The Consistency of Yields and Chemical Composition of HTL Bio-Oils from Lignins Produced by Different Preprocessing Technologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lignin Feedstock Overview
2.2. Lignin Samples and Pretreatment Procedures
2.2.1. Deacetylation of Rice Straw
2.2.2. Acid Precipitation
2.2.3. Acetone/Water Oxidation Pretreatment (Wet Oxidation)
2.2.4. Milox Pulping
2.3. Experimental Conditions in LtL Solvolysis
2.4. Silylation and Quantification
2.5. Gas Chromatography-Mass Spectrometry
2.6. Elemental Analysis
2.7. Chemometric Analysis
3. Results
3.1. Lignin Samples
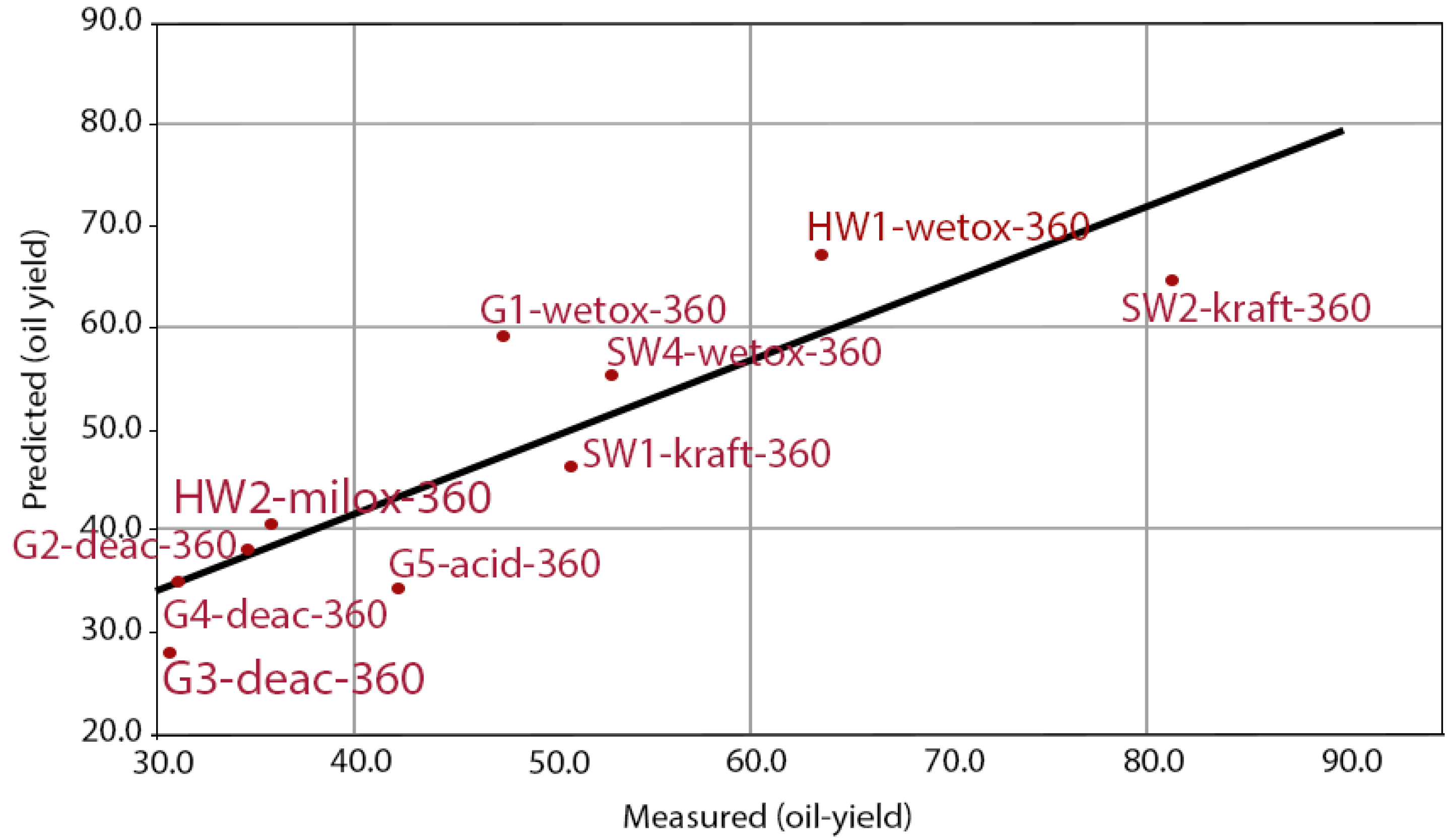
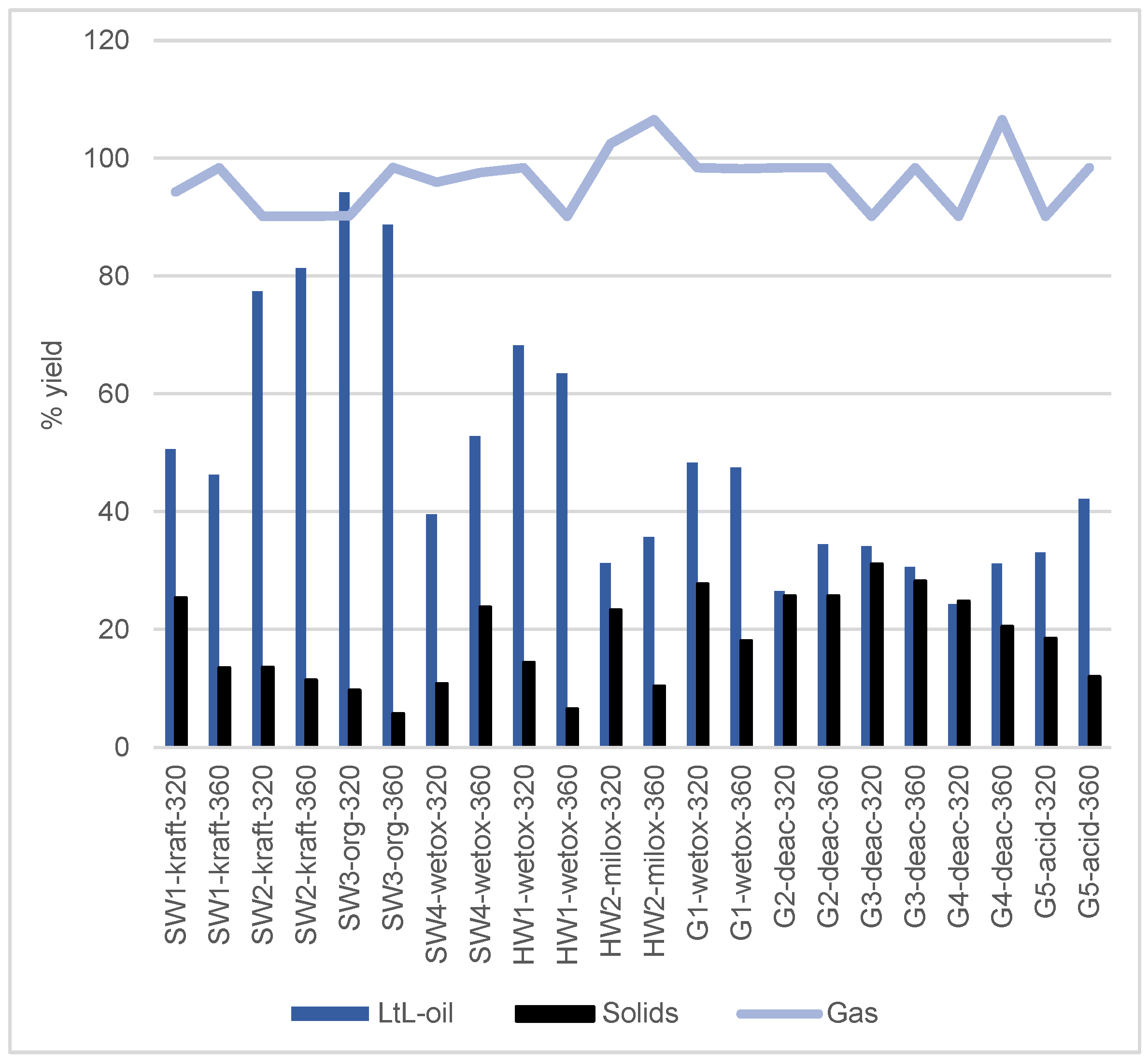
3.2. LtL-Experiment Yields
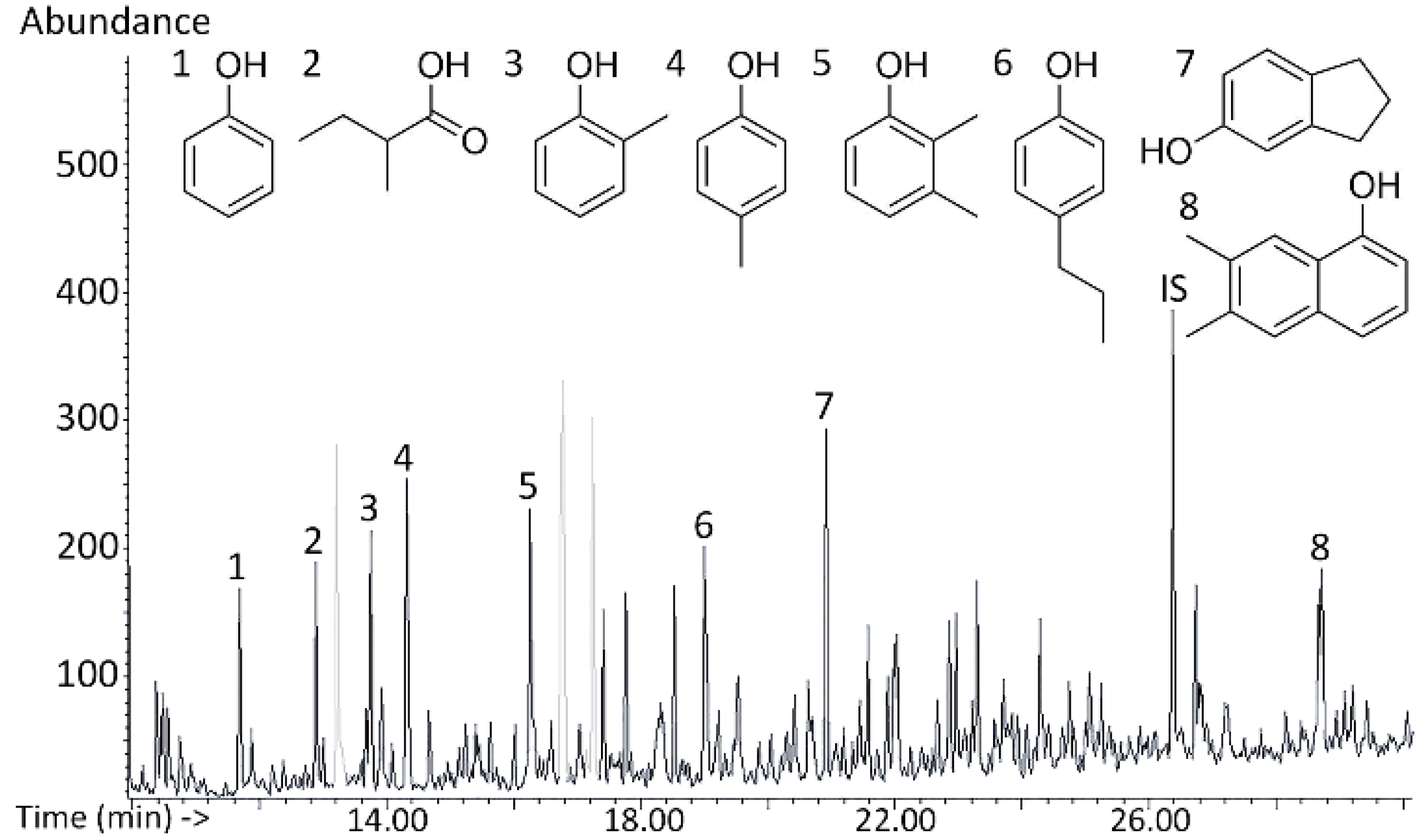
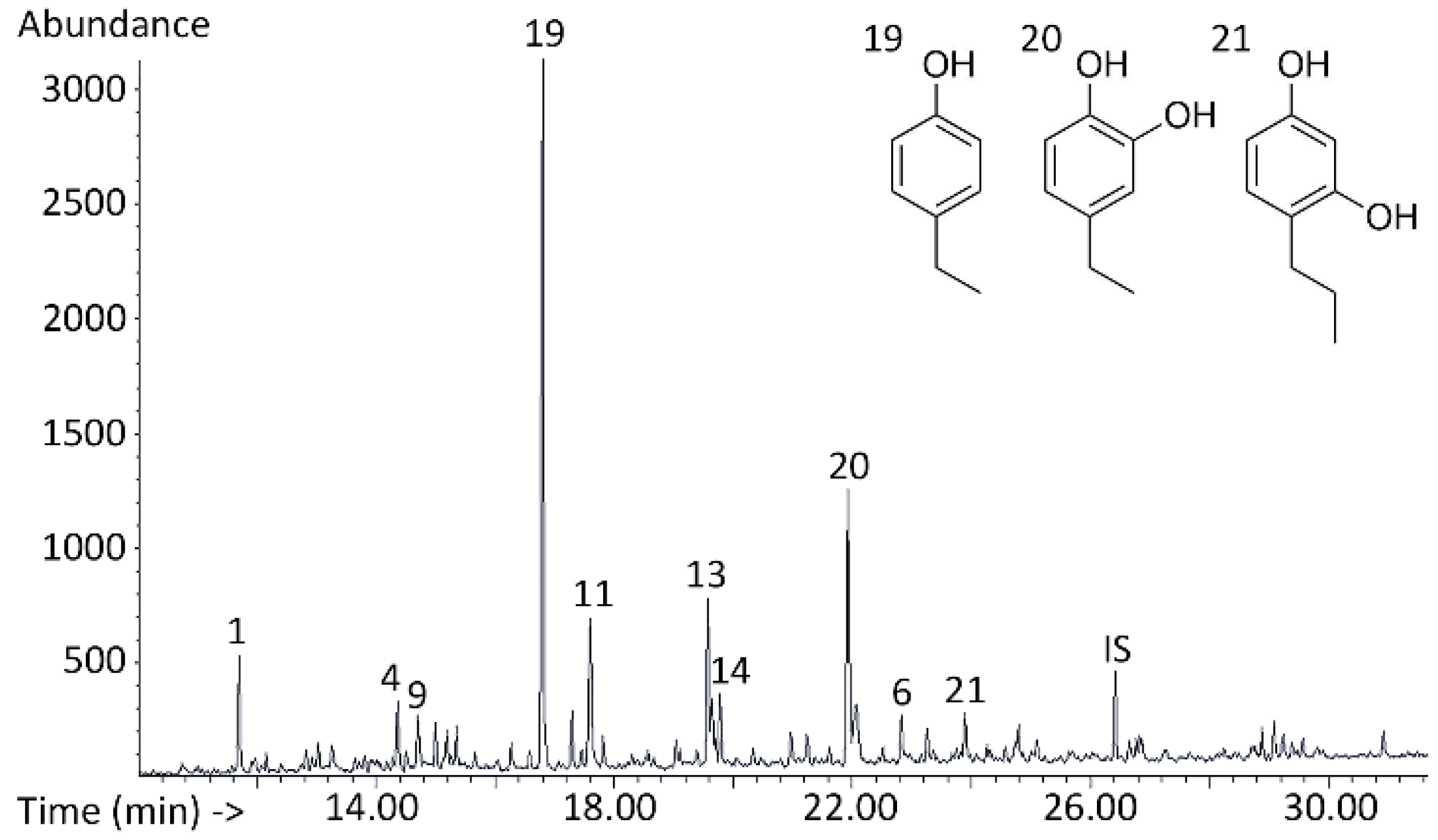
3.3. Qualitative Analysis of Bio-Oil Composition
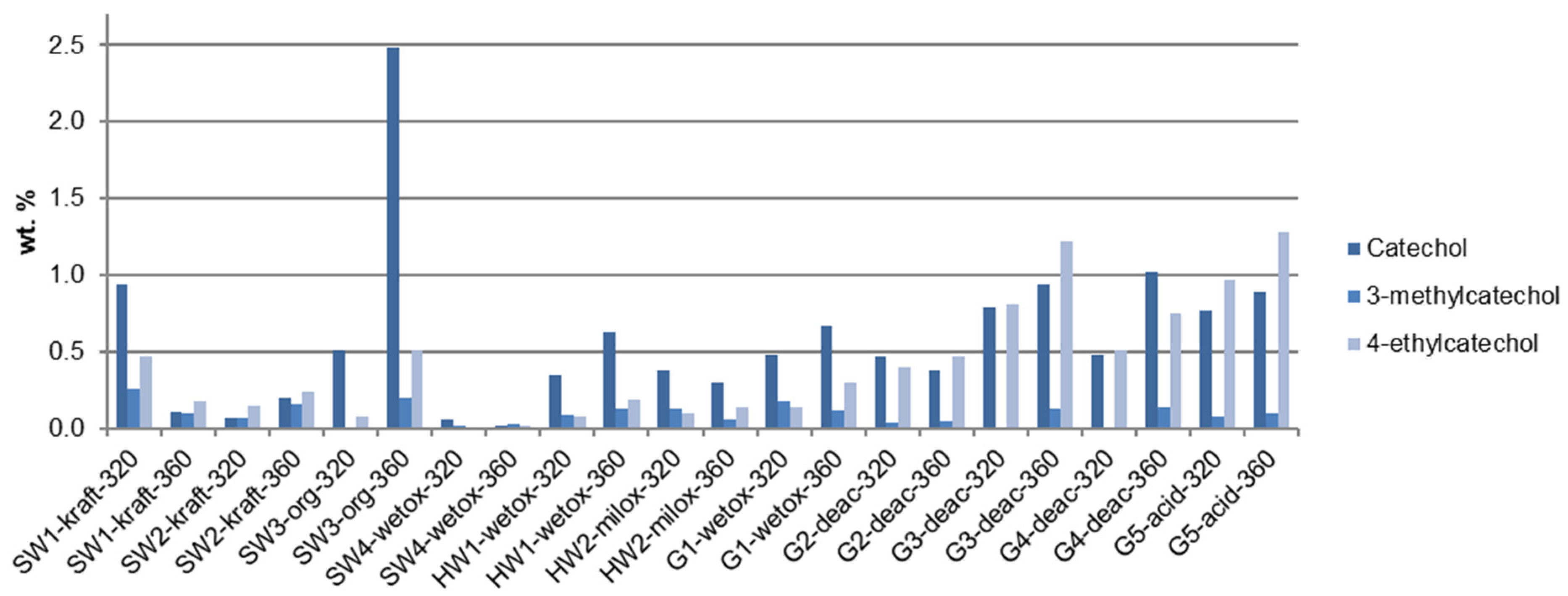
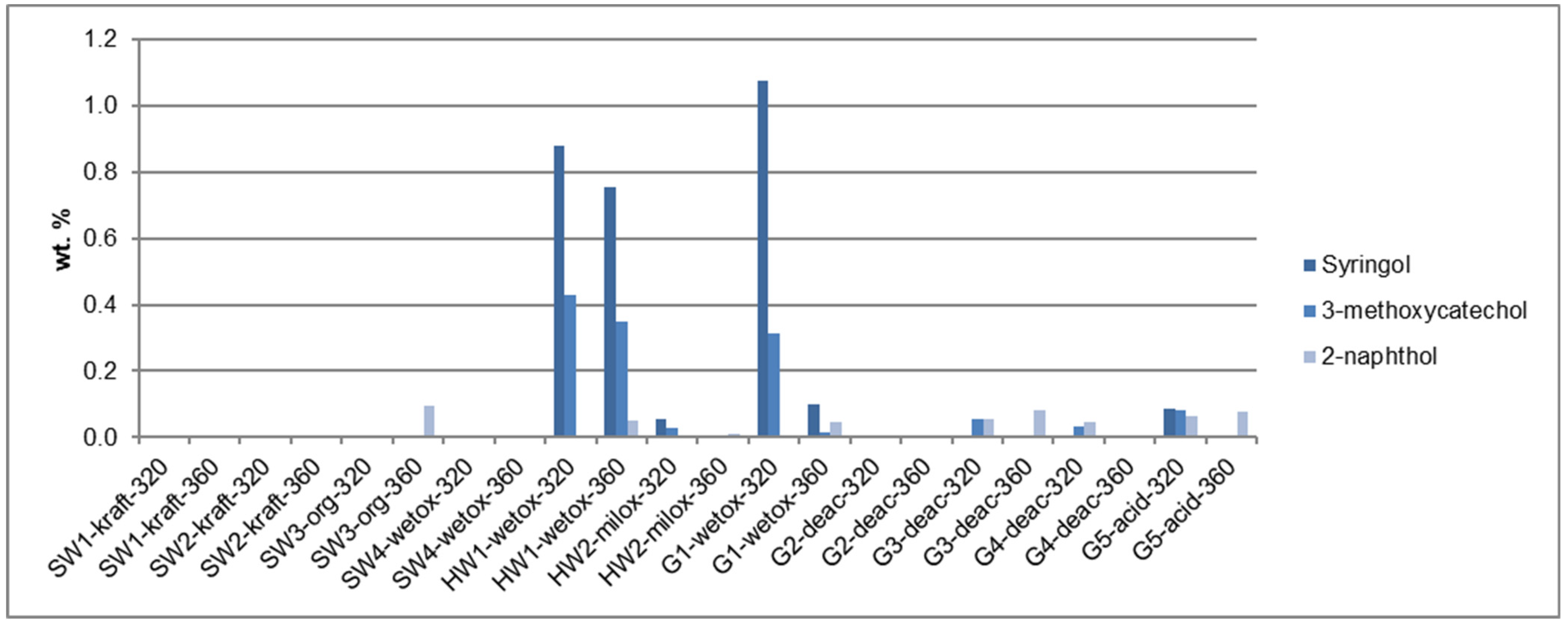
3.4. Quantitative Analysis: Monomer Composition
3.5. Elemental Analysis
4. Discussion
4.1. LtL-Experiments: Oil Yields
4.2. Quantitative Analysis: Monomer Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tian, X.; Fang, Z.; Smith, R.L.; Wu, Z.; Liu, M. Properties, Chemical Characteristics and Application of Lignin and Its Derivatives. In Production of Biofuels and Chemicals from Lignin; Fang, Z., Smith, J.R.L., Eds.; Springer: Singapore, 2016; pp. 3–33. [Google Scholar] [CrossRef]
- Varanasi, P.; Singh, P.; Auer, M.; Adams, P.D.; Simmons, B.A.; Singh, S. Survey of renewable chemicals produced from lig-nocellulosic biomass during ionic liquid pretreatment. Biotechnol. Biofuels 2013, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussatto, S.I.; Dragone, G.M. Chapter 1 Biomass Pretreatment, Biorefineries, and Potential Products for a Bioeconomy De-velopment. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsretdam, The Netherlands, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Yamakawa, C.K.; Qin, F.; Mussatto, S.I. Advances and opportunities in biomass conversion technologies and biorefineries for the development of a bio-based economy. Biomass Bioenergy 2018, 119, 54–60. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, S.S.; Zhang, S.; Ok, Y.S.; Matsagar, B.M.; Wu, K.C.W.; Tsang, D.C.W. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery. Bioresour. Technol. 2019, 291, 121878. [Google Scholar] [CrossRef] [PubMed]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Roberto, I.C. Lignin recovery from brewer’s spent grain black liquor. Carbohydr. Polym. 2007, 70, 218–223. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Mialon, L.; Vanderhenst, R.; Pemba, A.G.; Miller, S.A. Polyalkylenehydroxybenzoates (PAHBs): Biorenewable Aro-matic/Aliphatic Polyesters from Lignin. Macromol. Rapid Commun. 2011, 32, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.L.A.H. Compositional Variability of Lignin in Biomass. In Lignin—Trends and Applications; Matheus, P., Ed.; IntechOpem: London, UK, 2018; p. 308. [Google Scholar]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.J.; Samec, J.S.M. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Stone, M.L.; Katahira, R.; Reed, M.; Muchero, W.; Ramirez, K.J.; Beckham, G.T.; Román-Leshkov, Y. Differ-ences in S/G ratio in natural poplar variants do not predict catalytic depolymerization monomer yields. Nat. Commun. 2019, 10, 2033. [Google Scholar] [CrossRef] [Green Version]
- Gasson, J.R.; Forchheim, D.; Sutter, T.; Hornung, U.; Kruse, A.; Barth, T. Modeling the Lignin Degradation Kinetics in an Ethanol/Formic Acid Solvolysis Approach. Part 1. Kinetic Model Development. Ind. Eng. Chem. Res. 2012, 51, 10595–10606. [Google Scholar] [CrossRef]
- Goldmann, W.M.; Anthonykutty, J.M.; Ahola, J.; Komulainen, S.; Hiltunen, S.; Kantola, A.M.; Telkki, V.-V.; Tanskanen, J. Effect of Process Variables on the Solvolysis Depolymerization of Pine Kraft Lignin. Waste Biomass Valoriz. 2020, 11, 3195–3206. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.A.B.d.; Zabkova, M.; Araújo, J.D.; Cateto, C.A.; Barreiro, M.F.; Belgacem, M.N.; Rodrigues, A.E. An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.; Marianou, A.; Michailof, C.; Kalogianni, A.; Lappas, A. Lignocellulosic Biomass Fractionation as a Pretreatment Step for Production of Fuels and Green Chemicals. Waste Biomass Valoriz. 2015, 6, 781–790. [Google Scholar] [CrossRef]
- Li, M.-F.; Sun, S.-N.; Xu, F.; Sun, R.-C. Organosolv Fractionation of Lignocelluloses for Fuels, Chemicals and Materials: A Biorefinery Processing Perspective. In Biomass Conversion: The Interface of Biotechnology, Chemistry and Materials Science; Baskar, C., Baskar, S., Dhillon, R.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 341–379. [Google Scholar] [CrossRef]
- Kádár, Z.; Szengyel, Z.; Réczey, K. Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Ind. Crops Prod. 2004, 20, 103–110. [Google Scholar] [CrossRef]
- Nilsson, U.; Barron, N.; McHale, L.; McHale, A.P. The effects of phosphoric acid pretreatment on conversion of cellulose to ethanol at 45 °C using the thermotolerant yeast Kluyveromyces marxianus IMB3. Biotechnol. Lett. 1995, 17, 985–988. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [Green Version]
- Echresh Zadeh, Z.; Abdulkhani, A.; Saha, B. Characterization of Fast Pyrolysis Bio-Oil from Hardwood and Softwood Lignin. Energies 2020, 13, 887. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Chen, C.; Sun, Y.; Yu, M.; Yang, M.; Wang, X.; Zhou, J. Catalytic conversion of lignin to bio-oil over PTA/MCM-41 catalyst assisted by ultrasound acoustic cavitation. Fuel Process. Technol. 2020, 206, 106479. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Liu, T.; Mori, A.; Arai, R.; Asano, S.; Kudo, S.; Hayashi, J.-I. Selective Production of Phenolic Monomers and Biochar by Py-rolysis of Lignin with Internal Recycling of Heavy Oil. Energy Fuels 2020, 34, 7183–7189. [Google Scholar] [CrossRef]
- Camas, K.L.; Ullah, A. Depolymerization of lignin into high-value products. Biocatal. Agric. Biotechnol. 2022, 40, 102306. [Google Scholar] [CrossRef]
- Kleinert, M.; Barth, T. Towards a Lignincellulosic Biorefinery: Direct One-Step Conversion of Lignin to Hydrogen-Enriched Biofuel. Energy Fuels 2008, 22, 1371–1379. [Google Scholar] [CrossRef]
- Kleinert, M.; Barth, T. Phenols from Lignin. Chem. Eng. Technol. 2008, 31, 736–745. [Google Scholar] [CrossRef]
- Kleinert, M.; Gasson, J.R.; Barth, T. Optimizing solvolysis conditions for integrated depolymerisation and hydrodeoxygenation of lignin to produce liquid biofuel. J. Anal. Appl. Pyrolysis 2009, 85, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Liguori, L.; Barth, T. Palladium-Nafion SAC-13 catalysed depolymerisation of lignin to phenols in formic acid and water. J. Anal. Appl. Pyrolysis 2011, 92, 477–484. [Google Scholar] [CrossRef]
- Løhre, C.; Barth, T.; Kleinert, M. The effect of solvent and input material pretreatment on product yield and composition of bio-oils from lignin solvolysis. J. Anal. Appl. Pyrolysis 2016, 119, 208–216. [Google Scholar] [CrossRef]
- Oregui Bengoechea, M.; Hertzberg, A.; Miletić, N.; Arias, P.L.; Barth, T. Simultaneous catalytic de-polymerization and hydrodeoxygenation of lignin in water/formic acid media with Rh/Al2O3, Ru/Al2O3 and Pd/Al2O3 as bifunctional catalysts. J. Anal. Appl. Pyrolysis 2015, 113, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Oregui-Bengoechea, M.; Gandarias, I.; Arias, P.L.; Barth, T. Unraveling the Role of Formic Acid and the Type of Solvent in the Catalytic Conversion of Lignin: A Holistic Approach. ChemSusChem 2017, 10, 754–766. [Google Scholar] [CrossRef]
- Løhre, C.; Halleraker, H.V.; Barth, T. Composition of Lignin-to-Liquid Solvolysis Oils from Lignin Extracted in a Semi-Continuous Organosolv Process. Int. J. Mol. Sci. 2017, 18, 225. [Google Scholar] [CrossRef] [Green Version]
- Løhre, C.; Kleinert, M.; Barth, T. Organosolv extraction of softwood combined with lignin-to-liquid-solvolysis as a semi-continuous percolation reactor. Biomass Bioenergy 2017, 99, 147–155. [Google Scholar] [CrossRef]
- Castro, R.C.A.; Ferreira, I.S.; Roberto, I.C.; Mussatto, S.I. Isolation and physicochemical characterization of different lignin streams generated during the second-generation ethanol production process. Int. J. Biol. Macromol. 2019, 129, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Kalogiannis, K.G.; Stefanidis, S.D.; Michailof, C.M.; Lappas, A.A.; Sjöholm, E. Pyrolysis of lignin with 2DGC quantification of lignin oil: Effect of lignin type, process temperature and ZSM-5 in situ upgrading. J. Anal. Appl. Pyrolysis 2015, 115, 410–418. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Kalogiannis, K.G.; Kalogianni, A.; Lappas, A.A.; Topakas, E. Production of high concentrated cellulosic ethanol by acetone/water oxidized pretreated beech wood. Biotechnol. Biofuels 2017, 10, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimos, K.; Paschos, T.; Louloudi, A.; Kalogiannis, K.G.; Lappas, A.A.; Papayannakos, N.; Kekos, D.; Mamma, D. Effect of Various Pretreatment Methods on Bioethanol Production from Cotton Stalks. Fermentation 2019, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Abdi, H.; Williams, L.J. Principal component analysis. WIREs Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Grung, B. Det matematiske grunnlaget for latent variabel-metoder. In Anvendelse av Kjemometri Innen Forskning og Industri; Nortvedt, R., Brakstad, F., Kvalheim, O.M., Lundstedt, T., Eds.; Norsk Kjemisk Selskap and The Swedish Chemical Society: Bergen, Norway, 1996; pp. 121–144. [Google Scholar]
- Carlson, R.; Carlson, J.E. Chapter 18: Quantitative relations: Observed responses and experimental variations. In Design and Optimization in Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Oregui Bengoechea, M.; Miletíc, N.; Vogt, M.H.; Arias, P.L.; Barth, T. Analysis of the effect of temperature and reaction time on yields, compositions and oil quality in catalytic and non-catalytic lignin solvolysis in a formic acid/water media using experi-mental design. Bioresour. Technol. 2017, 234, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Holmelid, B.; Kleinert, M.; Barth, T. Reactivity and reaction pathways in thermochemical treatment of selected lig-nin-like model compounds under hydrogen rich conditions. JAAP 2012, 98, 37–44. [Google Scholar] [CrossRef]
- Løhre, C.; Laugerud, G.-A.A.; Huijgen, W.J.J.; Barth, T. Lignin-to-Liquid-Solvolysis (LtL) of Organosolv Extracted Lignin. ACS Sustain. Chem. Eng. 2018, 6, 3102–3112. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Guaiacol, CID = 460. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/guaiacol (accessed on 3 July 2020).
- National Center for Biotechnology Information. PubChem Database. 2-Methoxy-4-methylphenol, CID = 7144. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-Methoxy-4-methylphenol (accessed on 3 July 2020).
- National Center for Biotechnology Information. PubChem Database. 4-Ethyl-2-methoxyphenol, CID = 62465. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-Ethyl-2-methoxyphenol (accessed on 3 July 2020).
- National Center for Biotechnology Information. PubChem Database. 2-Methoxy-4-propylphenol, CID = 17739. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-Methoxy-4-propylphenol (accessed on 3 July 2020).
- Alhassan, Y.; Hornung, U.; Bugaje, I.M. Lignin Hydrothermal Liquefaction into Bifunctional Chemicals: A Concise Review. In Biorefinery Concepts; IntechOpen: London, UK, 2020. [Google Scholar]
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. Phenol Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Weber, M.; Weber, M. Phenols. In Phenolic Resins: A Century of Progress; Pilato, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 9–23. [Google Scholar] [CrossRef]





| Sample | Source | Delignification Method | Elemental Ratio (Moles) | |
|---|---|---|---|---|
| O/C | H/C | |||
| SW1 | Softwood | Kraft | 0.71 | 1.35 |
| SW2 | Softwood | Kraft | 0.38 | 1.14 |
| SW3 [a] | Spruce (90%) | Organosolv | 0.33 | 1.08 |
| SW4 | Spruce (90%) | Acetone/Water oxidation | 0.47 | 1.07 |
| HW1 | Beechwood | Acetone/Water oxidation | 0.42 | 1.19 |
| HW2 | Beechwood | Milox | 0.83 | 1.36 |
| G1 | Corn Stover | Acetone/Water oxidation | 0.42 | 1.07 |
| G2 | Rice straw | Deacetylation | 0.84 | 1.63 |
| G3 | Rice straw | Deacetylation | 0.98 | 1.55 |
| G4 | Rice straw | Deacetylation | 0.97 | 1.68 |
| G5 | Rice straw | Acid precipitated | 0.72 | 1.39 |
| Sample | Acid Insoluble Lignin (wt.%) | Acid Soluble Lignin (wt.%) | Glucan (wt.%) | Xylan (wt.%) |
|---|---|---|---|---|
| SW1 | 53.5 | 13.4 | * | * |
| SW2 | 90.7 | 2.0 | 0.4 | 1.3 |
| SW3 [36] | 94.9 | 0.5 | * | * |
| SW4 | 81.4 | 7.5 | * | * |
| HW1 | 88.3 | 3.8 | 0.1 | 0.5 |
| HW2 | 17.5 | 11.0 | 2.1 | 24.4 |
| G1 | 88.4 | 3.6 | 0.1 | 0.2 |
| G2 | 43.2 | 4.6 | 8.4 | 3.4 |
| G3 | 44.4 | 5.0 | 6.8 | 3.6 |
| G4 | 38.4 | 3.6 | 12.2 | 6.9 |
| G5 | 54.6 | 7.0 | 2.2 | 5.7 |
| Experiment | Lignin g | Water mL | Water g | Formic Acid mL | Formic Acid g | Time h | Temperature °C |
|---|---|---|---|---|---|---|---|
| SW1-kraft-320 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| SW1-kraft-360 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| SW2-kraft-320 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| SW2-kraft-360 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| SW3-org-320 [a] | 0.51 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| SW3-org-360 [a] | 0.51 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| SW4-wetox-320 | 0.50 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| SW4-wetox-360 | 0.50 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| HW1-wetox-320 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| HW1-wetox-360 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| HW2-milox-320 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| HW2-milox-360 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| G1-wetox-320 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| G1-wetox-360 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| G2-deac-320 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| G2-deac-360 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| G3-deac-320 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| G3-deac-360 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| G4-deac-320 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| G4-deac-360 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| G5-acid-320 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 320 |
| G5-acid-360 | 0.52 | 4.0 | 4.0 | 1.0 | 1.22 | 2 | 360 |
| Gas chromatograph | Agilent Technologies 7890A |
| Mass spectrometry detector | Agilent Technologies 5977A |
| Column | Agilent Technologies 30 m HP-5ms column with 250 µm ID and thickness of 0.25 µm |
| Mode | Splitless |
| Injection volume | 1 µL |
| Injector temperature | 280 °C |
| Carrier gas | Helium |
| Flow | 1 mL/min |
| Detector temperature | 250 °C |
| Qualitative Analysis | Solvent delay | 4.6 min | ||
| Start temperature | 40 °C | Hold time 5 min | ||
| Heating rate 1 | 6 °C/min | Hold time 0 min | Final temperature 280 °C | |
| Heating rate 2 | 40 °C/min | Hold time 5 min | Final temperature 300 °C | |
| Quantitative Analysis | Solvent delay | 11 min | ||
| Start temperature | 40 °C | Hold time 5 min | ||
| Heating rate 1 | 6 °C/min | Hold time 5 min | Final temperature 73 °C | |
| Heating rate 2 | 6 °C/min | Hold time 0 min | Final temperature 280 °C | |
| Heating rate 3 | 40 °C/min | Hold time 5 min | Final temperature 300 °C |
| Experiment | LtL-Oil wt.% of Lignin Input | Gas wt.% of FA Input | Aq. Ph. [c] wt.% of Solvent Input | Solids wt.% of Lignin Input | Total Mass Recovery wt.% |
|---|---|---|---|---|---|
| SW1-kraft-320 [a] | 50.6 ± 2.1 | 94.3 ± 4.1 | 87.6 | 25.4 ± 1.3 | 87.6 |
| SW1-kraft-360 [a] | 46.3 ± 9.6 | 98.4 ± 0 | 94.9 | 13.5 ± 1.7 | 92.1 |
| SW2-kraft-320 | 77.4 | 90.2 | 93.2 | 13.6 | 92.0 |
| SW2-kraft-360 | 81.3 | 90.2 | 94.7 | 11.4 | 93.2 |
| SW3-org-320 [b] | 94.2 | 90.2 | 99.1 | 9.7 | 97.6 |
| SW3-org-360 [b] | 88.7 | 98.4 | 96.6 | 5.7 | 96.8 |
| SW4-wetox-320 | 39.6 | 95.9 | 95.2 | 10.8 | 91.1 |
| SW4-wetox-360 | 52.8 | 97.5 | 92.9 | 23.8 | 92.0 |
| HW1-wetox-320 | 68.2 | 98.4 | 94.1 | 14.4 | 93.6 |
| HW1-wetox-360 | 63.5 | 90.2 | 97.4 | 6.5 | 93.0 |
| HW2-milox-320 [a] | 31.3 ± 0.6 | 102.5 ± 4.1 | 75.1 | 23.3 ± 1.2 | 78.8 |
| HW2-milox-360 [a] | 35.7 ± 1.4 | 106.6 ± 0 | 97.6 | 10.4 ± 1.1 | 94.5 |
| G1-wetox-320 | 48.3 | 98.4 | 90.7 | 27.7 | 90.6 |
| G1-wetox-360 [a] | 47.5 ± 2.7 | 98.2 ± 0.1 | 93.8 | 18.1 ± 3.2 | 92.9 |
| G2-deac-320 | 26.5 | 98.4 | 89.9 | 25.7 | 87.9 |
| G2-deac-360 | 34.5 | 98.4 | 89.9 | 25.7 | 87.9 |
| G3-deac-320 | 34.1 | 90.2 | 80.2 | 31.1 | 80.6 |
| G3-deac-360 | 30.6 | 98.4 | 80.3 | 28.2 | 81.9 |
| G4-deac-320 | 24.3 | 90.2 | 88.5 | 24.8 | 85.0 |
| G4-deac-360 | 31.2 | 106.6 | 87.3 | 20.5 | 87.8 |
| G5-acid-320 | 33.1 | 90.2 | 94.4 | 18.5 | 89.2 |
| G5-acid-360 | 42.2 | 98.4 | 83.5 | 12.0 | 83.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halleraker, H.V.; Kalogiannis, K.; Lappas, A.; Castro, R.C.A.; Roberto, I.C.; Mussatto, S.I.; Barth, T. The Consistency of Yields and Chemical Composition of HTL Bio-Oils from Lignins Produced by Different Preprocessing Technologies. Energies 2022, 15, 4707. https://doi.org/10.3390/en15134707
Halleraker HV, Kalogiannis K, Lappas A, Castro RCA, Roberto IC, Mussatto SI, Barth T. The Consistency of Yields and Chemical Composition of HTL Bio-Oils from Lignins Produced by Different Preprocessing Technologies. Energies. 2022; 15(13):4707. https://doi.org/10.3390/en15134707
Chicago/Turabian StyleHalleraker, Hilde Vik, Konstantinos Kalogiannis, Angelos Lappas, Rafael C. A. Castro, Ines C. Roberto, Solange I. Mussatto, and Tanja Barth. 2022. "The Consistency of Yields and Chemical Composition of HTL Bio-Oils from Lignins Produced by Different Preprocessing Technologies" Energies 15, no. 13: 4707. https://doi.org/10.3390/en15134707
APA StyleHalleraker, H. V., Kalogiannis, K., Lappas, A., Castro, R. C. A., Roberto, I. C., Mussatto, S. I., & Barth, T. (2022). The Consistency of Yields and Chemical Composition of HTL Bio-Oils from Lignins Produced by Different Preprocessing Technologies. Energies, 15(13), 4707. https://doi.org/10.3390/en15134707









