin-Tkaczuk M, Czapka T, Jaiswal AK, Vishwajeet, et al. Use of Hydrothermal Carbonization and Cold Atmospheric Plasma for Surface Modification of Brewer’s Spent Grain and Activated Carbon. Energies. 2022; 15(12):4396. https://doi.org/10.3390/en15124396
Chicago/Turabian StyleKrochmalny, Krystian, Halina Pawlak-Kruczek, Norbert Skoczylas, Mateusz Kudasik, Aleksandra Gajda, Renata Gnatowska, Monika Serafin-Tkaczuk, Tomasz Czapka, Amit K. Jaiswal, Vishwajeet, and et al. 2022. "Use of Hydrothermal Carbonization and Cold Atmospheric Plasma for Surface Modification of Brewer’s Spent Grain and Activated Carbon" Energies 15, no. 12: 4396. https://doi.org/10.3390/en15124396







