Investigation of an Intensified Thermo-Chemical Experimental Set-Up for Hydrogen Production from Biomass: Gasification Process Integrated to a Portable Purification System—Part II
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Gasification Test Campaigns
2.3. Simulation Model of the Integrated Plant
3. Results and Discussions
3.1. Process Performances
3.1.1. Biomass Gasification Tests with Catalytic Ceramic Candles
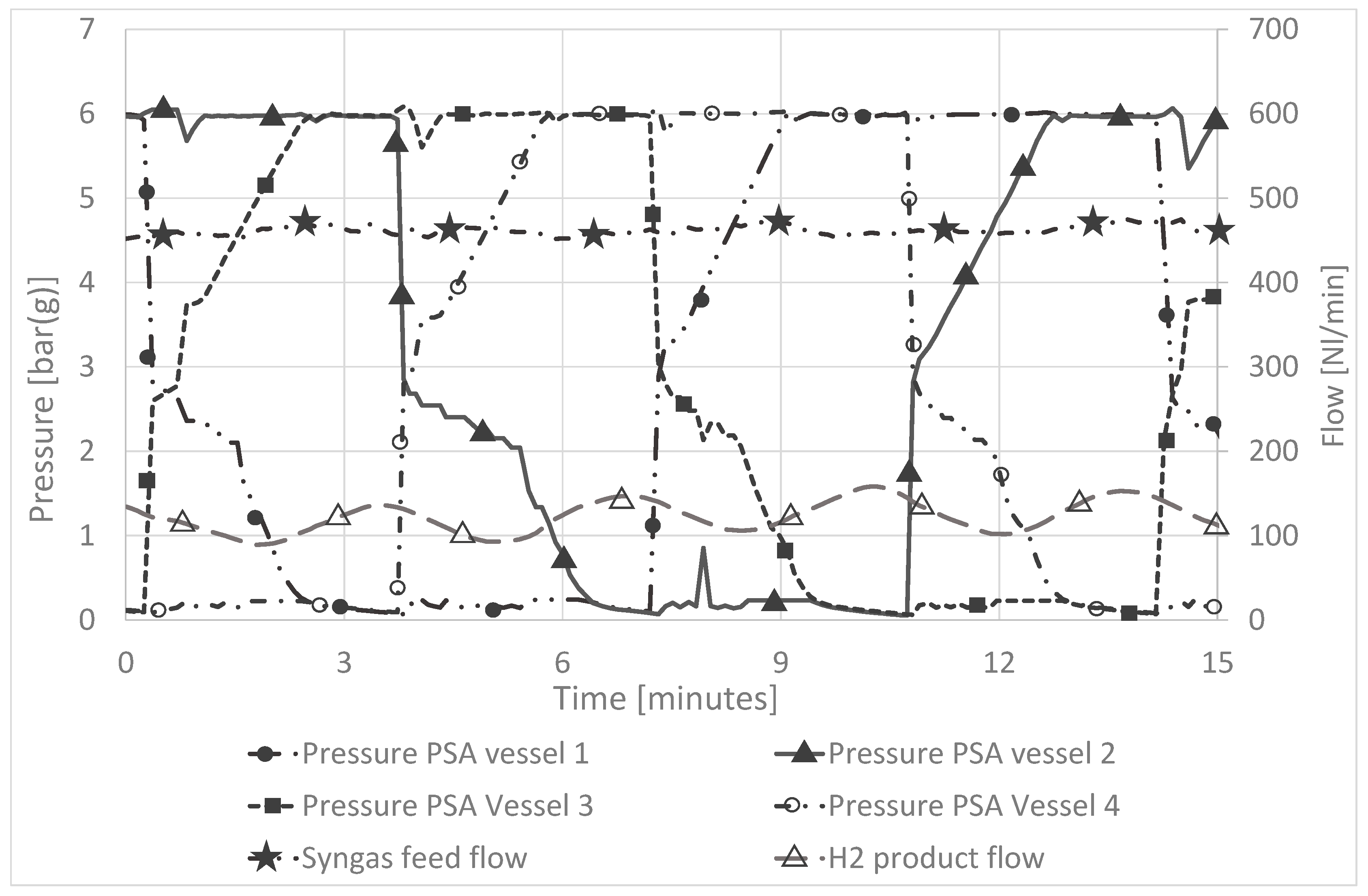
3.1.2. Operation of the PPS Unit with Real Product Gas from Biomass Gasification
3.1.3. Simulation vs. Experimental Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CHP | Combined Heat and Power |
| DT | Deformation Temperature |
| ER | Equivalence Ratio |
| FT | Flow Temperature |
| HT | Hemisphere Temperature |
| HHV | Higher Heating Value |
| LHV | Lower Heating Value |
| PSA | Pressure Swing Adsorption |
| PPS | Portable Purification System |
| S/B | Steam to Biomass ratio |
| SST | Shrinkage Starting Temperature |
| WGS | Water Gas Shift |
References
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Bocci, E.; Sisinni, M.; Moneti, M.; Vecchione, L.; Di Carlo, A.; Villarini, M. State of Art of Small Scale Biomass Gasification Power Systems: A Review of the Different Typologies. Energy Procedia 2014, 45, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Thapa, S.; Bhoi, P.R.; Kumar, A.; Huhnke, R.L. Effects of syngas cooling and biomass filter medium on tar removal. Energies 2017, 10, 349. [Google Scholar] [CrossRef] [Green Version]
- Orecchini, F.; Bocci, E. Biomass to hydrogen for the realization of closed cycles of energy resources. Energy 2007, 32, 1006–1011. [Google Scholar] [CrossRef]
- Bocci, E.; Carlo, A.D.; Marcelo, D. Power plant perspectives for sugarcane mills. Energy 2009, 34, 689–698. [Google Scholar] [CrossRef]
- Marcantonio, V.; De Falco, M.; Capocelli, M.; Bocci, E.; Colantoni, A.; Villarini, M. Process analysis of hydrogen production from biomass gasification in fluidized bed reactor with different separation systems. Int. J. Hydrogen Energy 2019, 44, 10350–10360. [Google Scholar] [CrossRef]
- Li, X.T.; Grace, J.R.; Lim, C.J.; Watkinson, A.P.; Chen, H.P.; Kim, J.R. Biomass gasification in a circulating fluidized bed. Biomass Bioenergy 2004, 26, 171–193. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Iversen, H.L.; Gøbel, B. Update on gas cleaning technologies. In Handbook Biomass Gasification; Biomass Technology Group: Enschede, The Netherlands, 2005. [Google Scholar]
- Reed, T.B. Principles and Technology of Biomass Gasification. Adv. Sol. Energy Annu. Rev. Res. Dev. 1985, 2, 125–174. [Google Scholar] [CrossRef]
- Qi, T.; Lei, T.; Yan, B.; Chen, G.; Li, Z.; Fatehi, H.; Wang ZBai, X.-S. Biomass steam gasification in bubbling fluidized bed for higher-H2 syngas: CFD simulation with coarse grain model. Int. J. Hydrogen Energy 2019, 44, 6448–6460. [Google Scholar] [CrossRef]
- Fremaux, S.; Beheshti, S.-M.; Ghassemi, H.; Shahsavan-Markadeh, R. An experimental study on hydrogen-rich gas production via steam gasification of biomass in a research-scale fluidized bed. Energy Convers. Manag. 2015, 91, 427–432. [Google Scholar] [CrossRef]
- Thao, N.T.N.L.; Chiang, K.-Y.; Wan, H.-P.; Hung, W.-C.; Liu, C.-F. Enhanced trace pollutants removal efficiency and hydrogen production in rice straw gasification using hot gas cleaning system. Int. J. Hydrogen Energy 2019, 44, 3363–3372. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Review and analysis of biomass gasification models. Renew. Sustain. Energy Rev. 2010, 14, 2841–2851. [Google Scholar] [CrossRef]
- An, H.; Song, T.; Shen, L.; Qin, C.; Yin, J.; Feng, B. Coal gasification with in situ CO2 capture by the synthetic CaO sorbent in a 1 kWth dual fluidised-bed reactor. Int. J. Hydrogen Energy 2012, 37, 14195–14204. [Google Scholar] [CrossRef]
- Klass, D.L. Energy Consumption, Reserves, Depletion, and Environmental Issues. In Biomass for Renewable Energy, Fuels, and Chemicals; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1–27. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef] [Green Version]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. A critical review on biomass gasification, co-gasification, and their environmental assessments. Biofuel Res. J. 2016, 3, 483–495. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/energy/sites/ener/files/hydrogen_strategy.pdf (accessed on 7 May 2022).
- Available online: https://ec.europa.eu/energy/topics/energy-system-integration/hydrogen_en (accessed on 27 May 2022).
- Caballero, M.A.; Corella, J.; Aznar, M.P.; Gil, J. Biomass gasification with air in fluidized bed. Hot gas cleanup with selected commercial and full-size nickel-based catalysts. Ind. Eng. Chem. Res. 2000, 39, 1143–1154. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Brown, R.C. Steam reforming of tar compounds over Ni/olivine catalysts doped with CeO2. Energy Convers. Manag. 2007, 48, 68–77. [Google Scholar] [CrossRef]
- Barisano, D.; Canneto, G.; Nanna, F.; Villone, A.; Fanelli, E.; Freda, C.; Grieco, M.; Cornacchia, G.; Braccio, G.; Marcantonio, V.; et al. Investigation of an Intensified Thermo-Chemical Experimental Set-Up for Hydrogen Production from Biomass: Gasification Process Performance—Part I. Process 2021, 9, 1104. [Google Scholar] [CrossRef]
- UNIQUE Cooperation Research Project, Contract N.211517 7FP. n.d.
- Bocci, E.; Di Carlo, A.; McPhail, S.J.; Gallucci, K.; Foscolo, P.U.; Moneti, M.; Villarini, M.; Carlini, M. Biomass to fuel cells state of the art: A review of the most innovative technology solutions. Int. J. Hydrogen Energy 2014, 39, 21876–21895. [Google Scholar] [CrossRef]
- Barisano, D.; Canneto, G.; Nanna, F.; Alvino, E.; Pinto, G.; Villone, A.; Carnevale, M.; Valerio, V.; Battafarano, A.; Braccio, G. Steam/oxygen biomass gasification at pilot scale in an internally circulating bubbling fluidized bed reactor. Fuel Process. Technol. 2016, 141, 74–81. [Google Scholar] [CrossRef]
- Available online: http://www.magnolithe.at/ (accessed on 7 May 2022).
- Fredriksson, H.O.A.; Lancee, R.J.; Thüne, P.C.; Veringa, H.J.; Niemantsverdriet, J.W.H. Olivine as tar removal catalyst in biomass gasification: Catalyst dynamics under model conditions. Appl. Catal. B Environ. 2013, 130, 168–177. [Google Scholar] [CrossRef]
- Lancee, R.J. Characterization and Reactivity of Olivine and Model Catalysts for Biomass Gasification. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Marcantonio, V.; Müller, M.; Bocci, E. A review of hot gas cleaning techniques for hydrogen chloride removal from biomass-derived syngas. Energies 2021, 14, 6519. [Google Scholar] [CrossRef]
- Marcantonio, V.; Bocci, E.; Ouweltjes, J.P.; Del Zotto, L.; Monarca, D. Evaluation of sorbents for high temperature removal of tars, hydrogen sulphide, hydrogen chloride and ammonia from biomass-derived syngas by using Aspen Plus. Int. J. Hydrogen Energy 2020, 45, 6651–6662. [Google Scholar] [CrossRef]
- Marcantonio, V.; Bocci, E.; Monarca, D. Development of a chemical quasi-equilibrium model of biomass waste gasification in a fluidized-bed reactor by using Aspen plus. Energies 2019, 13, 53. [Google Scholar] [CrossRef] [Green Version]
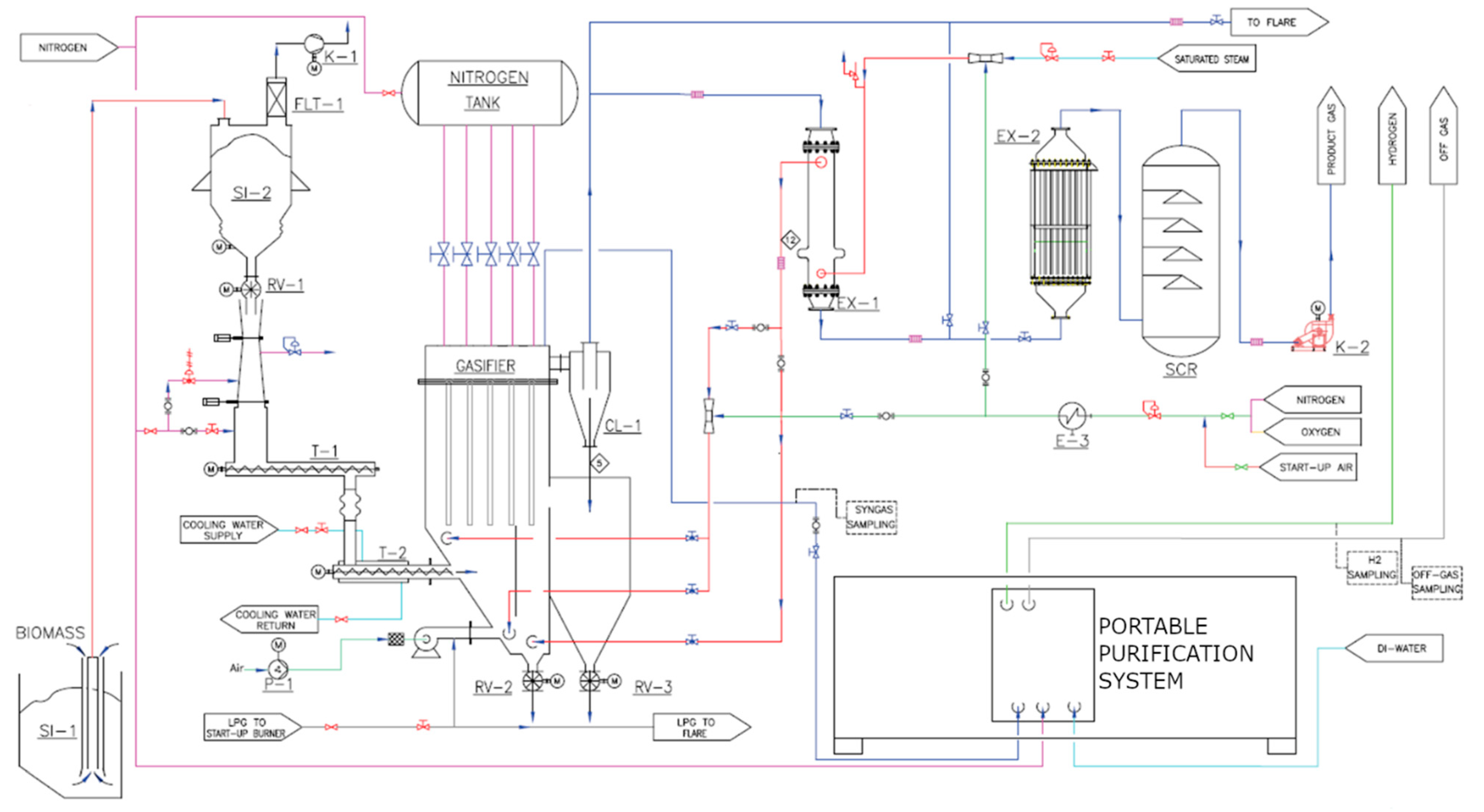
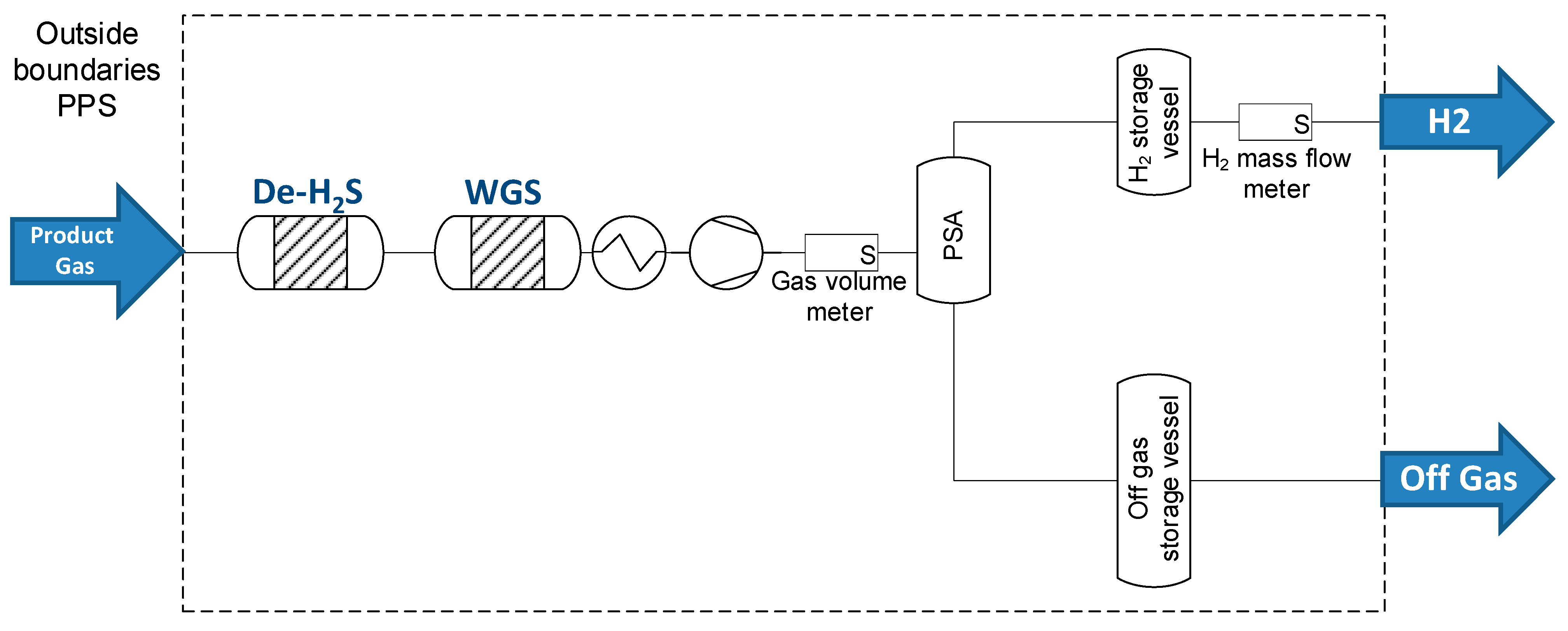



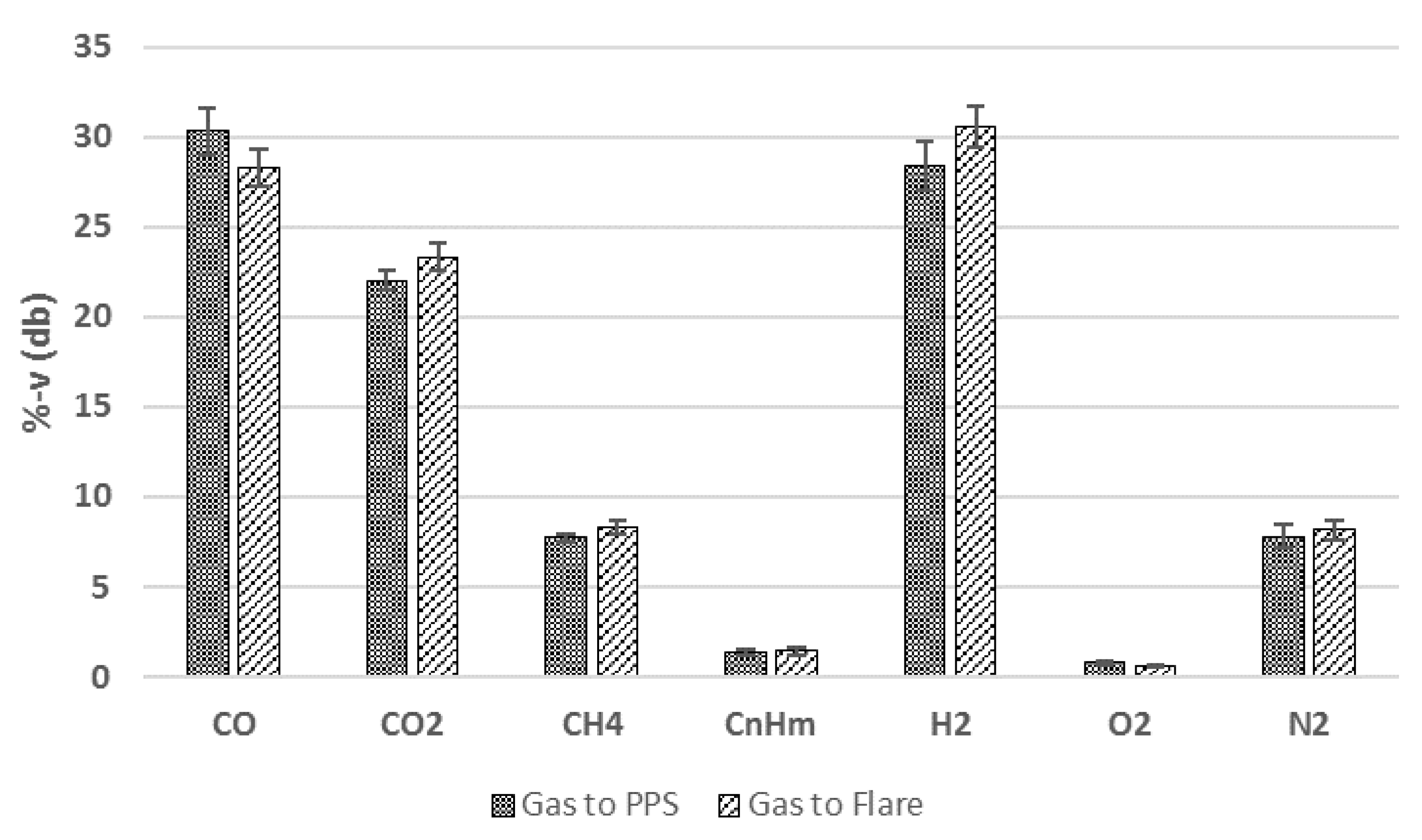

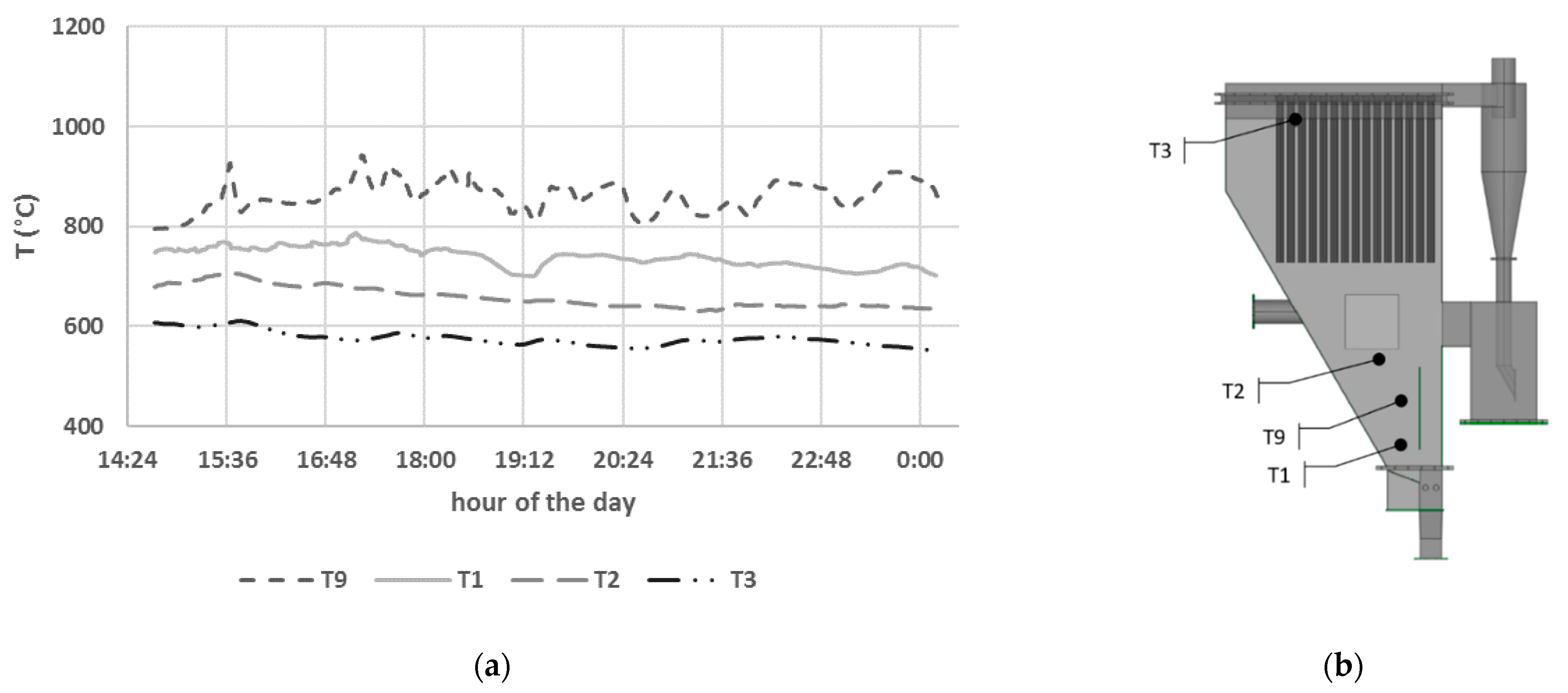

| Detection Tool | Range | Accuracy | Standard | |
|---|---|---|---|---|
| Temperature | Thermocouples K Types Model Transmitter: WT531 and WT532 Honeywell | 0 to 1200 °C | Class 2 precision | ANSI MC 96.1, IEC 60584-1, Class 2 |
| Pressure | Pressure Transmitter Honeywell Models WG511 Type 316 Stainless Steel Base and Diaphragm | 0 ÷ 3 bar | ±0.1% of full scale | - |
| Mass Flow | Calibrated Flanges Model Transmitter: WD520 and WD521 Honeywell | - | 2% of full-scale | ISO 5167:2003 and ASME MFC-14M-1995 |
| Detection Tool | Range | Accuracy | Standard | |
|---|---|---|---|---|
| Temperature | Thermocouples | 0 to 350 °C | Class 2 precision | IEC 60584-1, Class 2 |
| TC Type K | ||||
| Pressure | Pressure Transmitter Danfoss Model MBS 4701 Silicon based pressure transducer | Full scale = 10 bar | ≤±1% | - |
| Mass Flow Feed | Volume flow gas meter Dresser C-RM-G16 Rotary gas meter | MAX volumetric flow = 25 m3/h | ±2% | ISO 5167:2003 and ASME MFC-14M-1995 |
| Mass Flow Product | Mass Flow meter Bürkert 2712 (156 262) Thermal Mass Flow Controller | Full scale = 500 SL/min | ±0.3% full scale |
| Operating Parameter | Test I | Test II |
|---|---|---|
| Feeding rate (kgar/h) a | 175 | 135 |
| Steam/Biomass (S/B) b | 0.5–0.6 | 0.65–0.7 |
| Equivalence Ratio (ER) b | 0.24–0.25 | 0.22–0.25 |
| Bulk Density a (kg/m3) | Humidity a (%wt) | Chip Size Range (cm) | |||
|---|---|---|---|---|---|
| 450 ± 30 | 11 ± 2 | 2–3 | |||
| Proximate Analysis a (%wt., dry basis) | |||||
| Ash | Volatile Matter | Fixed Carbon b | |||
| 1.23 ± 0.06 | 80.6 ± 1.7 | 18.2 ± 1.8 | |||
| Ultimate Analysis a (%wt., dry basis) | |||||
| C | H | N | O b | Cl | S |
| 47.9 ± 0.7 | 6.3 ± 0.3 | 0.32 ± 0.02 | 44.3 ± 1.1 | 0.012 ± 0.002 | 0.015 ± 0.002 |
| Heating values a (kJ/kgdry) | |||||
| HHV | LHV c | ||||
| 19,510 ± 80 | 18,140 ± 140 | ||||
| Temperature (°C) | SST | DT | HT | FT |
| 915 ± 5 | 1000 ± 10 | 1180 ± 10 | 1210 ± 10 |
| Reaction | Reaction Name | Heat of Reaction | Reaction Number |
|---|---|---|---|
| Heterogeneous reaction | |||
| C + 0.5 O2 → CO | Char partial combustion | (−111 MJ kmol−1) | (R1) |
| C + H2O ↔ CO + H2 | Water–gas | (+172 MJ kmol−1) | (R2) |
| Homogeneous reactions | |||
| H2 + 0.5 O2 → H2O | H2 partial combustion | (−283 MJ kmol−1) | (R3) |
| CO + H2O ↔ CO2 + H2 | Water–gas shift | (−41 MJ kmol−1) | (R4) |
| CH4 + H2O → CO + 3H2 | Steam-methane reforming | (+206 MJ kmol−1) | (R5) |
| ASPEN Plus Name | Block ID | Description |
|---|---|---|
| RYIELD | DECOMP | Yield reactor—converts the non-conventional stream “BIOMASS” into its conventional components |
| RSTOIC | RSTOIC | RStoic reactor—simulates the production of NH3 and H2S |
| CANDLE | RStoic reactor—simulates the catalyst filter reaction | |
| TARPROD | RStoic reactor—simulates the production of toluene and benzene | |
| SEP | SEP | Separator—separates the biomass into three streams: VOLATILE; CHAR; and INORG |
| SEP | SEPH2 | Separator—separates 25% of hydrogen to produce tar |
| H2OREMOV | Separator—simulates a dryer which removes all the water | |
| PSA | Separator—simulates PSA process and extracts pure hydrogen with 70% efficiency | |
| MIXER | MIX | Mixer—mixes oxidizing fluid with VOL stream, that represents combustible fluid |
| MIX2 | Mixer—mixes the gas from gasifier with INORG and TAR streams | |
| MIX3 | Mixer—mixes the stream S3 and H2 | |
| FSPLIT | SPLIT | Splitter—splits char unreacted (UNREACT), char to gasifier (TOGASIF) and char which occurs in tar production (S3) |
| GASIF | Gibbs free energy reactor—simulates drying, pyrolysis, partial oxidation and gasification and restricts chemical equilibrium of the specified reactions to set the syngas composition by specifying a temperature approach for individual reactions | |
| REQUIL | H2SADSOR | REquil reactor—simulates the reaction of ZnO sorbent with H2S |
| WGS | REquil reactor—simulates water gas shift reaction | |
| COMPRESSOR | COMPR | Compressor—raises gas pressure upstream of PSA |
| Experimental Results | Simulation Results | |||
|---|---|---|---|---|
| Test I | Test II | Test I | Test II | |
| S/B | 0.5–0.6 | 0.65–0.7 | 0.55 | 0.68 |
| ER | 0.24–0.25 | 0.22–0.25 | 0.245 | 0.235 |
| H2 (%v, dry) | 28 | 39 | 30.1 | 42.1 |
| CO (%v, dry) | 30 | 22 | 32.2 | 23.7 |
| CO2 (%v, dry) | 22 | 35 | 20.8 | 31.1 |
| CH4 (%v, dry) | 7 | 3 | 5.4 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barisano, D.; Canneto, G.; Nanna, F.; Villone, A.; Fanelli, E.; Freda, C.; Grieco, M.; Lotierzo, A.; Cornacchia, G.; Braccio, G.; et al. Investigation of an Intensified Thermo-Chemical Experimental Set-Up for Hydrogen Production from Biomass: Gasification Process Integrated to a Portable Purification System—Part II. Energies 2022, 15, 4580. https://doi.org/10.3390/en15134580
Barisano D, Canneto G, Nanna F, Villone A, Fanelli E, Freda C, Grieco M, Lotierzo A, Cornacchia G, Braccio G, et al. Investigation of an Intensified Thermo-Chemical Experimental Set-Up for Hydrogen Production from Biomass: Gasification Process Integrated to a Portable Purification System—Part II. Energies. 2022; 15(13):4580. https://doi.org/10.3390/en15134580
Chicago/Turabian StyleBarisano, Donatella, Giuseppe Canneto, Francesco Nanna, Antonio Villone, Emanuele Fanelli, Cesare Freda, Massimiliano Grieco, Andrea Lotierzo, Giacinto Cornacchia, Giacobbe Braccio, and et al. 2022. "Investigation of an Intensified Thermo-Chemical Experimental Set-Up for Hydrogen Production from Biomass: Gasification Process Integrated to a Portable Purification System—Part II" Energies 15, no. 13: 4580. https://doi.org/10.3390/en15134580






