Removal of a Mixture of Seven Volatile Organic Compounds (VOCs) Using an Industrial Pilot-Scale Process Combining Absorption in Silicone Oil and Biological Regeneration in a Two-Phase Partitioning Bioreactor (TPPB)
Abstract
:1. Introduction
2. Materials and Methods
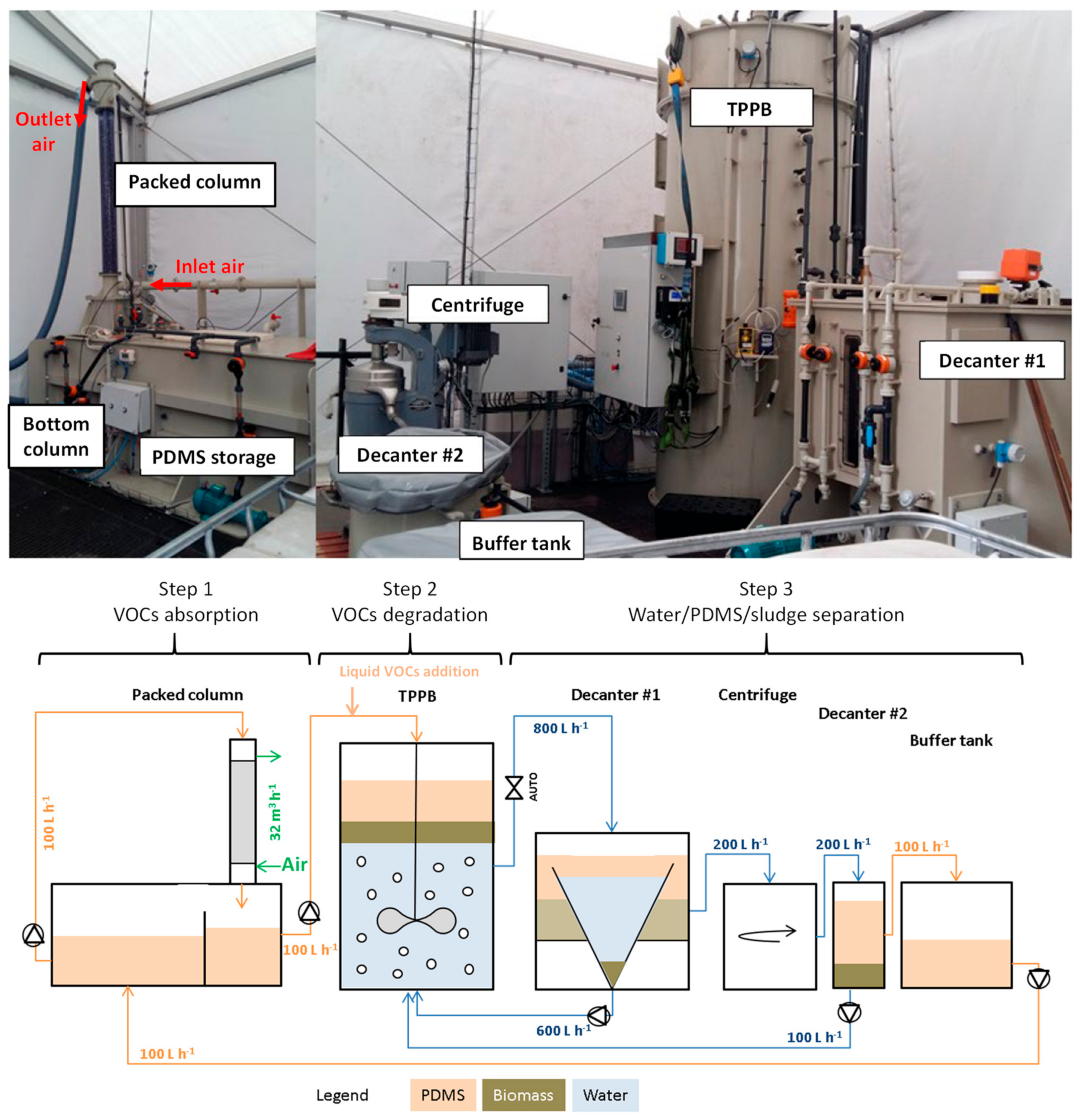
2.1. Pilot-Scale Installation
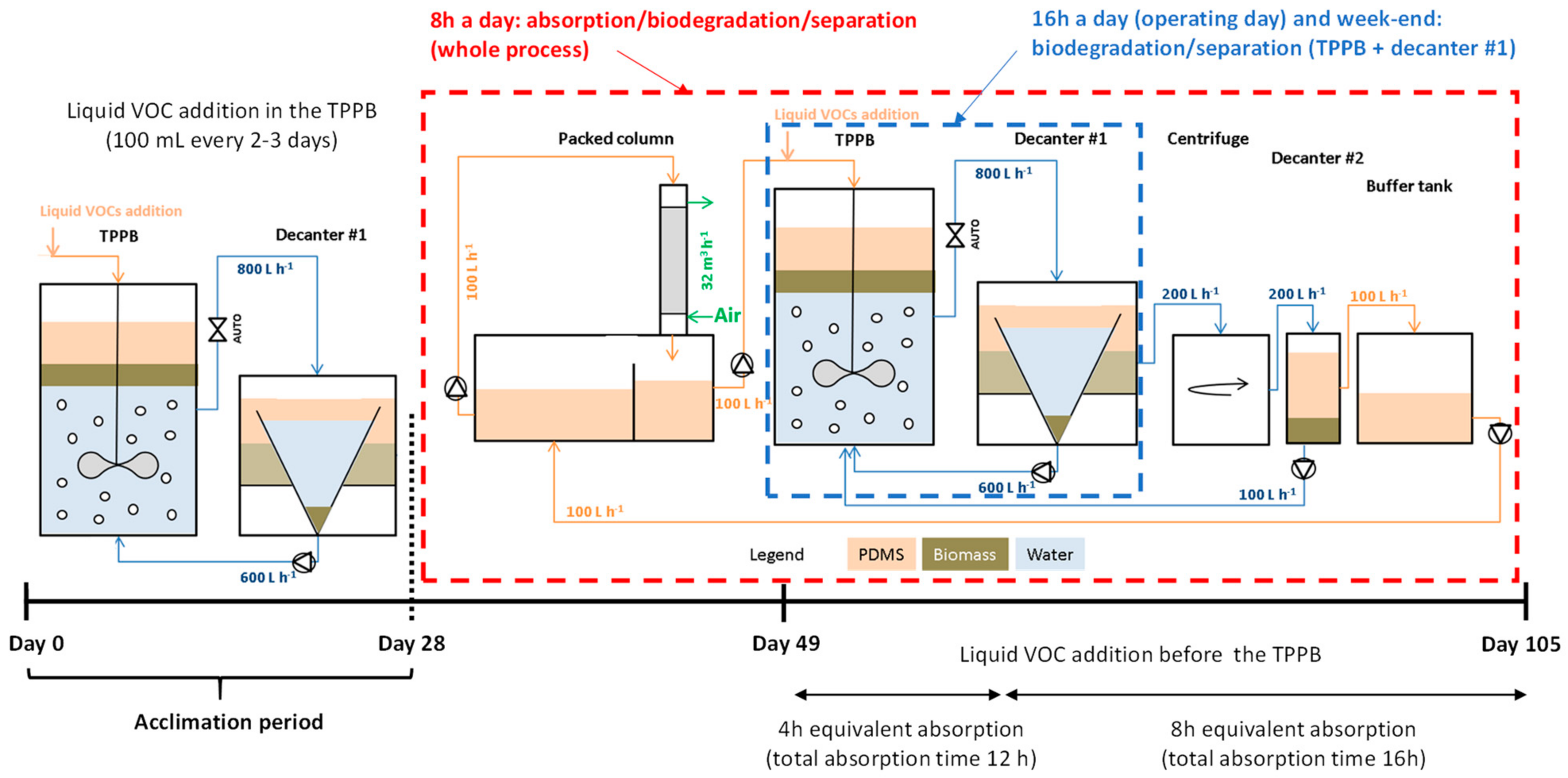
2.2. Operating Procedure
2.3. Measurements and Analyses
2.4. Characterization of Microbial Communities
2.5. Biosurfactant
3. Results and Discussion
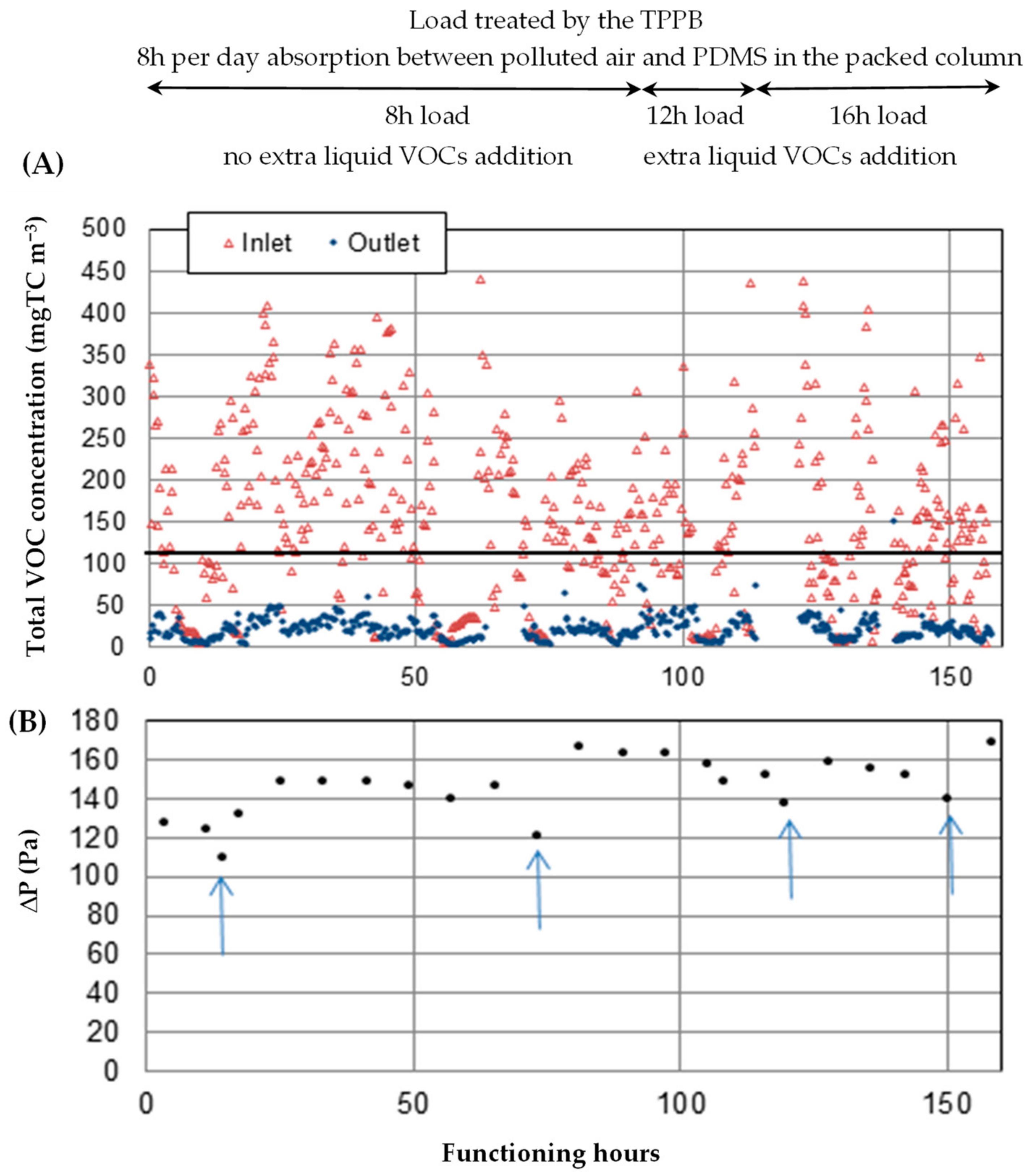
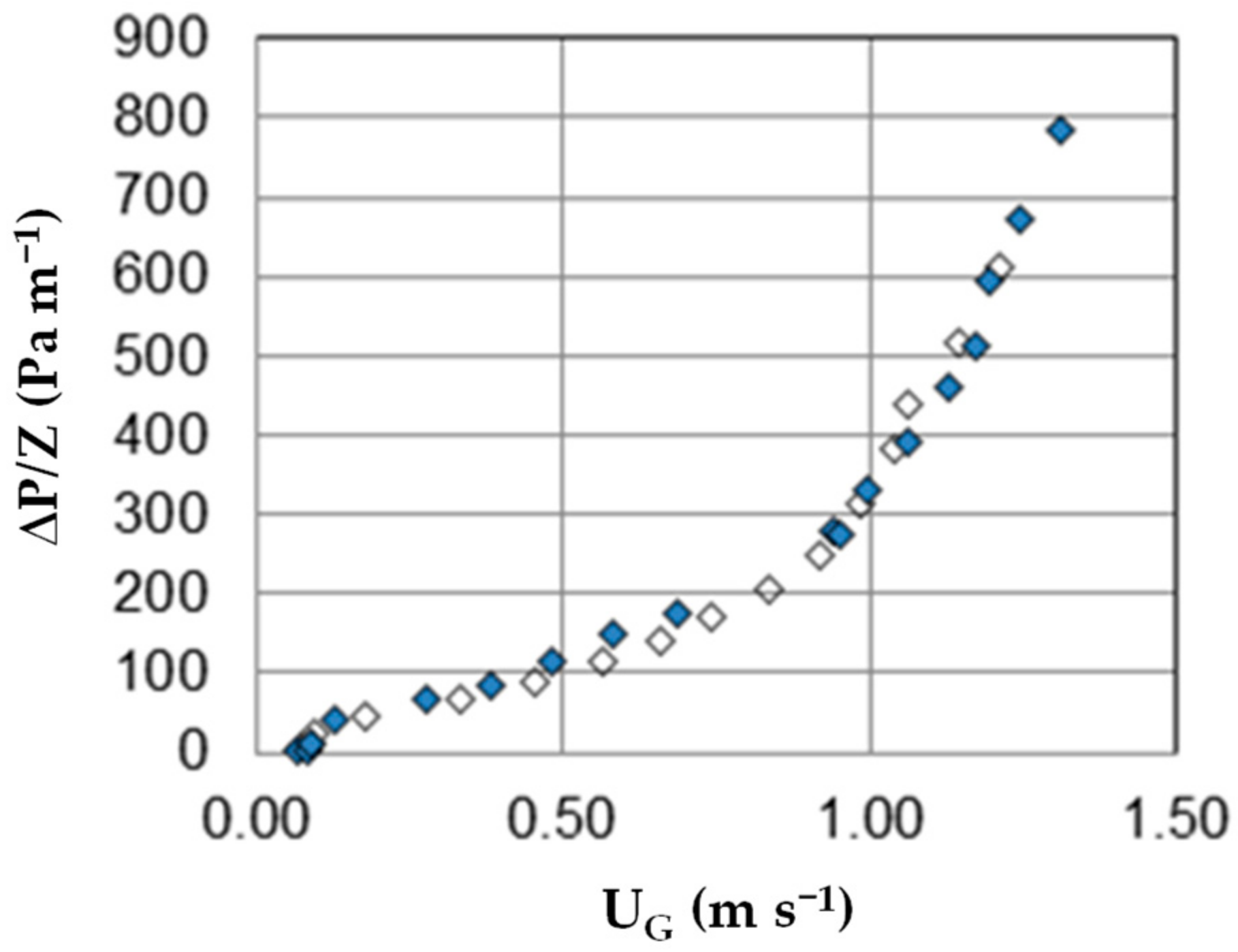
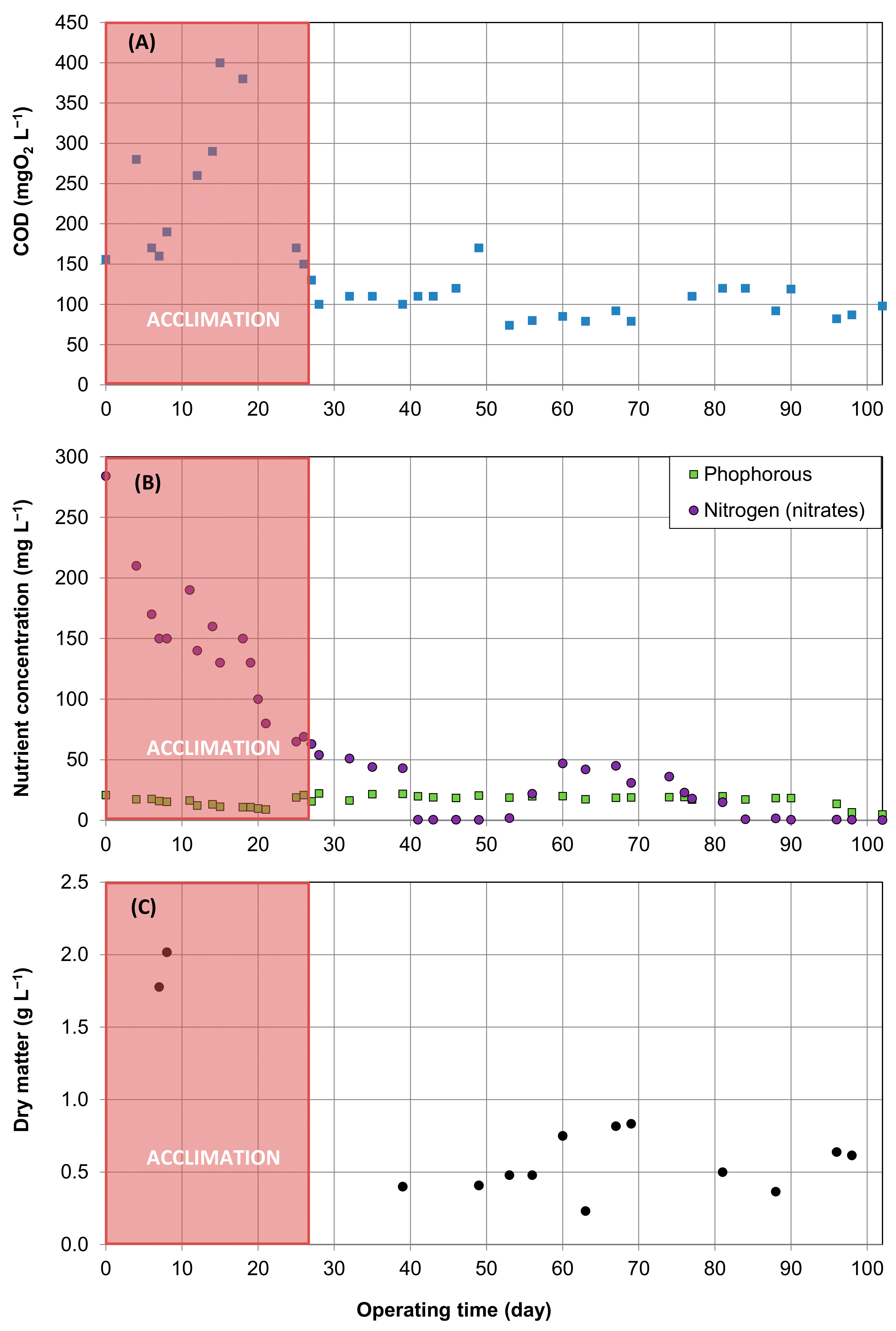
3.1. Absorption
3.2. Biological Regeneration of the PDMS
3.2.1. Aqueous Phase Content
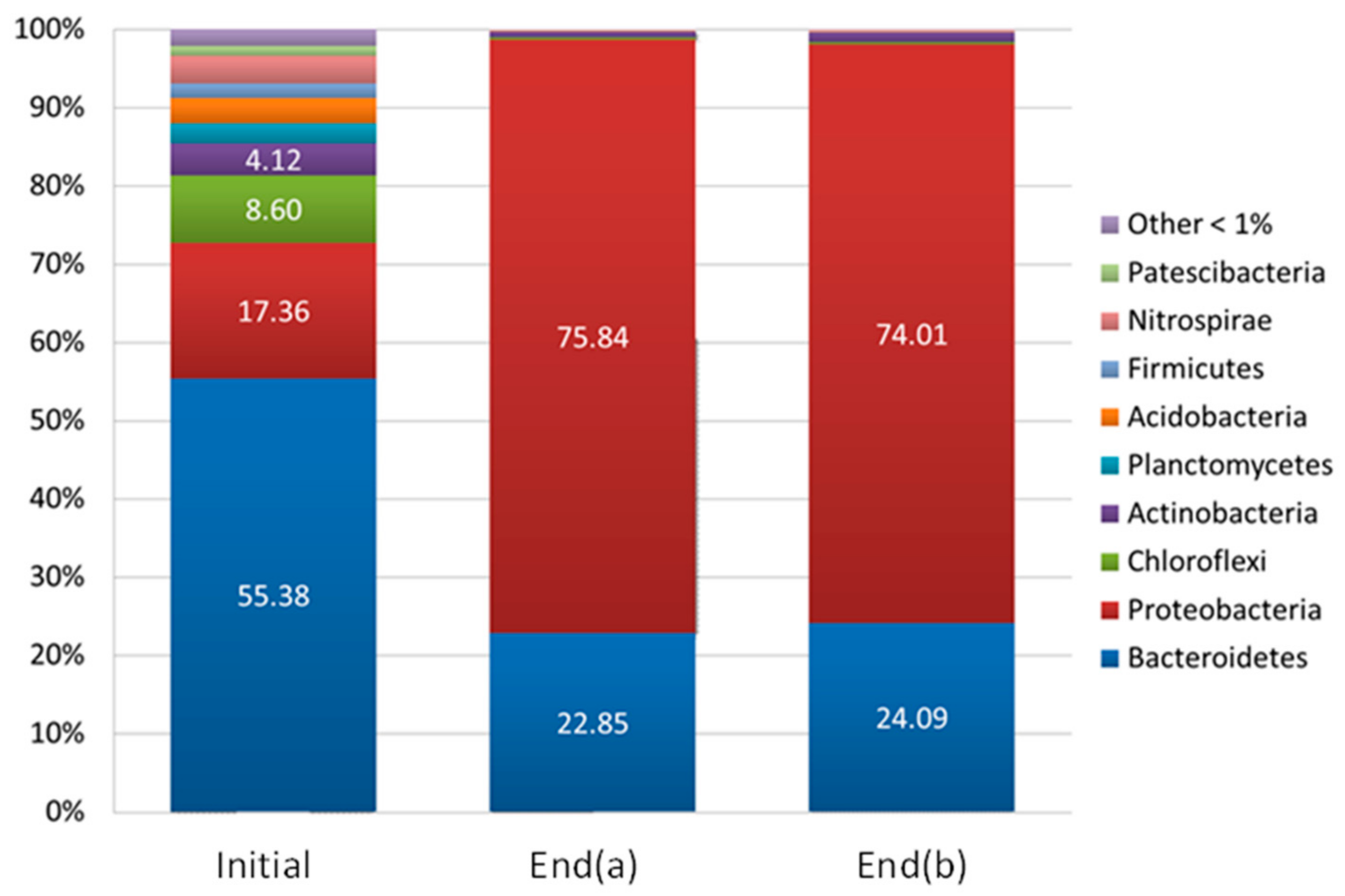
3.2.2. Microbial Community Dynamics
- (1)
- Alcaligenes faecalis subsp. Phenolicus, which represented 0.17% of the total sequences of the “initial” sludge, was enriched up to 37% and 30% of the total sequences of the acclimated sludge (“End(a)” and “End(b)”, respectively). It has to be noted that bacterial species belonging to the genus Alcaligenes have demonstrated versatile pollutant bioremediation ability, including phenols, polyaromatic hydrocarbon, and pesticides [29,30]. Because Alcaligenes faecalis subsp. Phenolicus is a denitrifying bacterium able to degrade phenol, it is possible that this bacterium is able to grow on the aromatic cycle molecules characterizing the composition of the real flow emitted on the industrial site, e.g., toluene, m-xylene, and 1,3,5-trimethylbenzene (1,3,5-TMB).
- (2)
- Thiopseudomonas denitrificans, which was present at 0.04% in the “initial” sludge, made up to 12 and 15% of the total sequences after sludge acclimation (“End(a)” and “End(b)”, respectively). Thiopseudomonas denitrificans is a denitrifying species able to oxidize sulfurs [31]. Its development was unexpected because the culture medium did not contain sulfur compounds. However, the presence of denitrifying bacteria such as Thiopseudomonas denitrificans could explain the nitrogen consumption previously reported (Figure 5B). Moreover, this presence suggests that anaerobic zones certainly existed in the pilot, probably due to an inadequate sludge recirculation in the settling tank. As a result, investigation could be carried out to elucidate the enrichment of this microbial species in the reactor.
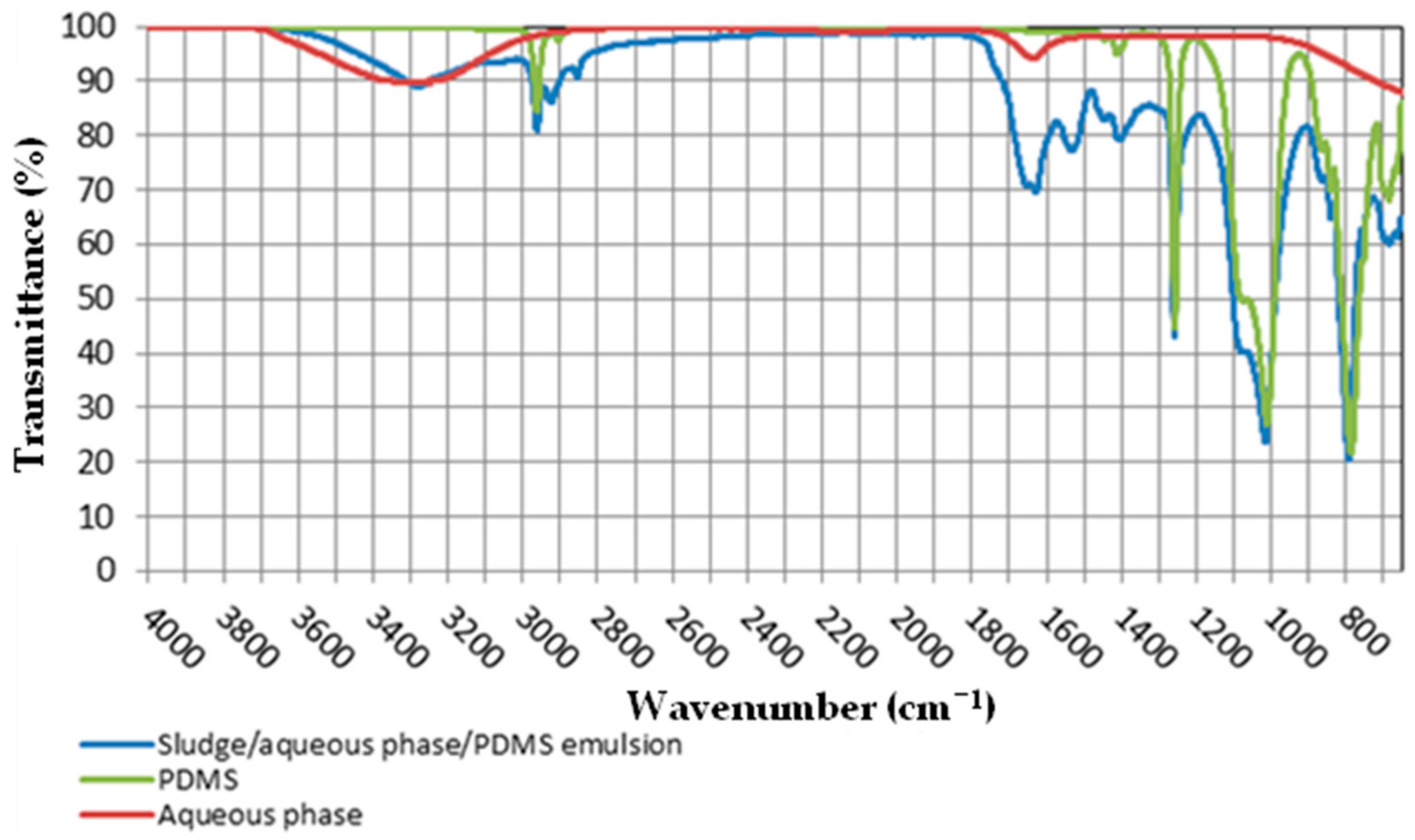
3.3. Separation
3.4. Comparison to the Conventional Technologies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz, R.; Daugulis, A.J.; Hernández, M.; Quijano, G. Recent Advances in Two-Phase Partitioning Bioreactors for the Treatment of Volatile Organic Compounds. Biotechnol. Adv. 2012, 30, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Mudliar, S.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for Treatment of VOCs and Odours—A Review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef] [PubMed]
- Chikh, R.; Couvert, A.; Aït Amar, H.; Amrane, A. Toluene Biodegradation in a Two Phase Partitioning System-Use of a Biodegradable Solvent. Environ. Prog. Sustain. Energy 2011, 30, 303–308. [Google Scholar] [CrossRef]
- Collins, L.D.; Daugulis, A.J. Simultaneous Biodegradation of Benzene, Toluene, and p-Xylene in a Two-Phase Partitioning Bioreactor: Concept Demonstration and Practical Application. Biotechnol. Prog. 1999, 15, 74–80. [Google Scholar] [CrossRef]
- Guillerm, M.; Couvert, A.; Amrane, A.; Dumont, É.; Norrant, E.; Lesage, N.; Juery, C. Characterization and Selection of PDMS Solvents for the Absorption and Biodegradation of Hydrophobic VOCs: PDMS Solvents for Absorption and Biodegradation of Hydrophobic VOCs. J. Chem. Technol. Biotechnol. 2016, 91, 1923–1927. [Google Scholar] [CrossRef]
- Lhuissier, M.; Couvert, A.; Amrane, A.; Kane, A.; Audic, J.-L. Characterization and Selection of Waste Oils for the Absorption and Biodegradation of VOC of Different Hydrophobicities. Chem. Eng. Res. Des. 2018, 138, 482–489. [Google Scholar] [CrossRef]
- Arca-Ramos, A.; Eibes, G.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Vegetable Oils as NAPLs in Two Phase Partitioning Bioreactors for the Degradation of Anthracene by Laccase. Chem. Eng. J. 2014, 240, 281–289. [Google Scholar] [CrossRef]
- Daugulis, A.J.; Janikowski, T.B. Scale-up Performance of a Partitioning Bioreactor for the Degradation of Polyaromatic Hydrocarbons by Sphingomonas aromaticivorans. Biotechnol. Lett. 2002, 24, 591–594. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wu, J.Y. Use of N-Hexadecane as an Oxygen Vector to Improve Phaffia Rhodozyma Growth and Carotenoid Production in Shake-Flask Cultures. J. Appl. Microbiol. 2006, 101, 1033–1038. [Google Scholar] [CrossRef]
- Guihéneuf, S.; Castillo, A.S.R.; Paquin, L.; Biard, P.-F.; Couvert, A.; Amrane, A. Absorption of Hydrophobic Volatile Organic Compounds in Ionic Liquids and Their Biodegradation in Multiphase Systems. In Production of Biofuels and Chemicals with Ionic Liquids; Fang, Z., Smith, R.L., Qi, X., Eds.; Biofuels and Biorefineries; Springer: Dordrecht, The Netherlands, 2014; Volume 1, pp. 305–337. ISBN 978-94-007-7710-1. [Google Scholar]
- Aldric, J.-M.; Thonart, P. Performance Evaluation of a Water/Silicone Oil Two-Phase Partitioning Bioreactor Using Rhodococcus erythropolis T902.1 to Remove Volatile Organic Compounds from Gaseous Effluents. J. Chem. Technol. Biotechnol. 2008, 83, 1401–1408. [Google Scholar] [CrossRef]
- Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Dumont, E.; Andres, Y.; Le Cloirec, P. Silicone Oil: An Effective Absorbent for the Removal of Hydrophobic Volatile Organic Compounds. J. Chem. Technol. Biotechnol. 2010, 85, 309–313. [Google Scholar] [CrossRef]
- Dumont, E.; Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Andrès, Y.; Le Cloirec, P. Hydrophobic VOC Absorption in Two-Phase Partitioning Bioreactors; Influence of Silicone Oil Volume Fraction on Absorber Diameter. Chem. Eng. Sci. 2012, 71, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Guillerm, M.; Couvert, A.; Amrane, A.; Norrant, E.; Breton, A.; Dumont, É. Toluene Degradation by a Water/Silicone Oil Mixture for the Design of Two Phase Partitioning Bioreactors. Chin. J. Chem. Eng. 2017, 25, 1512–1518. [Google Scholar] [CrossRef]
- Lebrero, R.; Osvaldo, D.F.; Pérez, V.; Cantera, S.; Estrada, J.M.; Muñoz, R. Biological Treatment of Gas Pollutants in Partitioning Bioreactors. In Advances in Chemical Engineering; Elsevier: Cambridge, MA, USA, 2019; Volume 54, pp. 239–274. ISBN 978-0-12-814996-6. [Google Scholar]
- Lalanne, F.; Malhautier, L.; Roux, J.-C.; Fanlo, J.-L. Absorption of a Mixture of Volatile Organic Compounds (VOCs) in Aqueous Solutions of Soluble Cutting Oil. Bioresour. Technol. 2008, 99, 1699–1707. [Google Scholar] [CrossRef]
- Nourmohammadi, M.; Golbabaei, F.; Karimi, A.; Pourmand, M.R.; Noor Poor, A.; Rahimi Foroushani, A.; Nourmohammadi, E. Absorption and Biodegradation of Toluene in a Two-Phase Low-Pressure Bioscrubber Using Cutting Oil as the Organic Phase. Health Scope 2019, 8, e65219. [Google Scholar] [CrossRef] [Green Version]
- Nourmohammadi, M.; Karimi, A.; Golbabaei, F.; Pourmand, M.R.; Rahimi Foroushanid, A.; Nourmohammadi, E. Biodegradation of Toluene in a Two-Phase Low-Pressure Bioscrubber with Using Silicon Oil as Organic Phase. Int. J. Environ. Anal. Chem. 2021, 1–13. [Google Scholar] [CrossRef]
- Lhuissier, M.; Couvert, A.; Kane, A.; Amrane, A.; Audic, J.-L.; Biard, P.-F. Volatile Organic Compounds Absorption in a Structured Packing Fed with Waste Oils: Experimental and Modeling Assessments. Chem. Eng. Sci. 2021, 238, 116598. [Google Scholar] [CrossRef]
- Darracq, G.; Couvert, A.; Couriol, C.; Thomas, D.; Amrane, A.; Dumont, E.; Andres, Y.; Le Cloirec, P. Optimization of the Volume Fraction of the NAPL, Silicone Oil, and Biodegradation Kinetics of Toluene and DMDS in a TPPB. Int. Biodeterior. Biodegrad. 2012, 71, 9–14. [Google Scholar] [CrossRef]
- Fisgativa, H.; Tremier, A.; Le Roux, S.; Bureau, C.; Dabert, P. Understanding the Anaerobic Biodegradability of Food Waste: Relationship between the Typological, Biochemical and Microbial Characteristics. J. Environ. Manag. 2017, 188, 95–107. [Google Scholar] [CrossRef]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- du Noüy, P.L. An interfacial tensiometer for universal use. J. Gen. Physiol. 1925, 7, 625–631. [Google Scholar] [CrossRef]
- Zuidema, H.; Waters, G. Ring Method for the Determination of Interfacial Tension. Ind. Eng. Chem. Anal. Ed. 1941, 13, 312–313. [Google Scholar] [CrossRef]
- Mackowiak, J. Fluid Dynamics of Packed Columns: Principles of the Fluid Dynamic Design of Columns for Gas/Liquid and Liquid/Liquid Systems; Chemische Technik/Verfahrenstechnik; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9783540887805. [Google Scholar]
- Urbain, V.; Block, J.C.; Manem, J. Bioflocculation in Activated Sludge: An Analytic Approach. Water Res. 1993, 27, 829–838. [Google Scholar] [CrossRef]
- Hu, M.; Wang, X.; Wen, X.; Xia, Y. Microbial Community Structures in Different Wastewater Treatment Plants as Revealed by 454-Pyrosequencing Analysis. Bioresour. Technol. 2012, 117, 72–79. [Google Scholar] [CrossRef]
- Basharat, Z.; Yasmin, A.; He, T.; Tong, Y. Genome Sequencing and Analysis of Alcaligenes faecalis Subsp. Phenolicus MB207. Sci. Rep. 2018, 8, 3616. [Google Scholar] [CrossRef]
- Durán, R.E.; Méndez, V.; Rodríguez-Castro, L.; Barra-Sanhueza, B.; Salvà-Serra, F.; Moore, E.R.B.; Castro-Nallar, E.; Seeger, M. Genomic and Physiological Traits of the Marine Bacterium Alcaligenes aquatilis QD168 Isolated From Quintero Bay, Central Chile, Reveal a Robust Adaptive Response to Environmental Stressors. Front. Microbiol. 2019, 10, 528. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.-B.; Jiang, Z.; Chen, C.; Yuan, Y.; Gao, L.-F.; Wang, H.-F.; Cheng, J.; Li, W.-J.; Wang, A.-J. Thiopseudomonas denitrificans Gen. Nov., Sp. Nov., Isolated from Anaerobic Activated Sludge. Int. J. Syst. Evol. Microbiol. 2015, 65, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameotra, S.S.; Makkar, R.S. Synthesis of Biosurfactants in Extreme Conditions. Appl. Microbiol. Biotechnol. 1998, 50, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.J.; Al-Wahaibi, Y.M.; Al-Bahry, S.N.; Elshafie, A.E.; Al-Bemani, A.S.; Al-Bahri, A.; Al-Mandhari, M.S. Production, Characterization, and Application of Bacillus licheniformis W16 Biosurfactant in Enhancing Oil Recovery. Front. Microbiol. 2016, 7, 1853. [Google Scholar] [CrossRef] [PubMed]
- Park, O.-J.; Lee, Y.-E.; Cho, J.-H.; Shin, H.-J.; Yoon, B.-D.; Yang, J.-W. Purification and Structural Characterization of Glycolipid Biosurfactants from Pseudomonas aeruginosa YPJ-80. Biotechnol. Bioprocess Eng. 1998, 3, 61–66. [Google Scholar] [CrossRef]
- Pemmaraju, S.C.; Sharma, D.; Singh, N.; Panwar, R.; Cameotra, S.S.; Pruthi, V. Production of Microbial Surfactants from Oily Sludge-Contaminated Soil by Bacillus subtilis DSVP23. Appl. Biochem. Biotechnol 2012, 167, 1119–1131. [Google Scholar] [CrossRef]
- Dumont, E.; Picard, C.; Guillerm, M.; Granero Fernandez, E.; Stavrakakis, C.; Norrant, E.; Juery, C.; Lesage, N.; Rouxel, F.; Balannec, B.; et al. Separation of Silicone Oil Droplets Dispersed in Activated Sludge. Sep. Sci. Technol. 2020, 55, 2369–2380. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of Various Types of VOCs by Adsorption/Catalytic Oxidation: A Review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-Temperature Catalysis for VOCs Removal in Technology and Application: A State-of-the-Art Review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Tomatis, M.; Moreira, M.T.; Xu, H.; Deng, W.; He, J.; Parvez, A.M. Removal of VOCs from Waste Gases Using Various Thermal Oxidizers: A Comparative Study Based on Life Cycle Assessment and Cost Analysis in China. J. Clean. Prod. 2019, 233, 808–818. [Google Scholar] [CrossRef]
| Molecules | Water | PDMS | Ratio water/PDMS |
|---|---|---|---|
| n-heptane | 2.08 × 105 | 2.34 | 88,888 |
| Ethyl acetate | 14.9 | 6.39 | 2.33 |
| Isopropanol | 0.31 | 19.93 | 0.016 |
| Methylisobutylketone (MIBK) | 21.1 | 1.78 | 11.8 |
| Toluene | 510.0 | 2.15 | 237 |
| m-xylene | 644.5 | 0.71 | 907 |
| 1,3,5-triméthylbenzène | 714.3 | 0.22 | 3246 |
| Sample | Acclimation Duration | Final Sequence Number | Cover Rate | OTU Number (Richness) | Simpson Index Number |
|---|---|---|---|---|---|
| Initial | - | 8899 | 0.98 | 529 | 0.98 |
| End(a) | 3 months | 12,856 | 0.99 | 179 | 0.83 |
| End(b) | 3 months | 11,464 | 0.99 | 205 | 0.87 |
| Process | VOCs Concen-trations (g m−3) | Issues |
|---|---|---|
| Absorption in PDMS + TPPB | <1 | PDMS cost PDMS/sludges separation step |
| Thermal oxidation | >5 | Natural gas cost (T ≈ 850 °C) Impact of natural gas to climate change |
| Catalytic oxidation | >1 | Natural gas cost (T from 200 to 400 °C) Catalyst cost Catalyst poisoning |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lhuissier, M.; Couvert, A.; Dabert, P.; Amrane, A.; Kane, A.; Audic, J.-L.; Dumont, E. Removal of a Mixture of Seven Volatile Organic Compounds (VOCs) Using an Industrial Pilot-Scale Process Combining Absorption in Silicone Oil and Biological Regeneration in a Two-Phase Partitioning Bioreactor (TPPB). Energies 2022, 15, 4576. https://doi.org/10.3390/en15134576
Lhuissier M, Couvert A, Dabert P, Amrane A, Kane A, Audic J-L, Dumont E. Removal of a Mixture of Seven Volatile Organic Compounds (VOCs) Using an Industrial Pilot-Scale Process Combining Absorption in Silicone Oil and Biological Regeneration in a Two-Phase Partitioning Bioreactor (TPPB). Energies. 2022; 15(13):4576. https://doi.org/10.3390/en15134576
Chicago/Turabian StyleLhuissier, Margaux, Annabelle Couvert, Patrick Dabert, Abdeltif Amrane, Abdoulaye Kane, Jean-Luc Audic, and Eric Dumont. 2022. "Removal of a Mixture of Seven Volatile Organic Compounds (VOCs) Using an Industrial Pilot-Scale Process Combining Absorption in Silicone Oil and Biological Regeneration in a Two-Phase Partitioning Bioreactor (TPPB)" Energies 15, no. 13: 4576. https://doi.org/10.3390/en15134576








