Self-Supporting NiFe Layered Double Hydroxide “Nanoflower” Cluster Anode Electrode for an Efficient Alkaline Anion Exchange Membrane Water Electrolyzer
Abstract
:1. Introduction
2. Experimental
2.1. Chemical and Materials
2.2. Catalyst Synthesis
2.2.1. Synthesis of NiFe LDHs/Ni Fiber
2.2.2. Synthesis of NiCo LDHs/Ni Fiber
2.2.3. Synthesis of NiMn LDHs/Ni Fiber
2.2.4. Synthesis of NiFe LDHs/Ni Foam
2.2.5. Synthesis of IrO2/Ni Fiber
2.2.6. Preparation of the AEMWE Single-Cell
2.3. Material Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
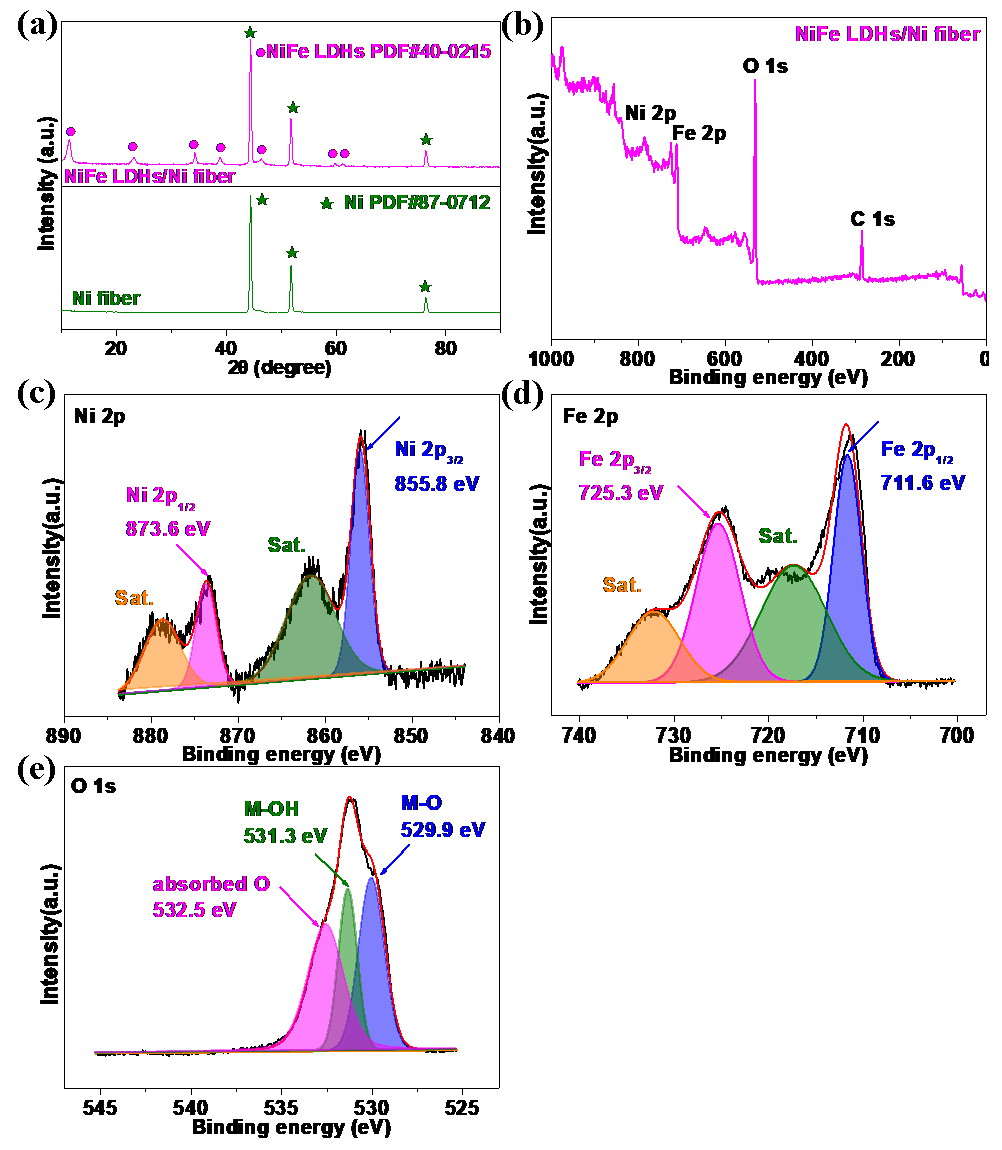
3.1. Structure and Characterization
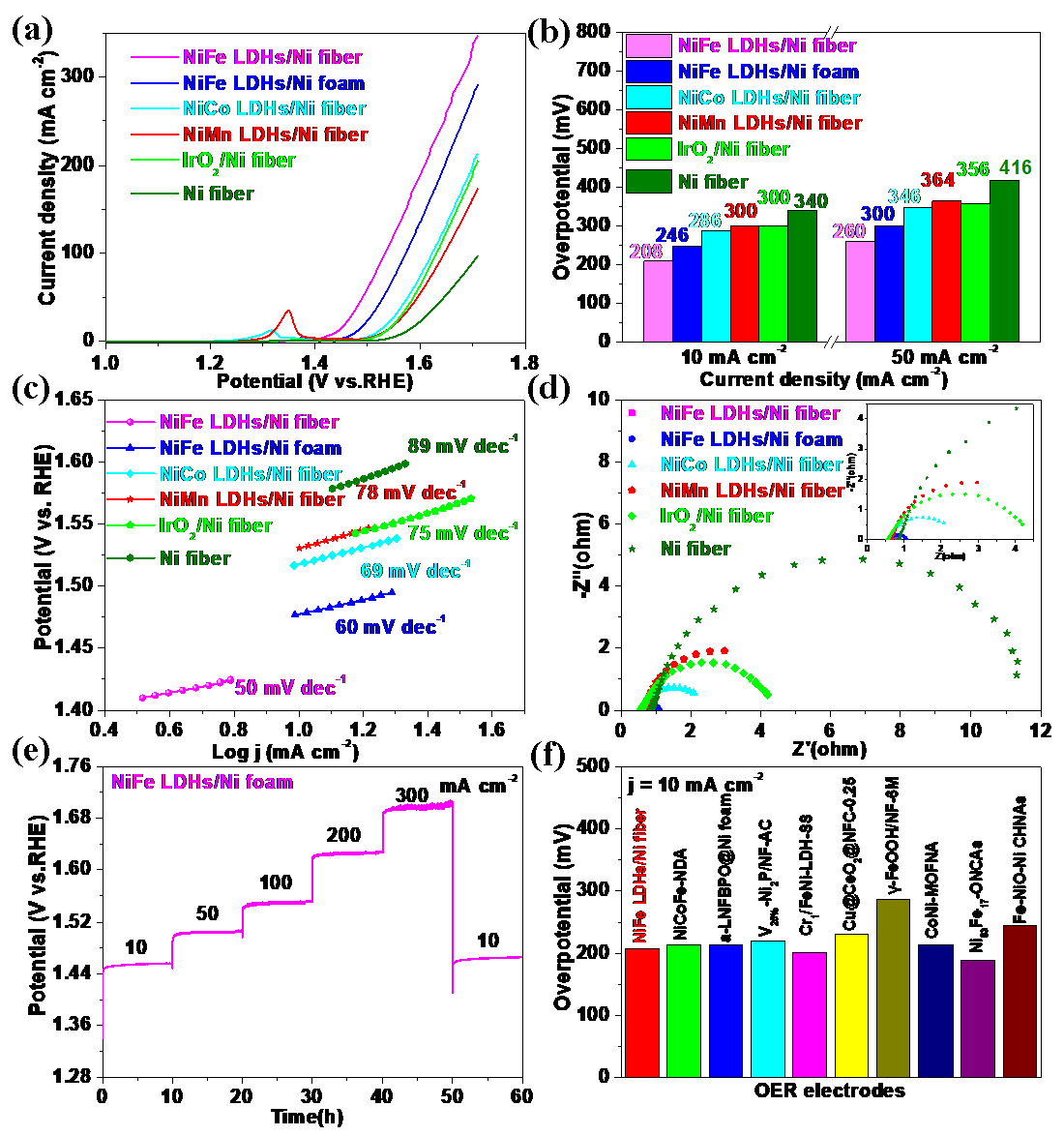
3.2. OER Performance in Alkaline Media
3.3. Performance of NiFe LDHs/Ni Fiber Electrode as the Anode of the AEMWE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, W.; Gao, Y.; Chen, Z.; Zhao, Y.; Wu, Z.; Wang, L. Strategies on improving the electrocatalytic hydrogen evolution performances of metal phosphides. Chin. J. Catal. 2021, 42, 1876–1902. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Li, Z.; Bu, X. Recent progress on NiFe-based electrocatalysts for the oxygen evolution reaction. Small 2020, 16, 2003916–2003938. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, W.; Shao, W.; Bai, M.; Zhou, M.; Li, S.; Ma, T.; Ma, L.; Cheng, C.; Liu, X. Synthesis and electronic modulation of nanostructured layered double hydroxides for efficient electrochemical oxygen evolution. ChemSusChem 2021, 14, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.S.; Lim, J.; Kang, P.W.; Lee, J.W.; Kang, G.; Lee, H. Design principles of NiFe-layered double hydroxide anode catalysts for anion exchange membrane water electrolyzers. ACS Appl. Mater. Interfaces 2021, 13, 37179–37186. [Google Scholar] [CrossRef]
- Liu, P.; Chen, B.; Liang, C.; Yao, W.; Cui, Y.; Hu, S.; Zou, P.; Zhang, H.; Fan, H.; Yang, C. Tip-enhanced electric field: A new mechanism promoting mass transfer in oxygen evolution reactions. Adv. Mater. 2021, 33, 2007377–2007385. [Google Scholar] [CrossRef]
- Yue, K.; Liu, J.; Zhu, Y.; Xia, C.; Wang, P.; Zhang, J.; Kong, Y.; Wang, X.; Yan, Y.; Xia, B.Y. In situ ion-exchange preparation and topological transformation of trimetal-organic frameworks for efficient electrocatalytic water oxidation. Energy Environ. Sci. 2021, 14, 6546–6553. [Google Scholar] [CrossRef]
- Gong, L.; Koh, J.; Yeo, B.S. Mechanistic study of the synergy between iron and transition metals for the catalysis of the oxygen evolution reaction. ChemSusChem 2018, 11, 3790–3795. [Google Scholar] [CrossRef]
- Chen, Y.; Rui, K.; Zhu, J.; Dou, S.; Sun, W. Recent progress on nickel-based oxide/(oxy)hydroxide electrocatalysts for the oxygen evolution reaction. Chem. Eur. J. 2019, 25, 703–713. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, X.; Wan, H.; Wang, S.; Zhao, Y.; Zhang, J.; Zhou, D.; Gao, W.; Ma, R.; Sasaki, T.; et al. Interface modulation of two-dimensional superlattices for efficient overall water splitting. Nano Lett. 2019, 19, 4518–4526. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J. A review on NiFe-based electrocatalysts for efficient alkaline oxygen evolution reaction. J. Power Sources 2020, 448, 227375–227424. [Google Scholar] [CrossRef]
- Xu, D.; Stevens, M.B.; Cosby, M.R.; Oener, S.Z.; Smith, A.M.; Enman, L.J.; Ayers, K.E.; Capuano, C.B.; Renner, J.N.; Danilovic, N.; et al. Earth-abundant oxygen electrocatalysts for alkaline anion exchange-membrane water electrolysis: Effects of catalyst conductivity and comparison with performance in three-electrode cells. ACS Catal. 2019, 9, 7–15. [Google Scholar] [CrossRef]
- Zignani, S.C.; Faro, M.L.; Trocino, S.; Aricò, A.S. Investigation of NiFe-Based catalysts for oxygen evolution in anion-exchange membrane electrolysis. Energies 2020, 13, 1720. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, C.; Fang, Z.; Xu, L.; Lu, C.; Hou, W. Ultrafast room-temperature synthesis of self-supported NiFe-layered double hydroxide as large-current-density oxygen evolution electrocatalyst. Small 2021, 18, 2104354–2104363. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, E.; Gao, J.; Yang, J.; Wu, C.; Jiang, L.; Zhu, M.; Sun, G. An exceptionally facile synthesis of high efficient oxygen evolution electrodes for zinc-oxygen batteries. ChemElectroChem 2017, 4, 2190–2195. [Google Scholar] [CrossRef]
- Cao, D.; Xu, H.; Cheng, D. Branch-leaf-shaped CuNi@NiFeCu nanodendrites as highly efficient electrocatalysts for overall water splitting. Appl. Catal. B 2021, 398, 120600–120609. [Google Scholar] [CrossRef]
- Zhang, R.; Duan, J.; Feng, J.; Mei, L.; Zhang, Q.; Wang, A. Walnut kernel-like iron-cobalt-nickel sulfide nanosheets directly grown on nickel foam: A binder-free electrocatalyst for high-efficiency oxygen evolution reaction. J. Colloid Interface Sci. 2021, 587, 141–149. [Google Scholar] [CrossRef]
- Guo, W.; Kim, J.; Kim, H.; Han, G.H.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Sandwich-like Co(OH)x/Ag/Co(OH)2 nanosheet composites for oxygen evolution reaction in anion exchange membrane water electrolyzer. J. Alloys Compd. 2021, 889, 161674–161681. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.; Park, Y.S.; Kwon, N.; Woo, S.; Selvam, N.C.S.; Han, G.S.; Jung, H.S.; Yoo, P.J.; Choi, S.M.; et al. Chemical transformation approach for high-performance ternary NiFeCo metal compound-based water splitting electrodes. Appl. Catal. B 2021, 294, 120246–120254. [Google Scholar] [CrossRef]
- Kim, J.-C.; Kim, J.; Park, J.C.; Ahn, S.H.; Kim, D.-W. Ru2P nanofibers for high-performance anion exchange membrane water electrolyzer. Chem. Eng. J. 2021, 420, 130491–130498. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, G.; Liu, X.; Ning, B.; Shi, C.; Pan, L.; Zhang, X.; Huang, Z.-F.; Zou, J.-J. Self-supporting NiFe LDH-MoSx integrated electrode for highly efficient water splitting at the industrial electrolysis conditions. Chin. J. Catal. 2021, 42, 1732–1741. [Google Scholar] [CrossRef]
- Meena, A.; Thangavel, P.; Nissimagoudar, A.S.; Singh, A.N.; Jana, A.; Jeong, D.S.; Im, H.; Kim, K.S. Bifunctional oxovanadate doped cobalt carbonate for high-efficient overall water splitting in alkaline-anion-exchange-membrane water-electrolyzer. Chem. Eng. J. 2022, 430, 132623–132630. [Google Scholar] [CrossRef]
- Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z.; et al. Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting. Nat. Commun. 2021, 12, 4587–4597. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, X.; Gao, H.; Chen, S.; Cheng, P.; Wang, P.; Zhao, Z.; Dang, R.; Wang, G. In situ semi-sacrificial template-assisted growth of ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution. Chem. Eng. J. 2021, 426, 131348–131358. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Ma, X.; Liu, F.; Xiao, H.; Zhang, J.; Lin, Z.; Hao, Z. Engineering ultrafine NiFe-LDH into self-supporting nanosheets: Separation-and-reunion strategy to expose additional edge sites for oxygen evolution. Small 2021, 17, 2103785–2103792. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Kuang, P.; Wang, L.; Yu, J. Hierarchical porous nickel supported NiFeOxHy nanosheets for efficient and robust oxygen evolution electrocatalyst under industrial condition. Appl. Catal. B 2021, 299, 120668–120675. [Google Scholar] [CrossRef]
- Dou, Y.; He, C.; Zhang, L.; Yin, H.; Al-Mamun, M.; Ma, J.; Zhao, H. Approaching the activity limit of CoSe2 for oxygen evolution via Fe doping and Co vacancy. Nat. Commun. 2020, 11, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Wang, L.; Li, R.; Zhang, K.; Zhao, D.; Li, Y.; Li, X.; Huang, X.; Wang, G. Constructing a hetero-interface composed of oxygen vacancy enriched Co3O4 and crystalline-amorphous NiFe-LDH for oxygen evolution reaction. ACS Catal. 2021, 11, 14338–14351. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, H.; Hu, W.; Yin, H.; Cao, G.; Wen, H.; Wang, J.; Wang, P. Highly dispersed Ni2−xMoxP nanoparticles on oxygen-defect-rich NiMoO4−y nanosheets as an active electrocatalyst for alkaline hydrogen evolution reaction. J. Power Sources 2019, 444, 227311–227317. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, M.; Sun, X.; Luo, Y.; Asiri, A.M. Efficient electrochemical water splitting catalyzed by electrodeposited NiFe nanosheets film. Int. J. Hydrogen Energy 2016, 41, 8785–8792. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Xu, K.; Tan, S.; Wang, D.; Li, Y. Regulating the tip effect on single-atom and cluster catalysts: Forming reversible oxygen species with high efficiency in chlorine evolution reaction. Angew. Chem. Int. Ed. 2022, 61, 202200366–202200373. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Y.; Xu, L.; Yang, X.; Han, Z.; Cao, R.; Li, G. Oxygen vacancy-rich amorphous FeNi hydroxide nanoclusters as an efficient electrocatalyst for water oxidation. J. Energy Chem. 2022, 71, 167–173. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H.; Jiang, G.; Jia, J.; Qin, B.; Yi, B.; Shao, Z. Construction of orderly hierarchical FeOOH/NiFe layered double hydroxides supported on cobaltous carbonate hydroxide nanowire arrays for a highly efficient oxygen evolution reaction. J. Mater. Chem. A 2018, 6, 3397–3401. [Google Scholar] [CrossRef]
- Minakshi, M. Lithium intercalation into amorphous FePO4 cathode in aqueous solutions. Electrochim. Acta 2010, 55, 9174–9178. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Zou, P.; Nairan, A.; Zhang, Y.; Liu, J.; Liu, K.; Hu, S.; Kang, F.; Fan, H.; Yang, C. Exceptional performance of hierarchical Ni-Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ. Sci. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Shi, G.; Yu, C.; Fan, Z.; Li, J.; Yuan, M. Graphdiyne-supported NiFe layered double hydroxide nanosheets as functional electrocatalysts for oxygen evolution. ACS Appl. Mater. Interfaces 2019, 11, 2662–2669. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, L.; Shearer, M.J.; Sheng, H.; Li, W.; Chen, J.; Xiao, P.; Zhang, Y.; Hamers, R.J.; Jin, S. Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 2018, 8, 1703189–1703197. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, F.; Ma, X.; Zhu, C.; Wang, Y.; Xie, Y.; Chou, S.; Huang, Y.; Chen, Y. Regulation of morphology and electronic structure of FeCoNi layered double hydroxides for highly active and stable water oxidization catalysts. Adv. Energy Mater. 2021, 11, 2102141–2102151. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, S.; Jia, Y.; Xiong, X.; Yang, H.; Liu, S.; Tang, J.; Zhang, J.; Liu, D.; Zheng, L.; et al. NiFe hydroxide lattice tensile strain: Enhancement of adsorption of oxygenated intermediates for efficient water oxidation catalysis. Angew. Chem. Int. Edit. 2019, 58, 736–740. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zhao, X.; Wang, Y.; Li, Q.; Wang, Q.; Tang, Y.; Lei, Y. Trimetallic oxyhydroxides as active sites for large-current-density alkaline oxygen evolution and overall water splitting. J. Mater. Sci. Technol. 2022, 110, 128–135. [Google Scholar] [CrossRef]
- Kwon, J.; Han, H.; Jo, S.; Choi, S.; Chung, K.Y.; Ali, G.; Park, K.; Paik, U.; Song, T. Amorphous nickel-iron borophosphate for a robust and efficient oxygen evolution reaction. Adv. Energy Mater. 2021, 11, 2100624–2100633. [Google Scholar] [CrossRef]
- Zhao, T.; Shen, X.; Wang, Y.; Hocking, R.K.; Li, Y.; Rong, C.; Dastafkan, K.; Su, Z.; Zhao, C. In situ reconstruction of V-doped Ni2P pre-catalysts with tunable electronic structures for water oxidation. Adv. Funct. Mater. 2021, 31, 2100614–2100623. [Google Scholar] [CrossRef]
- Xie, X.; Cao, C.; Wei, W.; Zhou, S.; Wu, X.; Zhu, Q. Ligand-assisted capping growth of self-supporting ultrathin FeNi-LDH nanosheet arrays with atomically dispersed chromium atoms for efficient electrocatalytic water oxidation. Nanoscale 2020, 12, 5817–5823. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhao, H.; Huang, B.; Xu, L.; Luo, M.; Wang, J.; Luo, F.; Du, Y.; Yan, C. Efficient optimization of electron/oxygen pathway by constructing ceria/hydroxide interface for highly active oxygen evolution reaction. Adv. Funct. Mater. 2020, 30, 1908367–1908375. [Google Scholar] [CrossRef]
- Wang, K.; Du, H.; He, S.; Liu, L.; Yang, K.; Sun, J.; Liu, Y.; Du, Z.; Xie, L.; Ai, W.; et al. Kinetically Controlled, Scalable synthesis of γ-FeOOH nanosheet arrays on nickel foam toward efficient oxygen evolution: The key role of in-situ-generated γ-NiOOH. Adv. Mater. 2021, 33, 2005587–2005596. [Google Scholar] [CrossRef]
- Huang, L.; Gao, G.; Zhang, H.; Chen, J.; Fang, Y.; Dong, S. Self-dissociation-assembly of ultrathin metal-organic framework nanosheet arrays for efficient oxygen evolution. Nano Energy 2020, 68, 104296–104304. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, T.; Ye, S.; Zheng, L.; Liao, P.; Xiong, W.; Hu, J.; Wang, Y.; Wang, J.; Ren, X.; et al. Engineering defect-rich Fe-doped NiO coupled Ni cluster nanotube arrays with excellent oxygen evolution activity. Appl. Catal. B 2021, 285, 119809–119818. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, L.; Khan, U.; Yu, Q.; Cheng, H.; Zou, X.; Liu, B. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat. Commun. 2019, 10, 269–277. [Google Scholar] [CrossRef]
- Chen, R.; Hung, S.; Zhou, D.; Gao, J.; Yang, C.; Tao, H.; Yang, H.; Zhang, L.; Zhang, L.; Xiong, Q.; et al. Layered structure causes bulk NiFe layered double hydroxide unstable in alkaline oxygen evolution reaction. Adv. Mater. 2019, 31, 1903909–1903915. [Google Scholar] [CrossRef]
- Park, Y.S.; Yang, J.; Lee, J.; Jang, M.J.; Jeong, J.; Choia, W.-S.; Kim, Y.; Yin, Y.; Seo, M.H.; Chen, Z.; et al. Superior performance of anion exchange membrane water electrolyzer: Ensemble of producing oxygen vacancies and controlling mass transfer resistance. Appl. Catal. B 2020, 278, 119276–119287. [Google Scholar] [CrossRef]
- Cossar, E.; Barnett, A.O.; Seland, F.; Safari, R.; Botton, G.A.; Baranova, E.A. Ionomer content optimization in nickel-iron-based anodes with and without ceria for anion exchange membrane water electrolysis. J. Power Sources 2021, 514, 230563–230574. [Google Scholar] [CrossRef]





| Electrocatalysts | Substrate | η for OER at Corresponding j (mV@mA cm−2) | Tafel Slope (mV dec−1) | Stability Tests | Reference |
|---|---|---|---|---|---|
| NiCoFe-NDA | nickel foam | 215@10 (iR-compensated) | 64.1 | 100 mA cm−2 for 50 h | [6] |
| a-LNFBPO@Ni foam | nickel foam | 215@10 (iR-compensated) | 37 | 10 mA cm−2 for 300 h | [40] |
| V25%-Ni2P/NF-AC | nickel foam | 221@10 (without-iR compensated) | 66 | 50 mA cm−2 for 20 h | [41] |
| Cr1/FeNi-LDH-SS | stainless steel mesh | 202@10 (iR-compensated) | 32.5 | various current densities for 17 h | [42] |
| Cu@CeO2@NFC-0.25 | copper foam | 231@10 (without iR compensated) | 32.7 | 10 and 20 mA cm−2 for 30 h | [43] |
| γ-FeOOH/NF-6M | nickel foam | 286@10 (iR compensated) | 51 | 10 and 50 mA cm−2 for 48 h | [44] |
| CoNi-MOFNA | nickel foam | 215@10 (iR-compensated) | 51.6 | 10 mA cm−2 for 300 h | [45] |
| Ni83Fe17-ONCAs | nickel foam | 190@10 (iR-compensated) | 39 | various current densities for 120 h | [5] |
| Fe-NiO-Ni CHNAs | carbon fiber cloth | 245@10 (iR-compensated) | 43.4 | 10 mA cm−2 for 24 h | [46] |
| NiFe LDHs/Ni fiber | Ni fiber | 208@10 (without iR compensated) | 50 | various current densities for 60 h | This work |
| (Anode || Cathode) | Cell Voltage (V @ A cm−2) | Cell Temperature (°C) | Stability Test (hours @ A·cm−2) | Reference |
|---|---|---|---|---|
| NiFeCo LDH || NiFeCo phosphide | 1.75 @ 0.5 | 50 | 40 @ 0.5 | [18] |
| FeOOH/NiFe LDHs@CCH NAs-NF || 70 wt% Pt/C (0.4 mg cm−2) | 1.768 @ 0.5 | 70 | 100 @ 0.5 | [32] |
| Co(OH)x/Ag/Co(OH)2 || 40 wt% Pt/C (not mention) | 1.8 @ 0.6 | 50 | 24 @ 0.6 | [17] |
| VCoCOx@NF || VCoCOx@NF | 2.01 @ 0.2 | 45 | 12 @ 0.25 | [21] |
| CE-CCO || 70% wt Pt/C (1 mg cm−2) | 1.8 @ 1.39 | 45 | 64 @ 0.5 | [49] |
| Ni90Fe10 (1 mg cm−2) || 60 wt% Pt/C (1 mg cm−2) | 1.72 @ 0.8 | 50 | not mention | [50] |
| M-NiFe-LDH (3 mg cm−2) || Pt/C (0.4 mg cm−2) | 1.69 @ 1 | 50 | 50 @ 1 | [4] |
| M-NiFe-LDH (3 mg cm−2) || Pt/C (0.4 mg cm−2) | 1.63 @ 1 | 80 | 50 @1 | [4] |
| NiFe LDH-MoSx/INF || 20 wt% Pt/C/CP (2 mg·cm−2) | 1.95 @ 1 | 60 | not mention | [20] |
| NiFe LDHs/Ni fiber || 70 wt% Pt/C (0.4 mg cm−2) | 1.68 @ 0.5 1.87 @ 1 | 70 70 | 200 @ 0.5 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Chi, J.; Yu, H.; Jiang, G.; Shao, Z. Self-Supporting NiFe Layered Double Hydroxide “Nanoflower” Cluster Anode Electrode for an Efficient Alkaline Anion Exchange Membrane Water Electrolyzer. Energies 2022, 15, 4645. https://doi.org/10.3390/en15134645
Guo D, Chi J, Yu H, Jiang G, Shao Z. Self-Supporting NiFe Layered Double Hydroxide “Nanoflower” Cluster Anode Electrode for an Efficient Alkaline Anion Exchange Membrane Water Electrolyzer. Energies. 2022; 15(13):4645. https://doi.org/10.3390/en15134645
Chicago/Turabian StyleGuo, Dandan, Jun Chi, Hongmei Yu, Guang Jiang, and Zhigang Shao. 2022. "Self-Supporting NiFe Layered Double Hydroxide “Nanoflower” Cluster Anode Electrode for an Efficient Alkaline Anion Exchange Membrane Water Electrolyzer" Energies 15, no. 13: 4645. https://doi.org/10.3390/en15134645





