Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment
Abstract
:1. Introduction
2. Materials and Methods
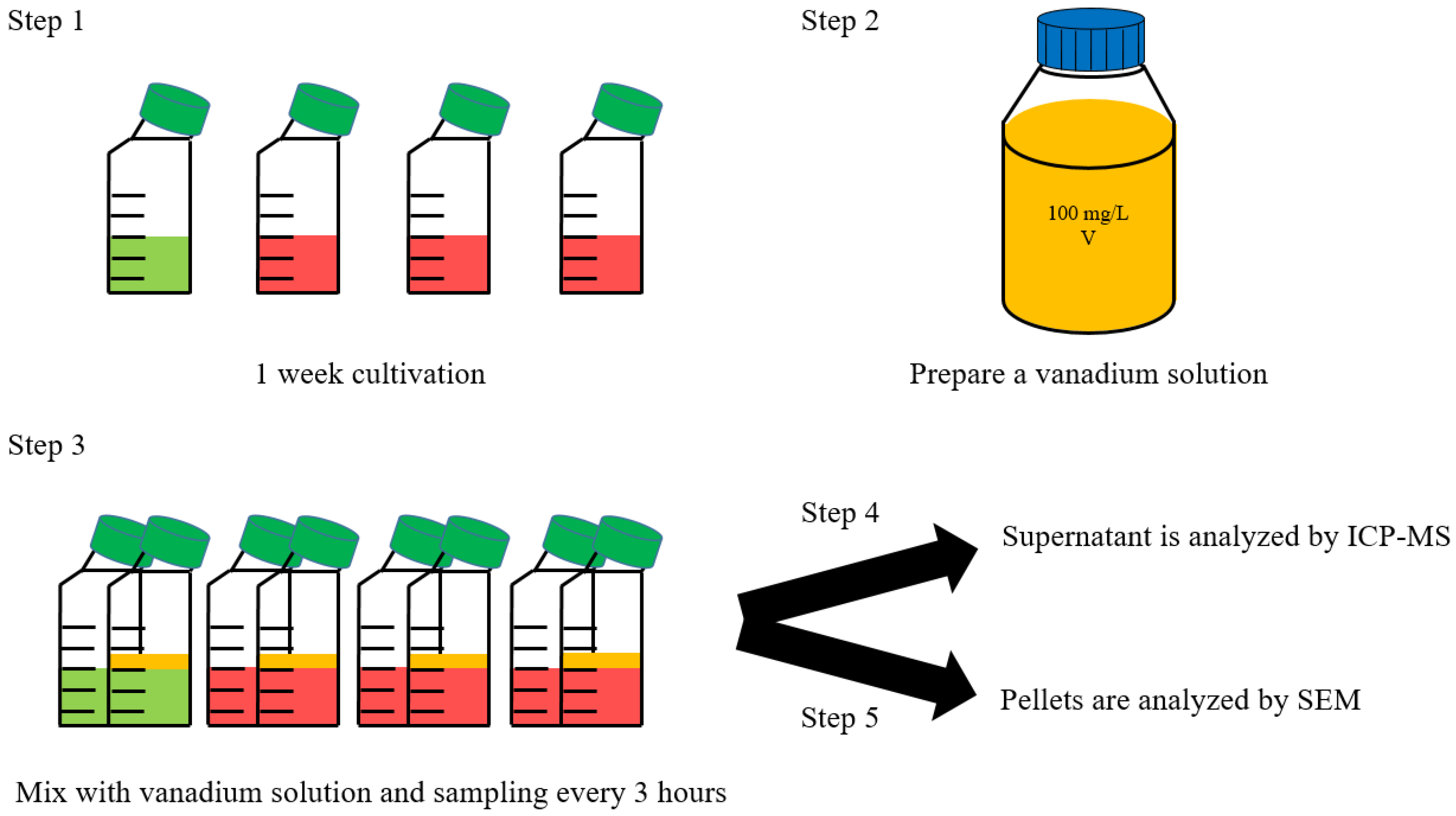
2.1. Strain and Maintenance Condition
2.2. Cellular Growth Measurement
2.3. Vanadium Ion Measurement Using ICP-MS
2.4. Scanning Electron Microscope (SEM)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Cellular Growth and Physiology of Microalgae
3.2. Vanadium Ion Concentration Measurement by ICP-MS
3.3. SEM Observation of Cellular Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, J.; Xiang, L.; Wang, X.; Ren, H.; Wei, L.; Chen, P. Residual effects of organochlorine pesticides (OCPs) in an e-waste recycling area compared with heavy metal pollution. Ecotoxicol. Environ. Saf. 2020, 198, 110651. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xiang, J.; Ko, J.H. Municipal plastic recycling at two areas in China and heavy metal leachability of plastic in municipal solid waste. Environ. Pollut. 2020, 260, 114074. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.-Y.; Xu, Z.; Bai, A.-P.; Resina-Gallego, M.; Ji, Z.-G. Separation and Recycling of Concentrated Heavy Metal Wastewater by Tube Membrane Distillation Integrated with Crystallization. Membranes 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Platzer, S.; Kar, M.; Leyma, R.; Chib, S.; Roller, A.; Jirsa, F.; Krachler, R.; MacFarlane, D.R.; Kandioller, W.; Keppler, B.K. Task-specific thioglycolate ionic liquids for heavy metal extraction: Synthesis, extraction efficacies and recycling properties. J. Hazard. Mater. 2017, 324, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Paulose, B.; Chhikara, S.; Coomey, J.; Jung, H.I.; Vatamaniuk, O.; Dhankher, O.P. A gamma-glutamyl cyclotransferase protects Arabidopsis plants from heavy metal toxicity by recycling glutamate to maintain glutathione homeostasis. Plant Cell 2013, 25, 4580–4595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Santos Andrade, T.; Keramidas, A.; Lianos, P. Use of Chalcogenide-Semiconductor-Sensitized Titania to Directly Charge a Vanadium Redox Battery. Nanomaterials 2020, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Duong, D.L.; Ha, D.M.; Singh, K.; Phan, T.L.; Choi, W.; Kim, Y.-M.; Lee, Y.H. Ferromagnetic Order at Room Temperature in Monolayer WSe2 Semiconductor via Vanadium Dopant. Adv. Sci. 2020, 7, 1903076. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zheng, B.; Sebastian, A.; Olson, D.H.; Liu, M.; Fujisawa, K.; Pham, Y.T.H.; Jimenez, V.O.; Kalappattil, V.; Miao, L.; et al. Monolayer Vanadium-Doped Tungsten Disulfide: A Room-Temperature Dilute Magnetic Semiconductor. Adv. Sci. 2020, 7, 2001174. [Google Scholar] [CrossRef]
- Cho, H.; Atanasov, V.; Krieg, H.M.; Kerres, J.A. Novel Anion Exchange Membrane Based on Poly(Pentafluorostyrene) Substituted with Mercaptotetrazole Pendant Groups and Its Blend with Polybenzimidazole for Vanadium Redox Flow Battery Applications. Polymers 2020, 12, 915. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.; Baek, S.H.; Han, Y.; Go, B.; Jeong, D.; Kim, S.M. Developing technology necessary to produce domestic vanadium resources. J. Korean Soc. Miner. Energy Resour. 2021, 58, 66–74. [Google Scholar] [CrossRef]
- Wold, A.; Ward, R. Perowskite-type oxides of cobalt, chromium and vanadium with some rare earth elements. J. Am. Chem. Soc. 1954, 76, 1029–1030. [Google Scholar] [CrossRef]
- Baroch, E.F. Updated by Staff. Vanadium and vanadium alloys. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 2000; pp. 1–18. [Google Scholar]
- Peng, H. A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 2020, 7, 103313. [Google Scholar] [CrossRef]
- Touarssi, I.; Mourtah, I.; Chaouqi, Y.; Kamal, O.; Sefiani, N.; Lebrun, L.; Hlaïbi, M. Conceptualization and quantification of oriented membrane processes for recovering vanadium ions from acidic industrial discharges. J. Environ. Chem. Eng. 2020, 7, 103182. [Google Scholar] [CrossRef]
- Petranikova, M.; Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Tunsu, C. Vanadium sustainability in the context of innovative recycling and sourcing development. Waste Manag. 2020, 113, 521–544. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiang, Q.; Gao, L.; Chen, J.; Peng, J.; Koppala, S.; Omran, M.; Chen, G. Investigations on the microwave absorption properties and thermal behavior of vanadium slag: Improvement in microwave oxidation roasting for recycling vanadium and chromium. J. Hazard. Mater. 2020, 395, 122698. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.K.; Li, J. An overview of the potential of eco-friendly hybrid strategy for metal recycling from WEEE. Resour. Conserv. Recycl. 2017, 126, 228–239. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae–A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Van Hille, R.P.; Boshoff, G.A.; Rose, P.D.; Duncan, J.R. A continuous process for the biological treatment of heavy metal contaminated acid mine water. Resour. Conserv. Recycl. 1999, 27, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.; Fukushi, K.; Ghosh, S. Stimulation of activated sludge cultures for enhanced heavy metal removal. Water Environ. Res. 1995, 67, 822–827. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Microalga-mediated bioremediation of heavy metal–contaminated surface waters. In Biomanagement of Metal-Contaminated Soils; Springer: Dordrecht, The Netherlands, 2011; pp. 365–385. [Google Scholar]
- Dirbaz, M.; Roosta, A. Adsorption, kinetic and thermodynamic studies for the biosorption of cadmium onto microalgae Parachlorella sp. J. Environ. Chem. Eng. 2018, 6, 2302–2309. [Google Scholar] [CrossRef]
- Hein, M.; Pedersen, M.F.; Sand-Jensen, K. Size-dependent nitrogen uptake in micro-and macroalgae. Mar. Ecol. Prog. Ser. 1995, 118, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Khoshmanesh, A.; Lawson, F.; Prince, I.G. Cell surface area as a major parameter in the uptake of cadmium by unicellular green microalgae. Chem. Eng. J. 1997, 65, 13–19. [Google Scholar] [CrossRef]
- Jia, W.; Wang, B.; Wang, C.; Sun, H. Tourmaline and biochar for the remediation of acid soil polluted with heavy metals. J. Environ. Chem. Eng. 2017, 5, 2107–2114. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.M.; Marques, A.P.; Castro, P.M.; Xavier Malcata, F. Characterization of Desmodesmus pleiomorphus isolated from a heavy metal-contaminated site: Biosorption of zinc. Biodegradation 2009, 20, 629–641. [Google Scholar] [CrossRef]
- Schiewer, S.; Wong, M.H. Ionic strength effects in biosorption of metals by marine algae. Chemosphere 2000, 41, 271–282. [Google Scholar] [CrossRef]
- Kim, D.; Nakamura, A.; Okamoto, T.; Komatsu, N.; Oda, T.; Iida, T.; Ishimatsu, A.; Muramatsu, T. Mechanism of superoxide anion generation in the toxic red tide phytoplankton Chattonella marina: Possible involvement of NAD (P) H oxidase. Biochim. Biophys. Acta 2000, 1524, 220–227. [Google Scholar] [CrossRef]
- Kim, D.; Sato, Y.; Oda, T.; Muramatsu, T.; Matsuyama, Y.; Honjo, T. Specific toxic effect of dinoflagellate Heterocapsa circularisquama on the rotifer Brachionus plicatilis. Biosci. Biotechnol. Biochem. 2000, 64, 2719–2722. [Google Scholar] [CrossRef]
- Samarakoon, K.W.; Kwon, O.-N.; Ko, J.-Y.; Lee, J.-H.; Kang, M.-C.; Kim, D.; Lee, J.B.; Lee, J.-S.; Jeon, Y.-J. Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochloropsis oculata) protein hydrolysate. J. Appl. Phycol. 2013, 25, 1595–1606. [Google Scholar] [CrossRef]
- Kim, D.; Watanabe, M.; Nakayasu, Y.; Kohata, K. Production of superoxide anion and hydrogen peroxide associated with cell growth of Chattonella antiqua. Aquat. Microb. Ecol. 2004, 35, 57–64. [Google Scholar] [CrossRef]
- Kim, D.; Choi, K.-S.; Hong, H.-K.; Jiang, Z.; Zou, Y.; Choi, K.-S.; Yamasaki, Y.; Matsuyama, Y.; Yamaguchi, K.; Oda, T. Comparative study on the toxic effects of red tide flagellates Heterocapsa circularisquama and Chattonella marina on the short-necked clam (Ruditapes philippinarum). Biosci. Biotechnol. Biochem. 2011, 75, 2052–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Oda, T. Production of Nitric Oxide by Marine Unicellular Red Tide Phytoplankton, Chattonella marina. In Nitric Oxide in Plants: Metabolism and Role in Stress Physiology; Springer: Cham, Switzerland, 2014; pp. 75–84. [Google Scholar]
- Cha, S.-H.; Kim, M.-J.; Yang, H.-Y.; Jin, C.-B.; Jeon, Y.-J.; Oda, T.; Kim, D.-K. ACE, α-glucosidase and cancer cell growth inhibitory activities of extracts and fractions from marine microalgae, Nannochloropsis oculata. Korean J. Fish. Aquat. Sci. 2010, 43, 437–444. [Google Scholar]
- Kim, H.S.; Park, W.; Lee, B.; Seon, G.; Suh, W.I.; Moon, M.; Chang, Y.K. Optimization of heterotrophic cultivation of Chlorella sp. HS2 using screening, statistical assessment, and validation. Sci. Rep. 2019, 9, 19383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šebestová, A.; Baron, D.; Pechancová, R.; Pluháček, T.; Petr, J. Determination of oxaliplatin enantiomers at attomolar levels by capillary electrophoresis connected with inductively coupled plasma mass spectrometry. Talanta 2019, 205, 120151. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Kosterink, N.; Wacka, N.T.; Vermuë, M.; Wijffels, R. Mechanism behind autoflocculation of unicellular green microalgae Ettlia texensis. J. Biotechnol. 2014, 174, 34–38. [Google Scholar] [CrossRef]
- Lee, H.; Nam, K.; Yang, J.-W.; Han, J.-I.; Chang, Y.K. Synergistic interaction between metal ions in the sea salts and the extracellular polymeric substances for efficient microalgal harvesting. Algal Res. 2016, 14, 79–82. [Google Scholar] [CrossRef]
- Huang, H.; Winchester, K.J.; Suvorova, A.; Lawn, B.R.; Liu, Y.; Hu, X.Z.; Dell, J.M.; Faraone, L. Effect of deposition conditions on mechanical properties of low-temperature PECVD silicon nitride films. Mater. Sci. 2006, 435, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Brauer, G. Vanadium, Niobium, Tantalum. In Handbook of Preparative Inorganic Chemistry V2, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012; p. 1252. [Google Scholar]
- Emission Permitted Standards, Article 34, Section 1, Chapter 3, Ministry of Environment Decree No. 553, Republic of KOREA. Available online: http://www.law.go.kr/lsInfoP.do?lsiSeq=153570&efYd=20140430#AJAX (accessed on 16 June 2022).
- Ellendersen, L.S.N.; Milinsk, M.C.; Feroldi, M.; Zadinelo, I.V.; Santos, L.D.; Muniz, G.I.B.; Gasparrini, L.J.; Alves, H.J. Biopolymer foam for remediation of aquatic environments contaminated with particulates and heavy metals. J. Environ. Chem. Eng. 2018, 6, 6131–6138. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, X.; Yang, H.; Sun, L. Bioremediation of heavy metal pollution utilizing composite microbial agent of Mucor circinelloides, Actinomucor sp. and Mortierella sp. J. Environ. Chem. Eng. 2017, 5, 3616–3621. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic modeling of azo dye adsorption on non-living cells of Nannochloropsis oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]


| Cell Condition | V2O5 Solution Condition (Vanadium Ion Concentration) | |||||
|---|---|---|---|---|---|---|
| OD | Cultivation Period (day) | Temperature (℃) | pH | Stock (mg L−1) | Inoculation (mg L−1) | pH |
| 0.9–0.95 | 7 | 20 | 7.5 | 100 | 5 | 4.3 |
| N. oculata | Vanadium Concentration (mg L−1) | |
|---|---|---|
| Supernatant | 0 h | 4.61 ± 0.11 |
| 24 h | 1.85 ± 0.21 | |
| Pellet | Sonication | 2.57 ± 0.27 |
| Acid * treatment | 1.01 ± 0.07 | |
| Base ** treatment | 2.34 ± 0.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.S.; Kim, M.; Park, W.-K.; Yang, W.-G.; Nayak, M.; Shin, H.H.; Cho, K.; Kim, D.; Oda, T. Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment. Energies 2022, 15, 4467. https://doi.org/10.3390/en15124467
Kim HS, Kim M, Park W-K, Yang W-G, Nayak M, Shin HH, Cho K, Kim D, Oda T. Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment. Energies. 2022; 15(12):4467. https://doi.org/10.3390/en15124467
Chicago/Turabian StyleKim, Hee Su, Minsik Kim, Won-Kun Park, Won-Geun Yang, Manoranjan Nayak, Hyeon Ho Shin, Kichul Cho, Daekyung Kim, and Tatsuya Oda. 2022. "Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment" Energies 15, no. 12: 4467. https://doi.org/10.3390/en15124467
APA StyleKim, H. S., Kim, M., Park, W.-K., Yang, W.-G., Nayak, M., Shin, H. H., Cho, K., Kim, D., & Oda, T. (2022). Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment. Energies, 15(12), 4467. https://doi.org/10.3390/en15124467








