Hybrid Battery Thermal Management System with NiTi SMA and Phase Change Material (PCM) for Li-ion Batteries
Abstract
:1. Introduction
2. Experimental Setup
3. System Design
4. Model Description
4.1. A Lumped Model
4.2. Governing Equations for Phase Change Material
4.3. NiTi SMA Model
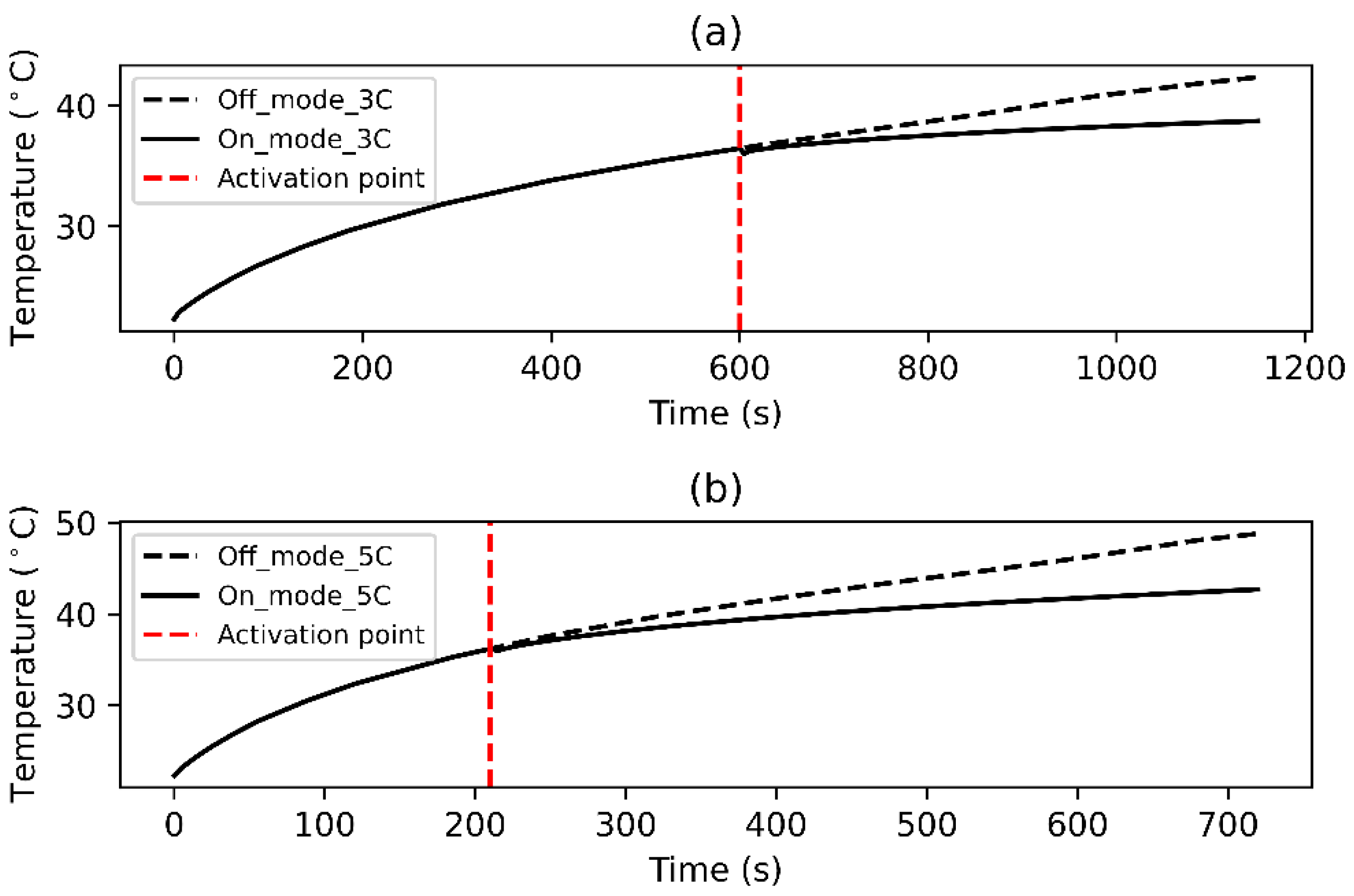
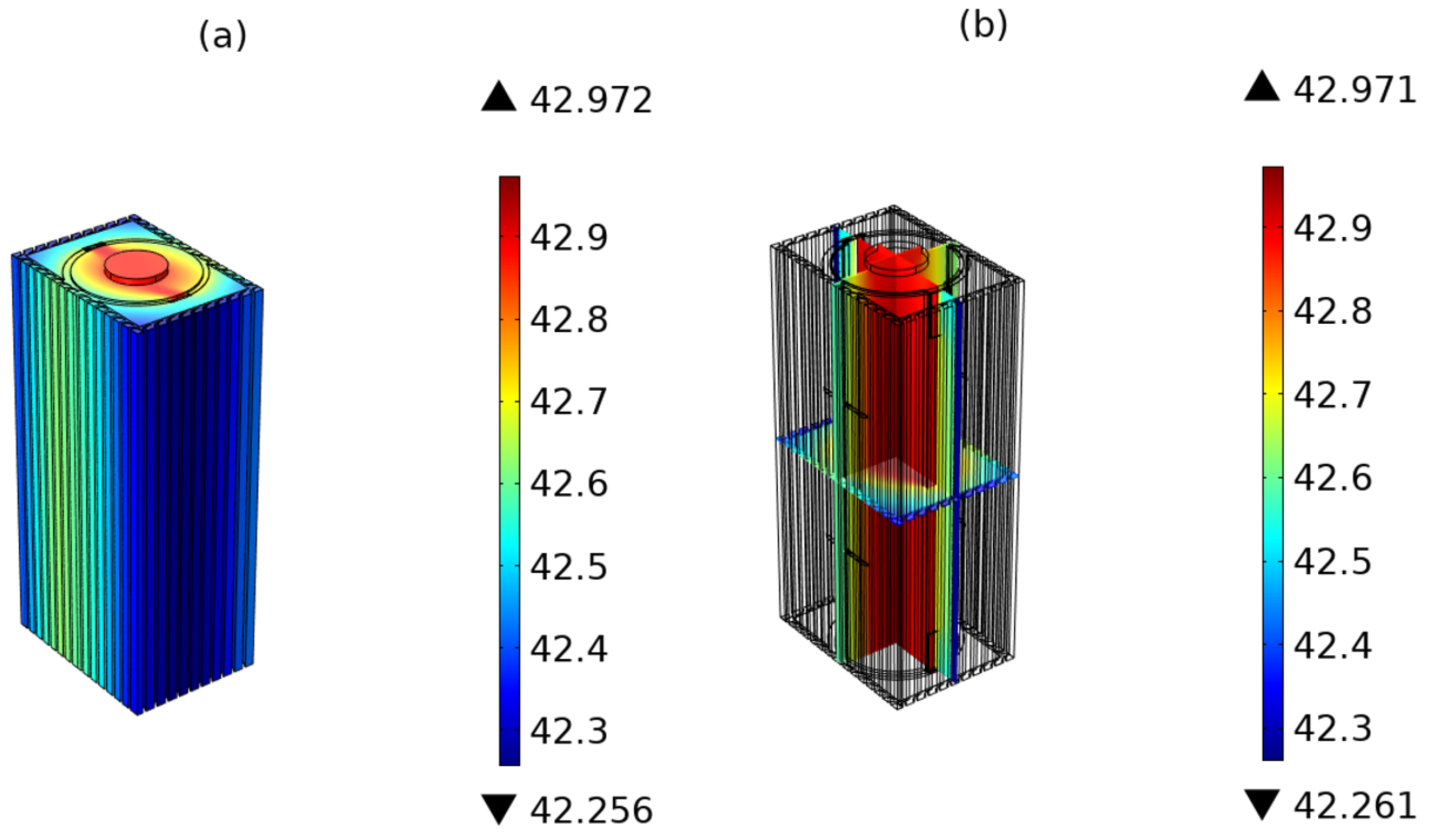
5. Results and Discussion
5.1. Model Validation
5.2. NiTi SMA Model Results
5.3. Battery Electrochemical and Thermal Behavior
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Chen, Q.; Wang, T. Effects of air cooling structure on cooling performance enhancement of prismatic lithium-ion battery packs based on coupled electrochemical-thermal model. Energy Sci. Eng. 2021, 9, 1450–1464. [Google Scholar] [CrossRef]
- Alipour, M.; Hassanpouryouzband, A.; Kizilel, R. Investigation of the Applicability of Helium-Based Cooling System for Li-Ion Batteries. Electrochem 2021, 2, 135–148. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Saw, L.H.; Ye, Y.; Tay, A.A.O. Integration issues of lithium-ion battery into electric vehicles battery pack. J. Clean. Prod. 2016, 113, 1032–1045. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, B.; Zheng, Y.; Huang, S.; Yan, T.; Liu, X. Hybrid Battery Thermal Management System in Electrical Vehicles: A Review. Energies 2020, 13, 6257. [Google Scholar] [CrossRef]
- Allcell, Lithium-Ion Battery Manufacturer|AllCell Technologies, LLC. Available online: https://www.allcelltech.com/ (accessed on 16 November 2021).
- Bai, F.; Chen, M.; Song, W.; Feng, Z.; Li, Y.; Ding, Y. Thermal management performances of PCM/water cooling-plate using for lithium-ion battery module based on non-uniform internal heat source. Appl. Therm. Eng. 2017, 126, 17–27. [Google Scholar] [CrossRef]
- Al-Hallaj, S.; Selman, J.R. Thermal modeling of secondary lithium batteries for electric vehicle/hybrid electric vehicle applications. J. Power Source 2002, 110, 341–348. [Google Scholar] [CrossRef]
- Goli, P.; Legedza, S.; Dhar, A.; Salgado, R.; Renteria, J.; Balandin, A.A. Graphene-enhanced hybrid phase change materials for thermal management of Li-ion batteries. J. Power Source 2014, 248, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Yang, X.; Zhang, G.; Ke, X.; Wang, Z.; Situ, W.; Li, X.; Zhang, J. An experimental study of thermal management system using copper mesh-enhanced composite phase change materials for power battery pack. Energy 2016, 113, 909–916. [Google Scholar] [CrossRef]
- Zhao, J.; Lv, P.; Rao, Z. Experimental study on the thermal management performance of phase change material coupled with heat pipe for cylindrical power battery pack. Exp. Therm. Fluid Sci. 2017, 82, 182–188. [Google Scholar] [CrossRef]
- Rangappa, R.; Rajoo, S. Effect of thermo-physical properties of cooling mass on hybrid cooling for lithium-ion battery pack using design of experiments. Int. J. Energy Environ. Eng. 2019, 10, 67–83. [Google Scholar] [CrossRef]
- Landini, S.; Leworthy, J.; O’Donovan, T.S. A Review of Phase Change Materials for the Thermal Management and Isothermalisation of Lithium-Ion Cells. J. Energy Storage 2019, 25, 100887. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase Change Materials Application in Battery Thermal Management System: A Review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef]
- Babapoor, A.; Azizi, M.; Karimi, G. Thermal management of a Li-ion battery using carbon fiber-PCM composites. Appl. Therm. Eng. 2015, 82, 281–290. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, J.; Fu, Y.; Cao, M.; Liu, M. Thermal optimization of composite phase change material/expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 2016, 108, 1119–1125. [Google Scholar] [CrossRef]
- Zou, D.; Ma, X.; Liu, X.; Zheng, P.; Hu, Y. Thermal performance enhancement of composite phase change materials (PCM) using graphene and carbon nanotubes as additives for the potential application in lithium-ion power battery. Int. J. Heat Mass Transf. 2018, 120, 33–41. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, R.; Yan, F.; Zhou, T.; Zheng, N. Thermal management of the lithium-ion battery by the composite PCM-Fin structures. Int. J. Heat Mass Transf. 2019, 145, 118739. [Google Scholar] [CrossRef]
- Heyhat, M.M.; Mousavi, S.; Siavashi, M. Battery thermal management with thermal energy storage composites of PCM, metal foam, fin and nanoparticle. J. Energy Storage 2020, 28, 101235. [Google Scholar] [CrossRef]
- Qin, P.; Liao, M.; Zhang, D.; Liu, Y.; Sun, J.; Wang, Q. Experimental and numerical study on a novel hybrid battery thermal management system integrated forced-air convection and phase change material. Energy Convers. Manag. 2019, 195, 1371–1381. [Google Scholar] [CrossRef]
- Fathabadi, H. High thermal performance lithium-ion battery pack including hybrid active–passive thermal management system for using in hybrid/electric vehicles. Energy 2014, 70, 529–538. [Google Scholar] [CrossRef]
- Du, X.; Qian, Z.; Chen, Z.; Rao, Z. Experimental investigation on mini-channel cooling–based thermal management for Li-ion battery module under different cooling schemes. Int. J. Energy Res. 2018, 42, 2781–2788. [Google Scholar] [CrossRef]
- Naresh, G.; Kumar, T.P.; Aadhithyan, B.; Utkarsh, S.; Nithin, J.V. Transient thermal analysis of passive air-cooled battery-pack for various casing material. IOP Conf. Ser. Mater. Sci. Eng. 2020, 993, 012131. [Google Scholar] [CrossRef]
- Huang, W. On the selection of shape memory alloys for actuators. Mater. Des. 2002, 23, 11–19. [Google Scholar] [CrossRef]
- Hao, M.; Li, J.; Park, S.; Moura, S.; Dames, C. Efficient thermal management of Li-ion batteries with a passive interfacial thermal regulator based on a shape memory alloy. Nat. Energy 2018, 3, 899–906. [Google Scholar] [CrossRef]
- Joula, M.; Dilibal, S.; Owusu-Danquah, J. Smart adaptronic thermal management system designs for the Li-ion battery packs. In Proceedings of the 2021 IEEE International Conference on Mechatronics (ICM), Kashiwa, Japan, 7 March 2021. [Google Scholar] [CrossRef]
- Panasonic Industry, Lithium-Ion Battery Manufacturer. Available online: https://industrial.panasonic.com/pt/lithium-ion/models/NCR18650GA (accessed on 16 November 2021).
- Pra, F.; al Koussa, J.; Ludwig, S.; de Servi, C.M. Experimental and Numerical Investigation of the Thermal Performance of a Hybrid Battery Thermal Management System for an Electric Van. Batteries 2021, 7, 27. [Google Scholar] [CrossRef]
- Ng, B.; Coman, P.T.; Mustain, W.E.; White, R.E. Non-destructive parameter extraction for a reduced order lumped electrochemical-thermal model for simulating Li-ion full-cells. J. Power Source 2020, 445, 227296. [Google Scholar] [CrossRef]
- Ekström, H.; Fridholm, B.; Lindbergh, G. Comparison of lumped diffusion models for voltage prediction of a lithium-ion battery cell during dynamic loads. J. Power Source 2018, 402, 296–300. [Google Scholar] [CrossRef]
- Owusu-Danquah, J.; Saleeb, A.F.; Soudah, M.A. Cyclic pseudoelastic training and two-way shape memory behavior of a NiTi alloy with small irrecoverable plastic strains: Numerical modeling. Int. J. Solids Struct. 2021, 217, 178–192. [Google Scholar] [CrossRef]
- Saleeb, F.; Soudah, M.A.; Owusu-Danquah, J.S. Stabilization of the Cyclic Response of the Ni 49.9 Ti 50.1 Shape Memory Actuators under Thermomechanical Loads. J. Aerosp. Eng. 2021, 34, 04020099. [Google Scholar] [CrossRef]
- Coman, P.T.; Darcy, E.C.; Strangways, B.; White, R.E. A Reduced-Order Lumped Model for Li-Ion Battery Packs during Operation. J. Electrochem. Soc. 2021, 168, 100525. [Google Scholar] [CrossRef]
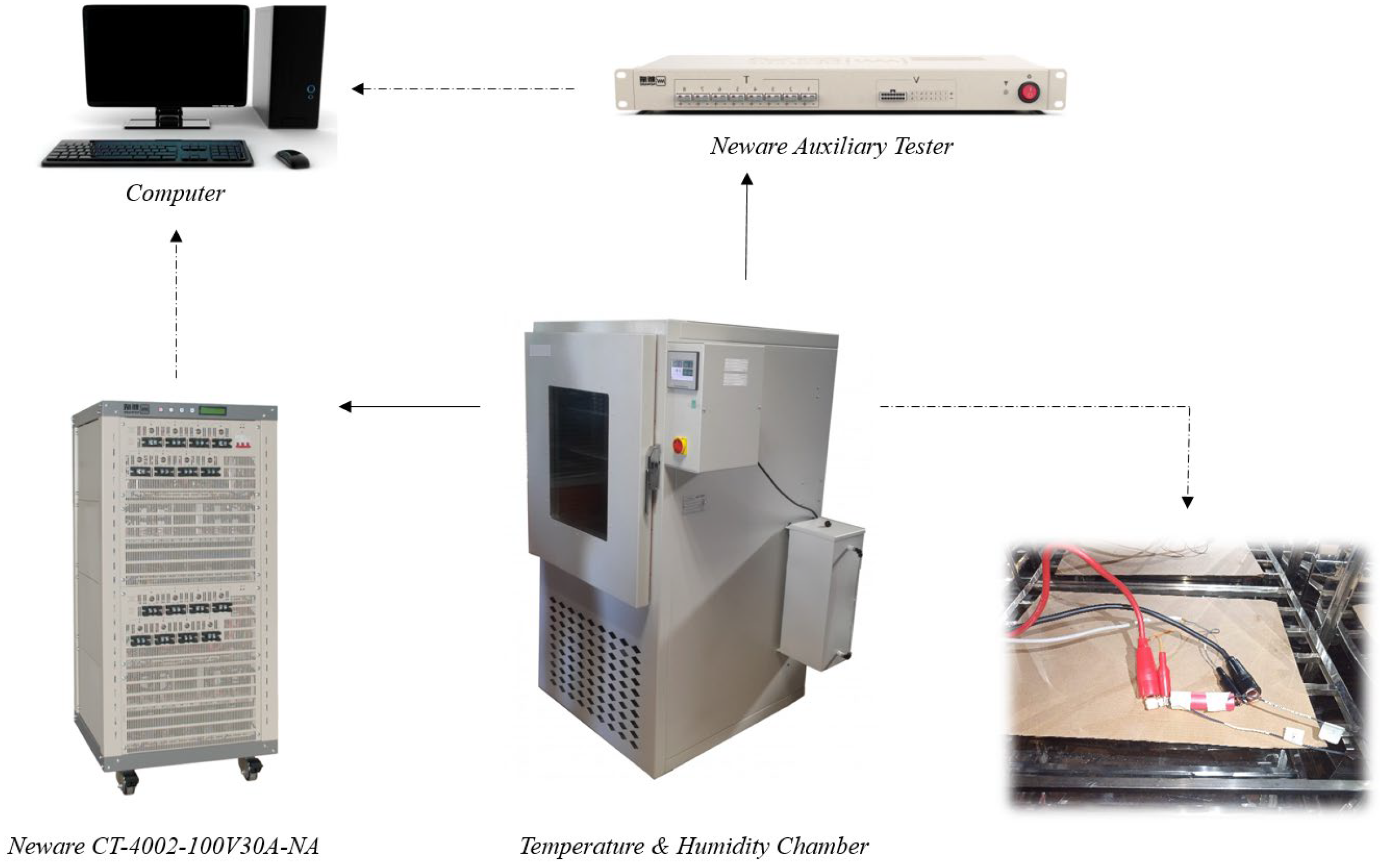
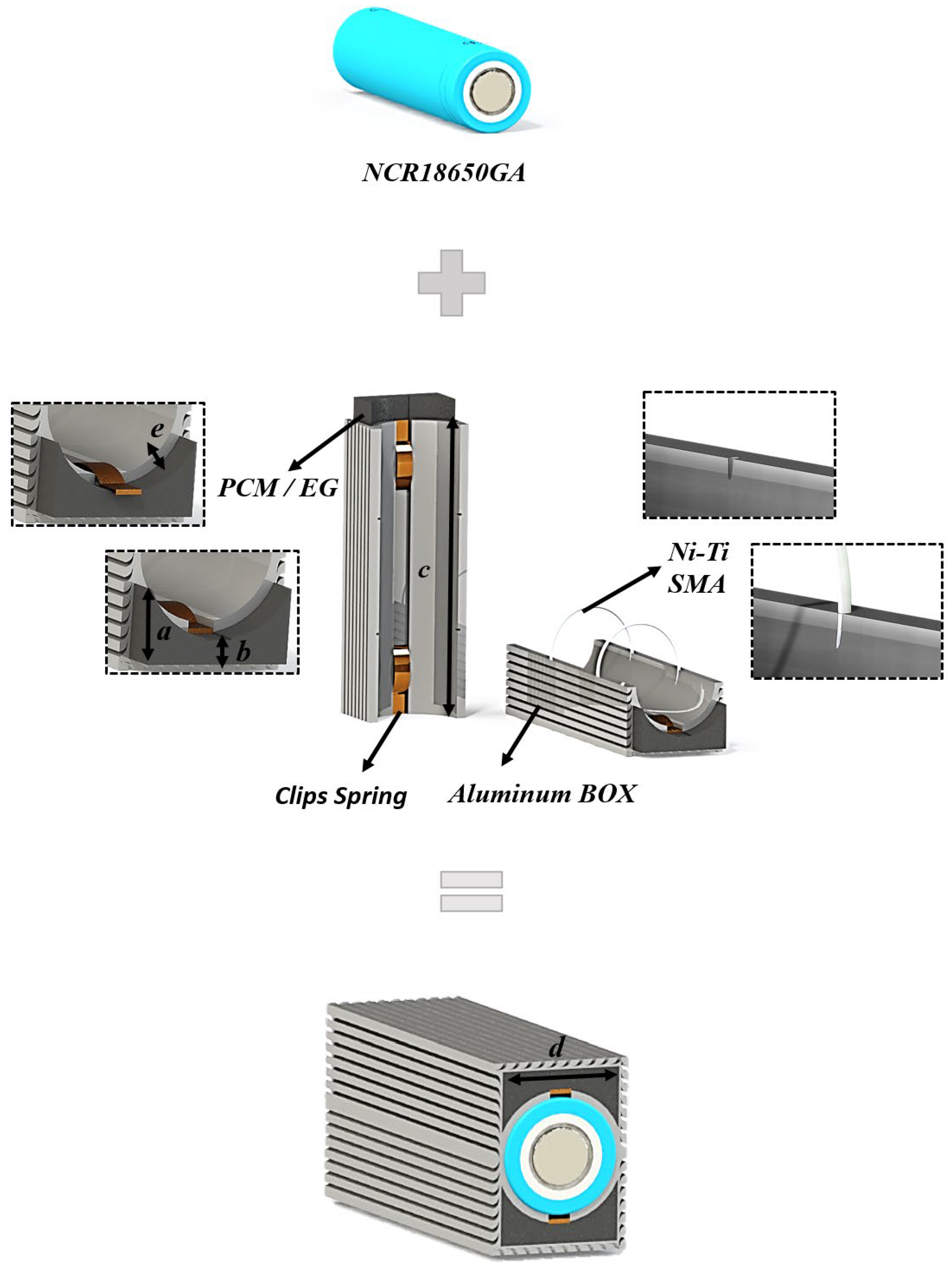
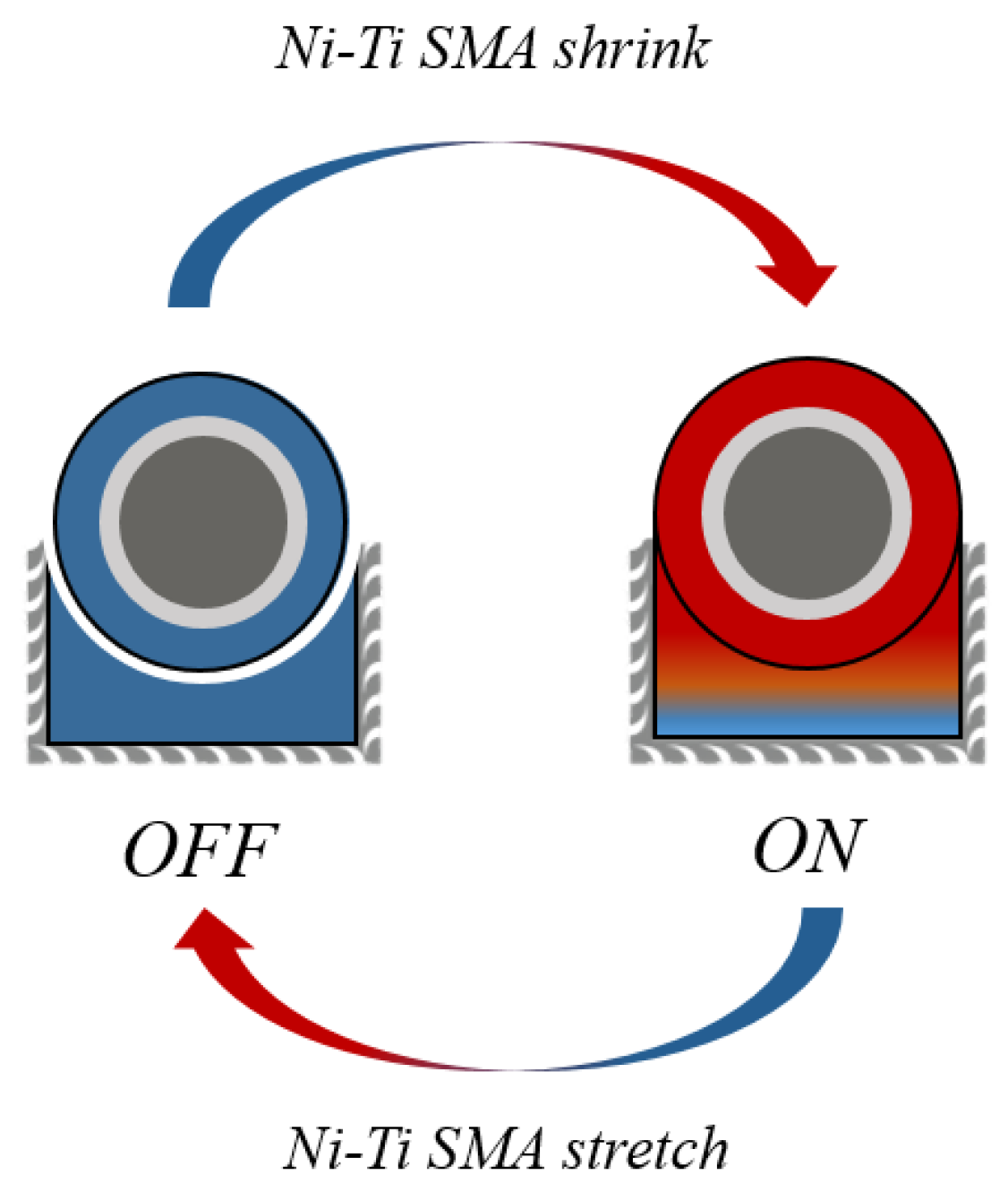


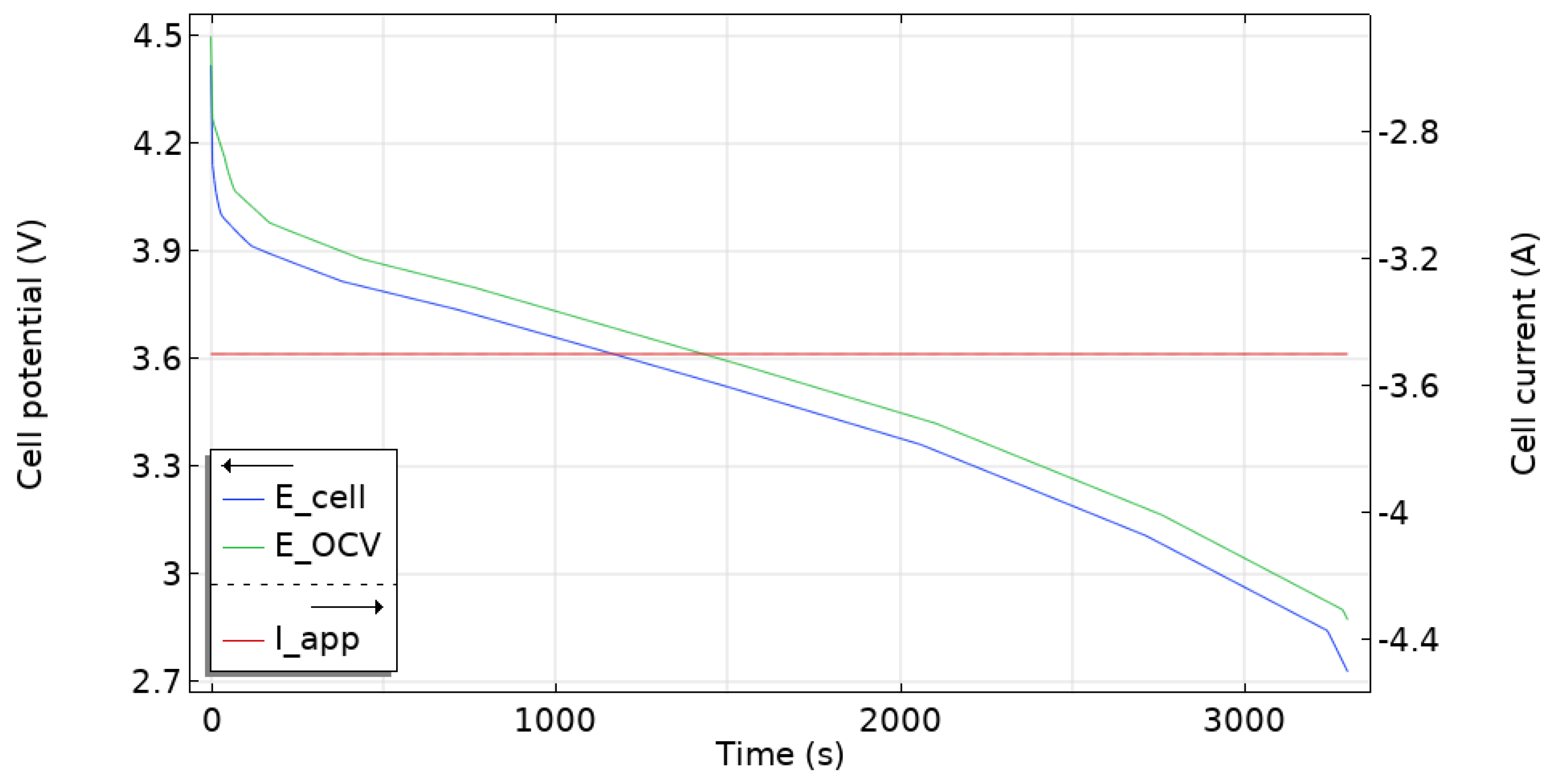

| ID | Step Name | Step Time | Voltage (V) | Current (A) | Stop Current (A) |
|---|---|---|---|---|---|
| 1 | Rest | 10 s | |||
| 2 | CCCV_Chg | 4 h 30 min | 4.2 | 1.4750 | 0.0670 |
| 3 | Rest | 30 min | |||
| 4 | CC_Dchg | 2.5 | 3.5 | ||
| 5 | Rest | 30 min | |||
| 6 | CCCV_Chg | 4 h 30 min | 4.2 | 1.4750 | 0.0670 |
| 7 | Rest | 30 min | |||
| 8 | CC_Dchg | 2.5 | 7.0 | ||
| 9 | Rest | 30 min | |||
| 10 | CCCV_Chg | 4 h 30 min | 4.2 | 1.4750 | 0.0670 |
| 11 | Rest | 30 min | |||
| 12 | CC_Dchg | 2.5 | 10.0 | ||
| 13 | Rest | 30 min | |||
| 14 | CCCV_Chg | 4 h 30 min | 3.52 | 1.4750 | 0.3500 |
| 15 | Rest | 30 min | |||
| 16 | End |
| Parameters | Values | Units | Details |
|---|---|---|---|
| (a) Battery Specifications: | |||
| Battery Model | Panasonic Sanyo | NCR18650GA | |
| Battery Chemistry | Li-ion | NCA | |
| Cathode Chemistry | LiNiCoAl | - | |
| Anode Chemistry | Carbon | - | |
| Capacity | 3450 | mAh | |
| Nominal Voltage | 3.6 | V | |
| Charging | 4.20 | V | |
| 1475 | mA | ||
| CC-CV | |||
| Weight | 47.06 | gr | |
| Energy Density | 224 | Wh/kg | |
| Internal Resistance | 26.40 | mOhm | |
| (b) PCM/EG Specifications: | |||
| PCM/EG Melting Point | 37 | °C | |
| PCM/EG Density | 895 | kg/m3 | At room temperature |
| PCM/EG Thermal Conductivity | 10–25 | W/m/K | Horizontal plane |
| Latent Heat | 160 | J/g | |
| Specific Heat | 1.91 | J/g/°C | Solid |
| Electrical Resistance | 0.41 | 10−3 Ωm | Horizontal plane |
| Parameter | Value | Description |
|---|---|---|
| (mm) | 18.5 | Battery diameter |
| (mm) | 64.3 | Battery height |
| (mm) | 9 | Positive tab diameter |
| (mm) | 1 | Positive tab height |
| (J⋅kg−1⋅K−1) | 910 | Battery heat capacity |
| (kg⋅m−3) | 2735 | Battery density |
| (mV) | 50 | Ohmic overpotential at 1 C and reference temperature |
| (kj⋅mol−1) | 40 | Activation energy for ohmic overpotential |
| (A ⋅m−2) | 1 | Local current density at reference temperature |
| (kj⋅mol−1) | −60 | Activation energy for local current density |
| (s) | 900 | Diffusion time constant at reference temperature |
| (kj⋅mol−1) | 40 | Activation energy for diffusion time constant |
| (W⋅ m−1⋅K−1) | 30.8 | Thermal conductivity, in plane |
| (W⋅m−1⋅K−1) | 1.38 | Thermal conductivity, cross plane |
| (W.⋅m−2⋅K−1) | 8 | Heat transfer coefficient |
| (°C) | 37 | Melting point |
| (°C) | 6 | Melting range |
| (°C) | 32 | Initial temperature |
| (°C) | 38 | Final temperature |
| (J⋅kg−1⋅K−1) | 1910 | Heat capacity, solid |
| (J⋅kg−1⋅K−1) | 2250 | Heat capacity, liquid |
| (kg⋅m3) | 895 | Density at room temperature |
| (J⋅kg−1) | 160,000 | Latent heat |
| (W⋅ m−1⋅K−1) | 10–25 | Thermal conductivity (horizontal plane) |
| (W⋅ m−1⋅K−1) | 6–12 | Thermal conductivity (vertical direction) |
| Parameters | Units | Values |
|---|---|---|
| Elastic Constants | ||
| Elastic modulus, E | GPa | 60 |
| Poisson’s ratio, ν | −W | 0.3 |
| Inelastic Constants | ||
| No. of mechanisms | - | 6 |
| n | - | 5 |
| μ | MPa.s | 1 × 105 |
| κ | MPa | 20 |
| κ(b), b = 3 | MPa | 1E21 |
| κ(b), b = 5 | MPa | 50 |
| κ(b), b = 6 | MPa | 72 |
| β(b), b = 1 to 6 | - | 1, 1, 10, 10, 1, 2.5 |
| H(b), b = 1 to 3 | MPa | 300 × 103, 300 × 103, 200 |
| H(b), b = 4 to 6 | MPa | 45 × 103, 2 × 103, 600 |
| Temperature (°C) | κ(b), b = 1, 2 | κ(b), b = 4 |
|---|---|---|
| 15 | 0.01 | 100 |
| 35 | 0.01 | 10 |
| 65 | 100 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joula, M.; Dilibal, S.; Mafratoglu, G.; Danquah, J.O.; Alipour, M. Hybrid Battery Thermal Management System with NiTi SMA and Phase Change Material (PCM) for Li-ion Batteries. Energies 2022, 15, 4403. https://doi.org/10.3390/en15124403
Joula M, Dilibal S, Mafratoglu G, Danquah JO, Alipour M. Hybrid Battery Thermal Management System with NiTi SMA and Phase Change Material (PCM) for Li-ion Batteries. Energies. 2022; 15(12):4403. https://doi.org/10.3390/en15124403
Chicago/Turabian StyleJoula, Mohammad, Savas Dilibal, Gonca Mafratoglu, Josiah Owusu Danquah, and Mohammad Alipour. 2022. "Hybrid Battery Thermal Management System with NiTi SMA and Phase Change Material (PCM) for Li-ion Batteries" Energies 15, no. 12: 4403. https://doi.org/10.3390/en15124403









