Recent Advances on CO2 Mitigation Technologies: On the Role of Hydrogenation Route via Green H2
Abstract
:1. Introduction
- Brief description of the detrimental role of CO2 as the main greenhouse gas in climate change and directives towards CO2 mitigation;
- Summary of the two main CO2 valorization routes, i.e., Carbon Capture and Storage (CCS) and Carbon Capture and Utilization (CCU);
- Details on the route of CO2 hydrogenation pathways via the exploitation of renewable energy sources for “green” H2 production, the so-called “Power-to-X, PtX” processes, exemplified by “Power-to-Gas, PtG”;
- Conclusions and future outlooks for the large-scale deployment of CO2 hydrogenation schemes by electrolytic hydrogen.
2. The Past, Present and Future Role of CO2
- Global surface temperature was approximately 1.1 °C higher in the second decade of the 21st century than between the period 1850–1900, and the last five years in particular were recorded as the hottest since 1850.
- The latest sea level rise rate has increased by nearly threefold compared to the period 1901–1971 and sea levels have increased by ca. 80 cm.
- Acidification of the oceans has intensified, as the current oceanic pH value is estimated at 7.65, compared to the value of 8.10 in the 1960s.
- The observed warming stems from anthropogenic emissions, with greenhouse gas warming masked in part by the effect of aerosol cooling.
- Human influence is indeed “very likely” the main cause of the global retreat of glaciers globally since the 1990s as well as the decrease in Arctic sea-ice.
- It is “virtually certain” that hot and extreme phenomena (mostly heatwaves) have become increasingly more frequent and more intense since 1950, whereas cold events follow the opposite trend.
3. Directives and Global Goals towards Carbon Neutrality
- Surface temperature globally is not expected to decrease until at least 2050. A global warming extent of 1.5 °C and 2 °C will be exceeded during this century, provided that deep reductions in emissions of CO2 and other GHGs do not take place soon.
- Several climate system changes will become more prominent, directly ascribed to the increasing global warming. These changes include increased frequency and intensity of hot extremes, heavy precipitation and marine heatwaves, ecological/agricultural droughts in some regions and intense tropical cyclones, as well as Arctic sea-ice reduction, permafrost and snow cover.
- The sustained global warming is projected to further intensify the water cycle, specifically its variability, the severity of wet/dry weather phenomena and monsoon precipitation.
- Under scenarios with increased CO2 atmospheric content, land and ocean carbon sinks are expected to be less effective at decelerating the accumulation of atmospheric CO2.
- A lot of changes attributed to past and future trends regarding greenhouse gas emissions are now irreversible at a time frame between centuries or even millennia, especially changes in global sea level, ocean and ice sheets.
4. CO2 Mitigation Technologies
4.1. Carbon Capture and Storage (CCS)
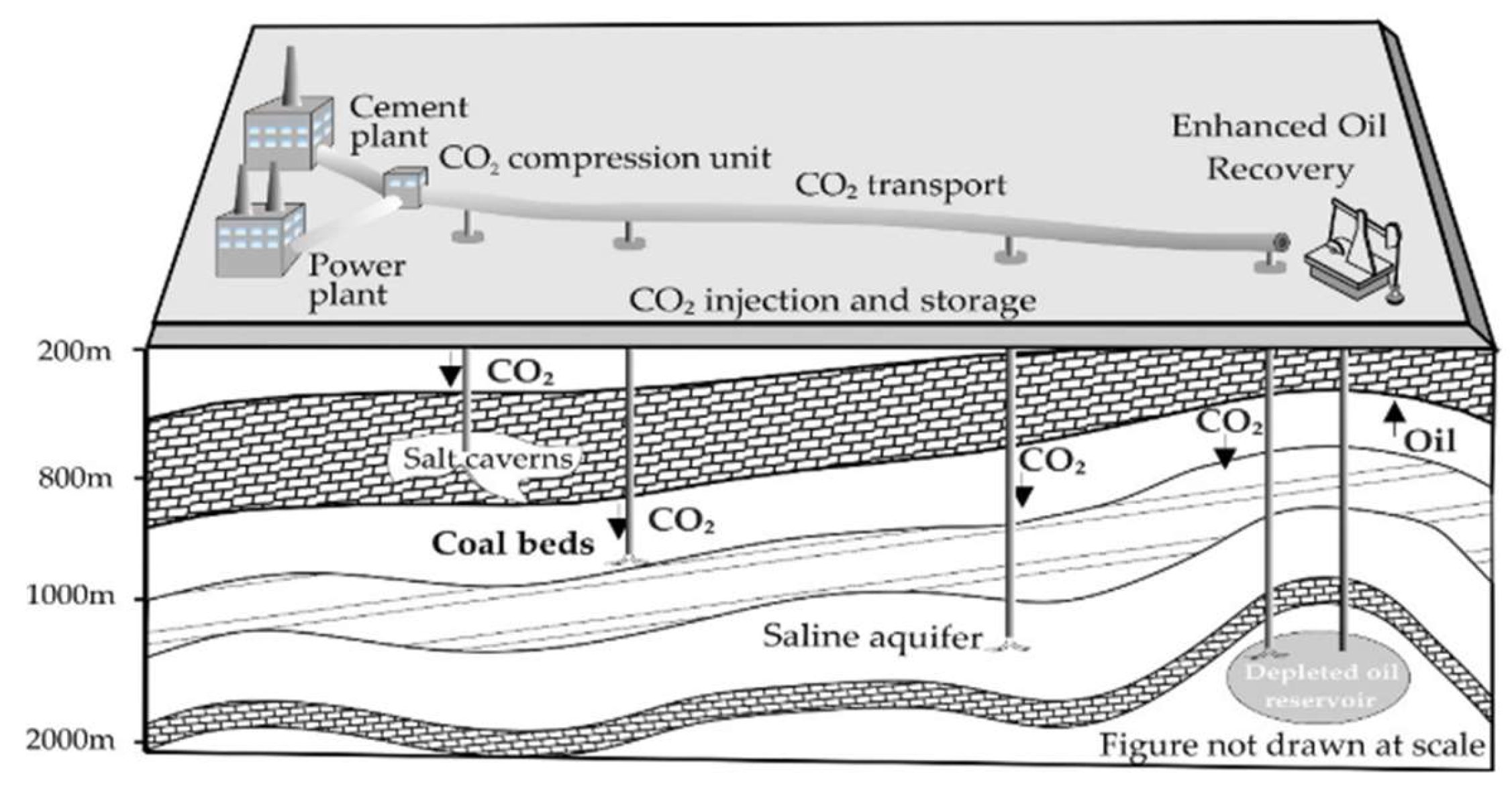
- Pre-combustion or fuel decarbonization is associated with the removal of carbon from the fuel and the eventual combustion of its hydrogen content in an energy conversion device. At this stage, a carbon-containing solid fuel is decarbonized before its utilization in a gaseous stream via a gasifier and a catalytic reactor. Firstly, syngas (a gas mixture containing predominantly CO, H2 and CO2) is formed in the gasifier under sub-stoichiometric oxygen conditions. Secondly, syngas reacts with steam in the shift reactor for its enrichment in CO2 via the water-gas shift reaction. Instead of solid fuels, natural gas can also be used for pre-combustion capture via the reaction of steam reforming, which also yields syngas [85]. The captured CO2 in the effluent can be compressed, dried and separated by the commercially available technique of physical adsorption. A summary of some important studies on pre-combustion processes is included in [86].
- Oxy-fuel combustion involves the burning of the fuel (mainly coal) in the presence of pure oxygen instead of air, thus eliminating nitrogen dilution of flue gases. The N2-free and O2-rich reaction atmosphere minimizes CO formation and results in a more concentrated CO2 stream in the final flue gases, largely reducing its purification demands [76]. CO2 capture may then take place from flue gasses by water condensation accompanied by compression and appropriate storage. This method was developed in the 1980s for the generation of high-purity CO2 for enhanced oil recovery. The required oxygen during the initial stage is provided by means of a cryogenic distillation unit and appropriate conditions are maintained by mixing the flue gases with pure O2 before combustion [87]. Oxy-fuel combustion is potentially highly advantageous in terms of feasibility, albeit the high requirements of pure oxygen inevitably increase the overall cost. However, together with pre-combustion capture, these approaches are adaptable to transportation applications [88].
- Post-combustion carbon capture (PCC). As the name suggests, CO2 in PCC is captured in the stage following combustion, i.e., from a CO2-rich exhaust gas. PCC is currently the most accessible option among all capture technologies, as it is an established process and currently in use in various industrial applications [89,90,91]. In fact, the proposal of PCC started in the 1970s as a potentially economical source of CO2, mainly for enhanced oil recovery (EOR) operations. Therefore, PCC differs from pre-combustion and oxyfuel methods, in the sense that the latter two are still in their developmental phase. PCC is also now the most accessible option for retrofitting already existing plants and lowering their carbon footprint, though this is not always applicable in a cost-effective way [92]. A few technologies have been proposed as alternative approaches for PCC and a comprehensive review by Mondal et al. provides detailed information about these approaches [93].

4.2. Carbon Capture and Utilization (CCU)
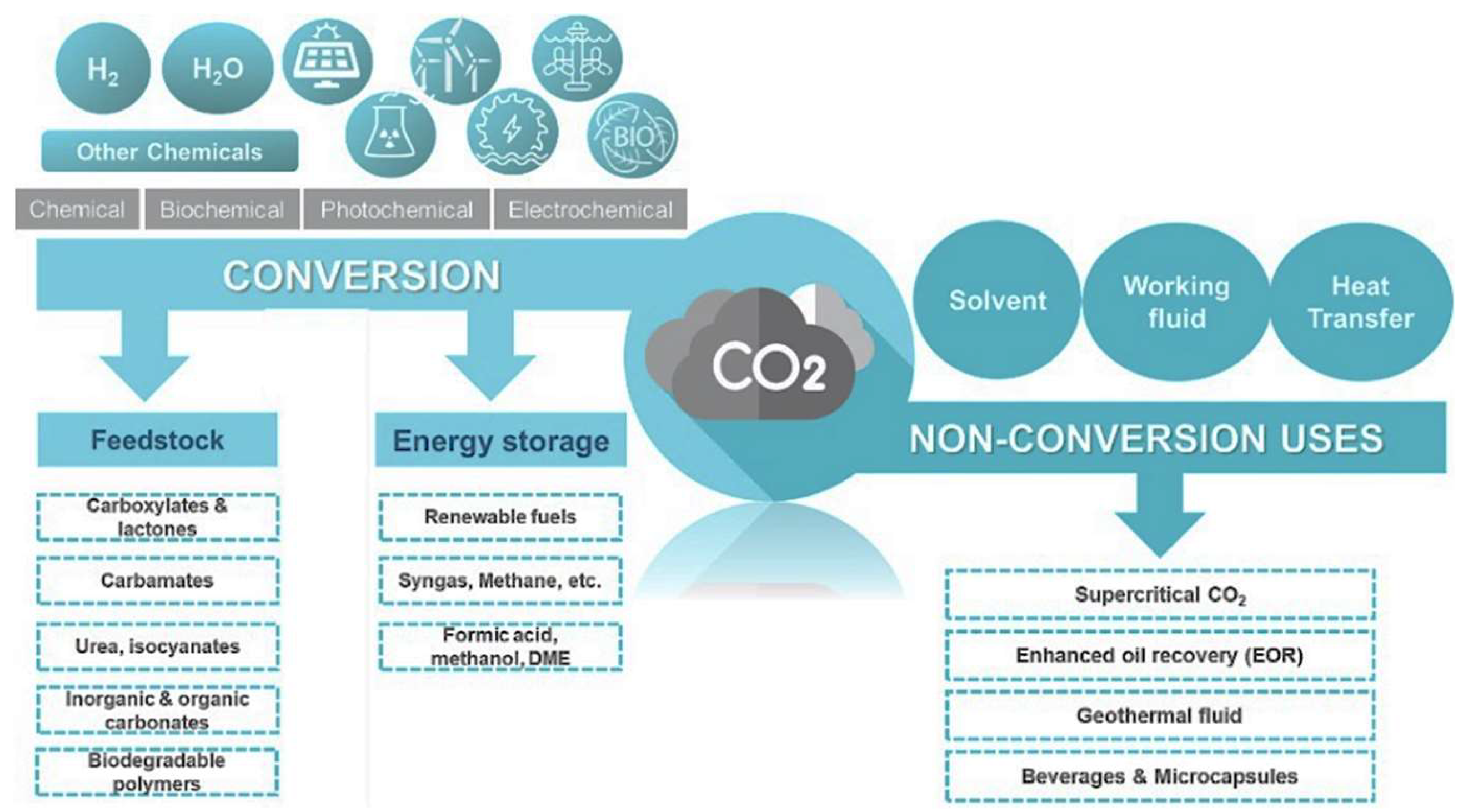
5. Valorization of CO2 Emissions via Hydrogenation
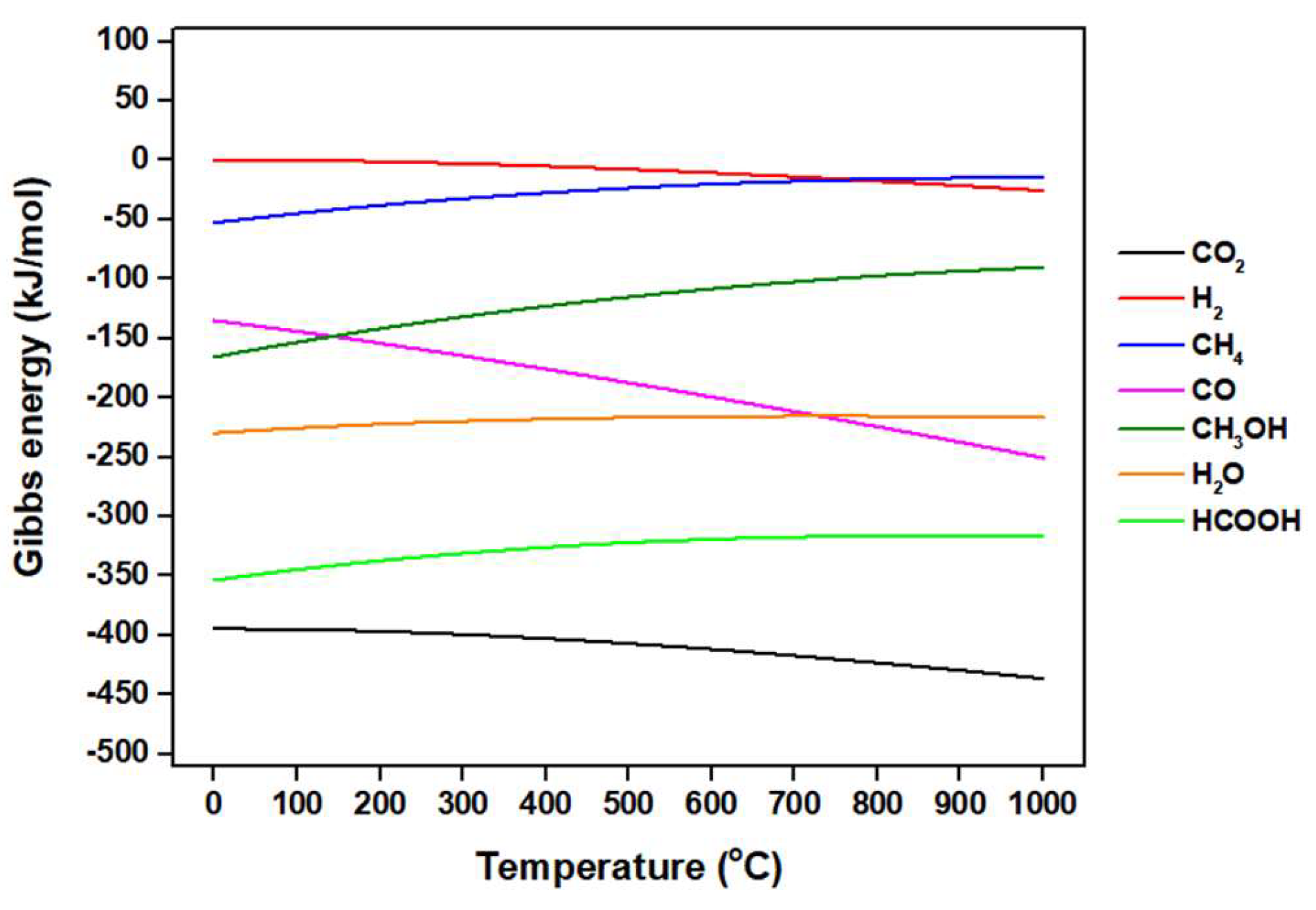
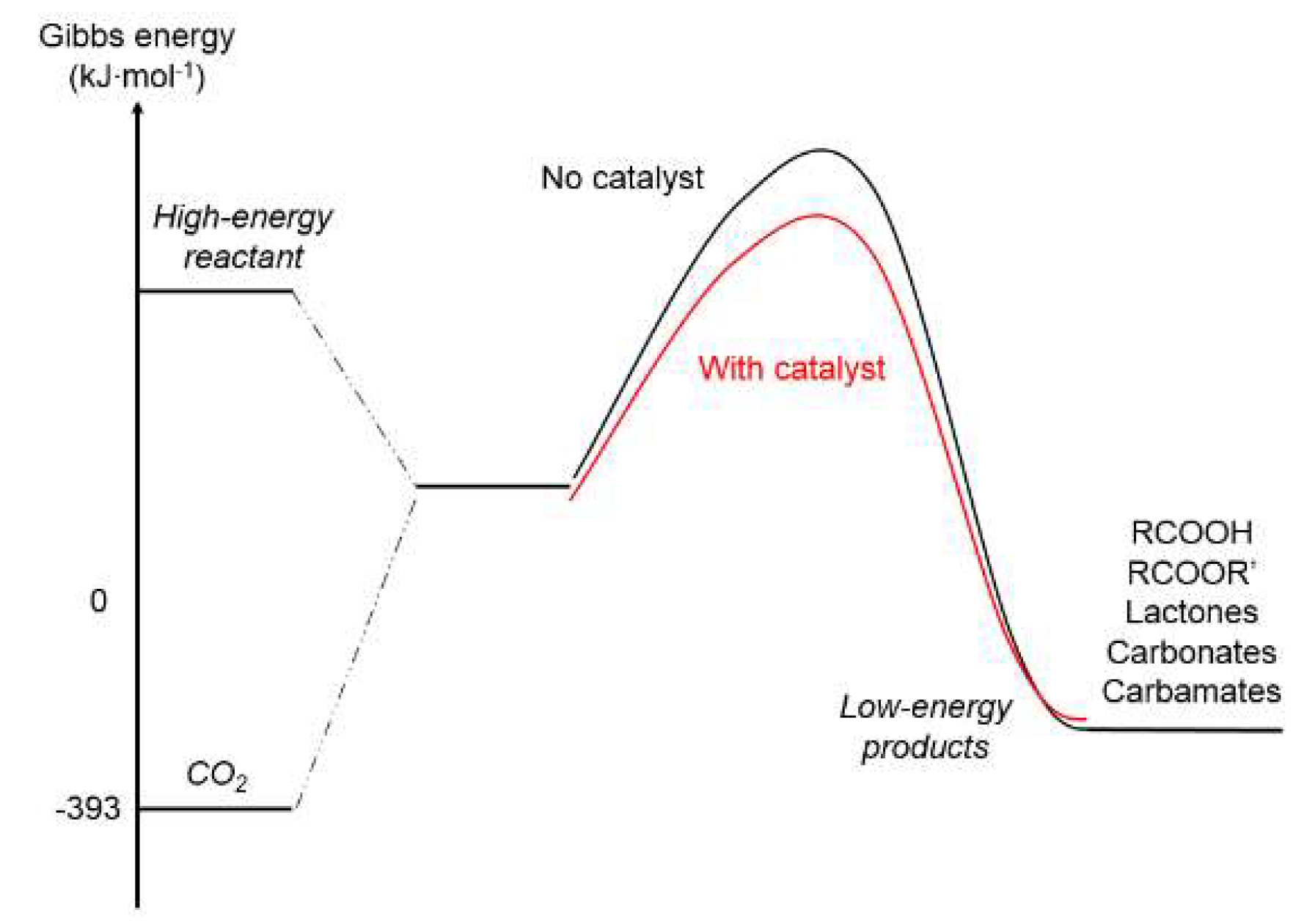
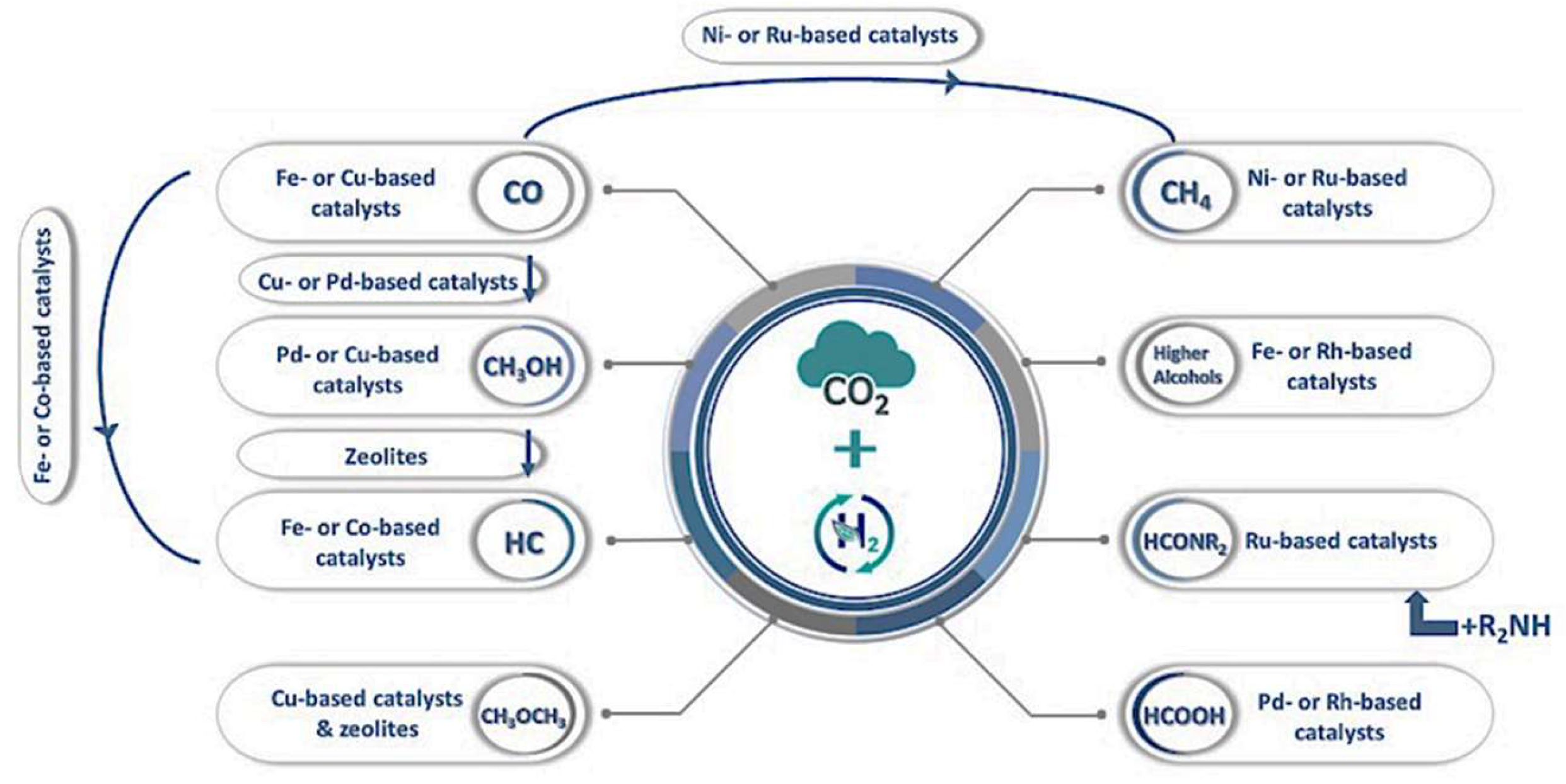
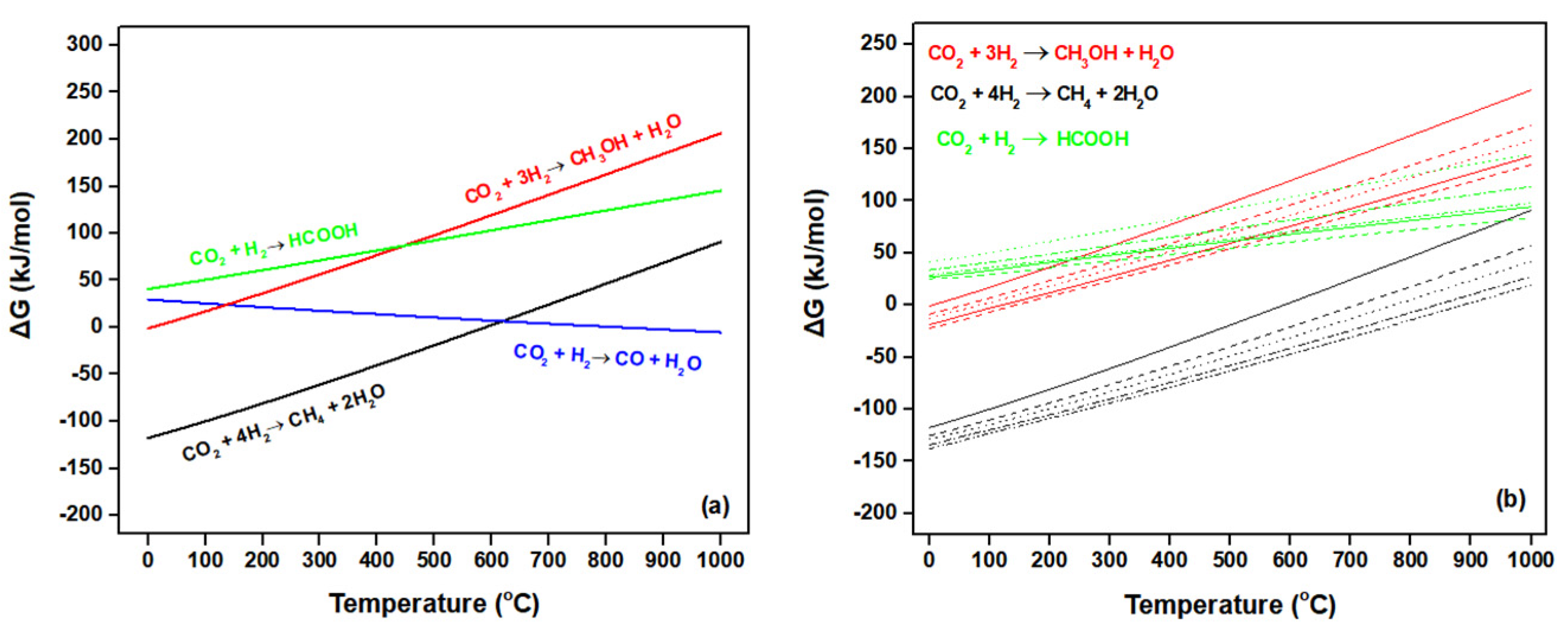
5.1. Fundamentals of CO2 Conversion Using H2
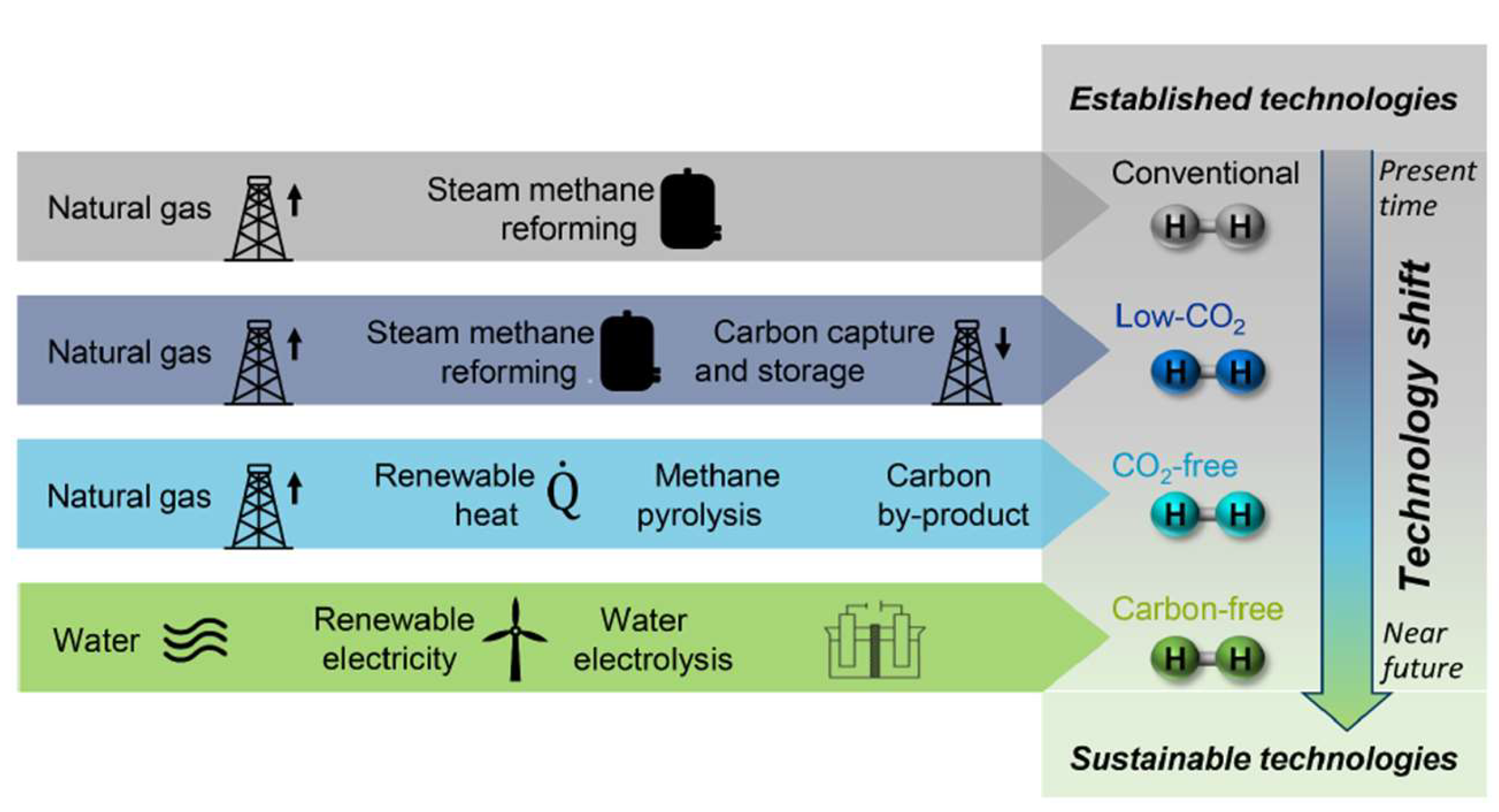
5.2. Sustainable H2 Production via Renewable Energy
6. The Scheme of CO2 Hydrogenation
6.1. Integration of Captured CO2 with RES-Derived H2
6.2. Power-to-X Processes
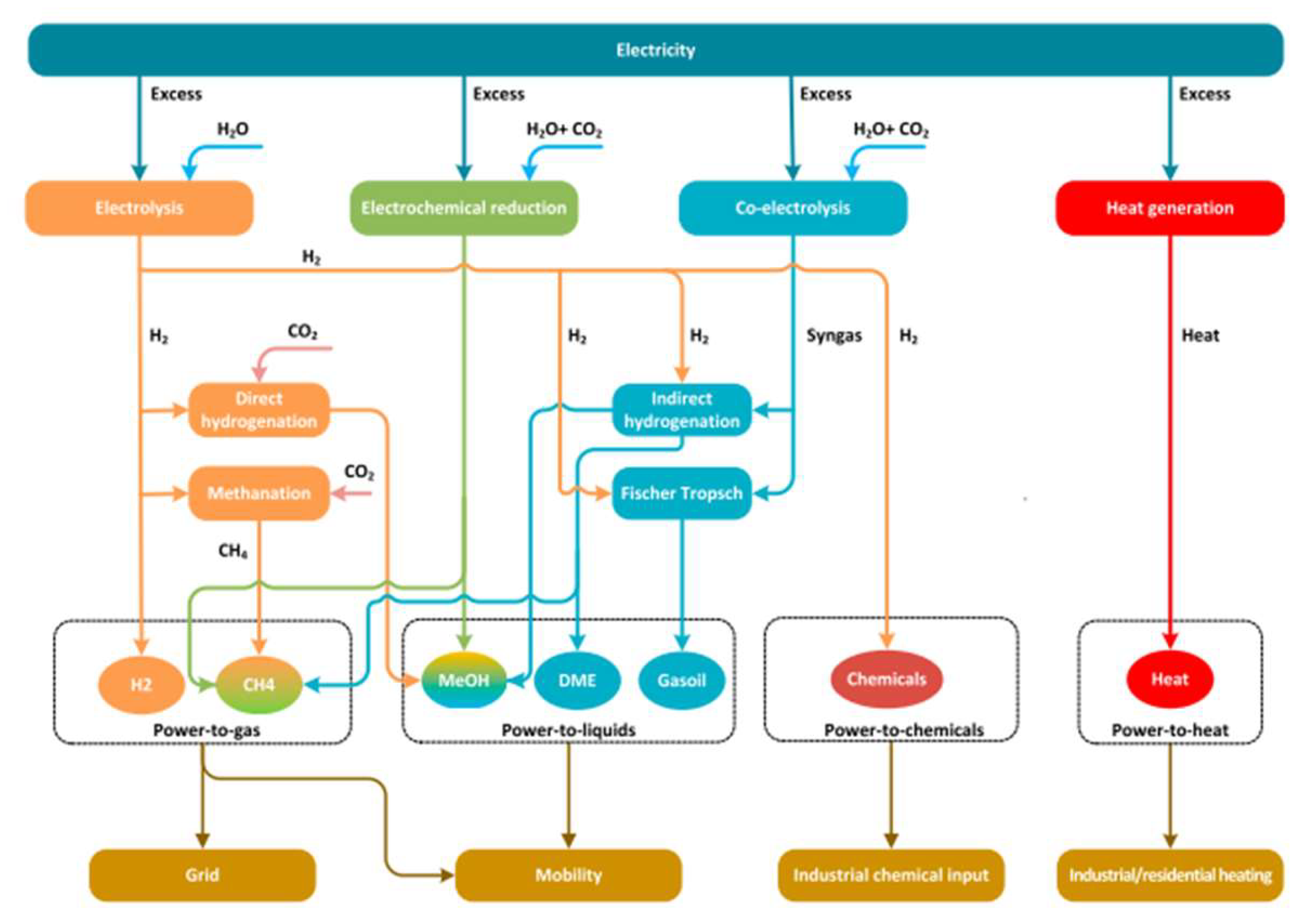
- Power-to-Heat (PtH): A coupling of the heat and power sectors appears to be a rather promising strategy for addressing energy decarbonization and RES-energy intermittency. To this end, several PtH technologies that can potentially contribute to decarbonizing heat supply along with integrating the variable renewable electricity, if sufficiently flexible, are available. In the scheme of PtH, typically, electric boilers, heat pumps or furnaces are used to convert RES energy to heat [197], which may be a significant product in regions with heat district networks (operating traditionally via the waste heat of coal power plants) [198,199] or for industrial facilities producing steam.
- Power-to-Liquids (PtL) or Power-to-Chemicals (PtC): The processes of PtL or PtC are associated with either electrolysis of H2O for H2 production and subsequent hydrogenation of captured CO2 emissions or the co-electrolysis of CO2 and water for the production of liquid hydrocarbons via the Fischer–Tropsch synthesis or the methanol-mediated rout towards specific fuels or chemicals. The produced fuels via the Power-to-Liquids process have the advantage of higher energy density products both in terms of volume and weight, rendering them ideal carbon-neutral substitutes for hard-to-abate sectors such as shipping, aviation, or heavy-duty trucks and lorries [200]. Moreover, many PtL products are considered value-added products which are conventionally produced from natural gas or coal. Notably, multi-source and multi-purpose alcohols are excellent candidate fuels, and CH3OH and CH3CH2OH are actionable first targets with a potential of Gtonne-scale production [117].
- Power-to-Hydrogen (PtH2): In the PtH2 scheme, the RES-derived electric energy is converted to hydrogen, which is considered as the final product without further transformation and can ultimately be used in the industry, mobility, electricity network (including storage of excess renewables) and heating, with the industrial sector accounting for approximately 90% of the total H2 demand. Specifically, the largest share comes from the chemicals sector for the production of ammonia and in refining for hydrocracking and fuel hydro-treating processes. Other industrial sectors include the production of iron/steel, electronics, glass, bulk and specialty chemicals, but their combined share in the global demand is not high [17,203].
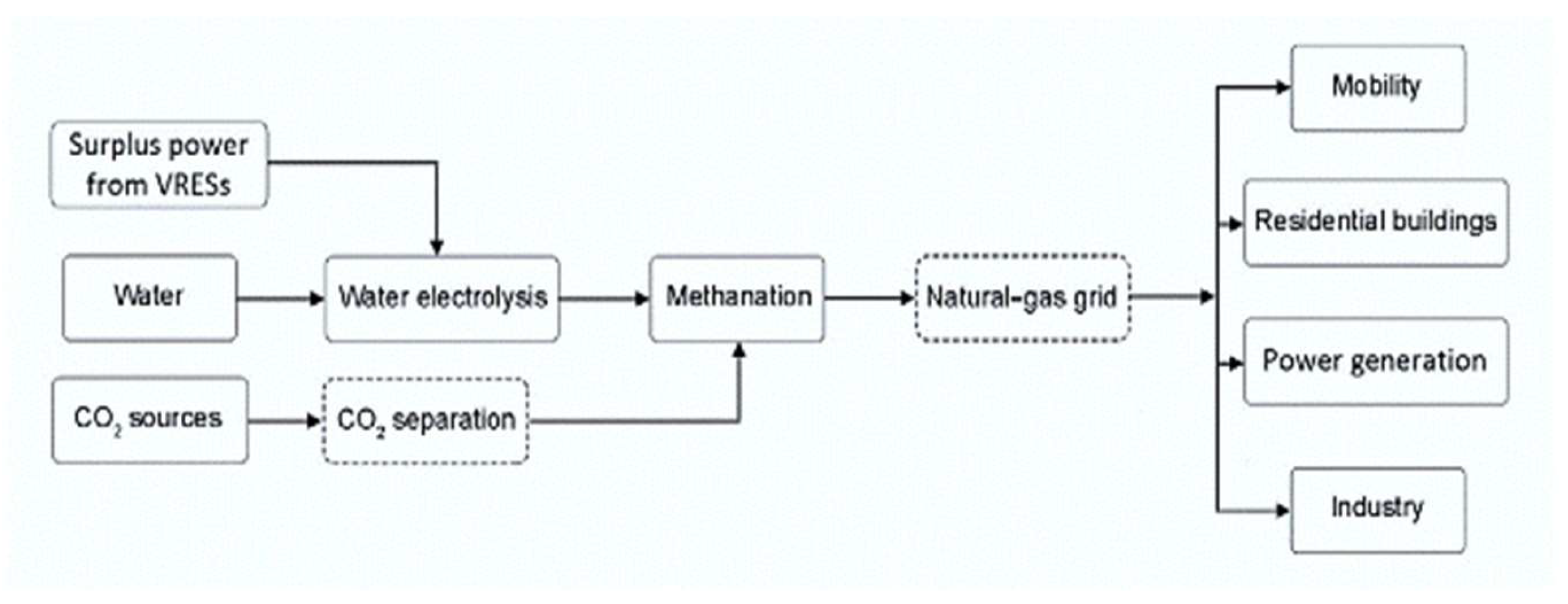
7. Power-to-Gas
7.1. Brief Overview
7.2. Thermochemical CO2 Methanation
7.3. Biological CO2 Methanation
- Continuously Stirred Tank Reactors;
- Fixed-Bed Reactors or Anaerobic Filters;
- Trickle-Bed Reactors.
8. Conclusions and Outlooks
- Deceleration and crucially reversion of the greenhouse effect caused predominantly by increased emissions of carbon dioxide and in turn the necessity for worldwide phasing out of carbon-based fossil fuels.
- Curtailment of large amounts of surplus electricity generated by inherently intermittent renewable sources, i.e., solar and wind.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations World: Total Population. Available online: https://population.un.org/wpp/Graphs/Probabilistic/POP/TOT/900 (accessed on 18 March 2022).
- International Energy Agency Data and Statistics. Available online: https://www.iea.org/data-and-statistics (accessed on 19 March 2022).
- Arrhenius, S. On the influence of carbonic acid in the air upon the temperature of the ground. Philos. Mag. 1896, 41, 373–393. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Zhang, Z. Carbon Capture, Utilization and Storage (CCUS). Appl. Energy 2019, 235, 1289–1299. [Google Scholar] [CrossRef]
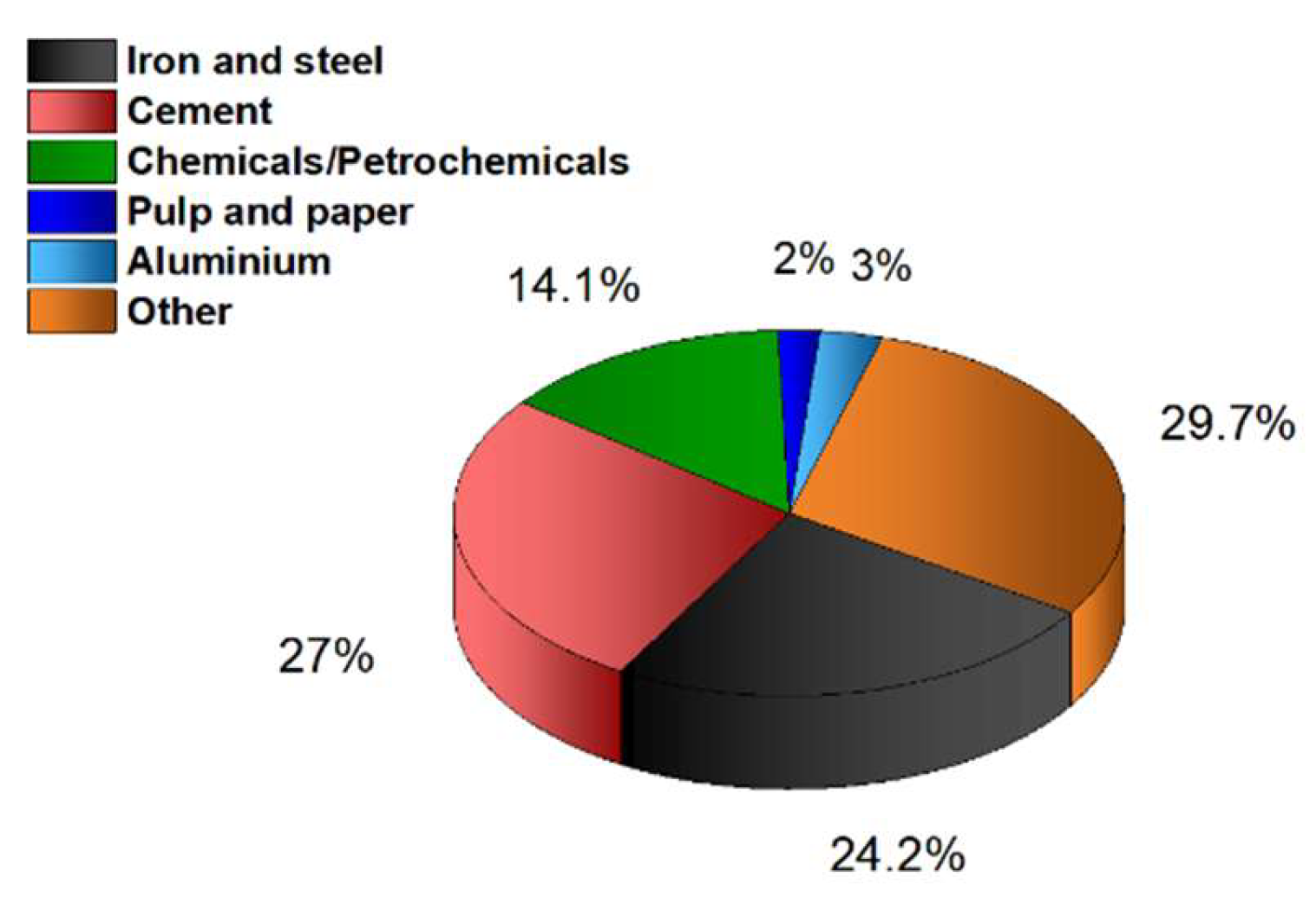
- Leeson, D.; Fennell, P.; Shah, N.; Petit, C.; Dowell, N. Mac A Techno-economic Analysis and Systematic Review of Carbon Capture and Storage (CCS) Applied to the Iron and Steel, Cement, Oil Refining and Pulp and Paper Industries. Energy Procedia 2017, 114, 6297–6302. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Vega, F.; Baena-Moreno, F.M.; Gallego Fernández, L.M.; Portillo, E.; Navarrete, B.; Zhang, Z. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale. Appl. Energy 2020, 260, 114313. [Google Scholar] [CrossRef]
- Global CCS Institute. Accelerating the Uptake of CCS: Industrial Use of Captured Carbon Dioxide; Global CCS Institute: Melbourne, Australia, 2011. [Google Scholar]
- Tapia, J.F.D.; Lee, J.Y.; Ooi, R.E.H.; Foo, D.C.Y.; Tan, R.R. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consum. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Laaksonen, A.; Krook-Riekkola, A.; Yang, Z.; Lu, X.; Ji, X. Carbon recycling—An immense resource and key to a smart climate engineering: A survey of technologies, cost and impurity impact. Renew. Sustain. Energy Rev. 2020, 131, 110010. [Google Scholar] [CrossRef]
- Lopes, J.V.M.; Bresciani, A.E.; Carvalho, K.M.; Kulay, L.A.; Alves, R.M.B. Multi-criteria decision approach to select carbon dioxide and hydrogen sources as potential raw materials for the production of chemicals Intergovernmental Panel on Climate Change. Renew. Sustain. Energy Rev. 2021, 151, 111542. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- World Energy Council. World Energy Scenarios—Composing Energy Futures to 2050—Executive Summary; World Energy Council: London, UK, 2013. [Google Scholar]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery Technologies for Grid-Level Large-Scale Electrical Energy Storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Chen, C.; Lu, H.T.; Wu, Y.; Liu, C.; Tao, L.; Men, Y.; He, G.; Li, K.G. A Review of Technical Advances, Barriers, and Solutions in the Power to Hydrogen (P2H) Roadmap. Engineering 2020, 6, 1364–1380. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline water electrolysis powered by renewable energy: A review. Processes 2020, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- International Renewable Energy Agency. Hydrogen from Renewable Power: Technology Outlook for the Energy Transition; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2018. [Google Scholar]
- Hydrogen Council. Hydrogen Scaling Up: A Sustainable Pathway for the Global Energy Transition; Hydrogen Council: Brussels, Belgium, 2017. [Google Scholar]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Gerard, F.; van Nuffel, L.; Smit, T.; Yearwood, J.; Cerny, O.; Michalski, J.; Altmann, M. Opportunities for Hydrogen Energy Technologies Considering the National Energy & Climate Plans; FCH: Rotterdam, The Netherlands, 2020. [Google Scholar]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Purcell, R. CO2 recycling by reaction with renewably-generated hydrogen. Int. J. Greenh. Gas Control 2010, 4, 44–50. [Google Scholar] [CrossRef]
- König, D.H.; Freiberg, M.; Dietrich, R.U.; Wörner, A. Techno-economic study of the storage of fluctuating renewable energy in liquid hydrocarbons. Fuel 2015, 159, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Klankermayer, J.; Wesselbaum, S.; Beydoun, K.; Leitner, W. Selective Catalytic Synthesis Using the Combination of Carbon Dioxide and Hydrogen: Catalytic Chess at the Interface of Energy and Chemistry. Angew. Chem.—Int. Ed. 2016, 55, 7296–7343. [Google Scholar] [CrossRef] [PubMed]
- Van Der Giesen, C.; Kleijn, R.; Kramer, G.J. Energy and climate impacts of producing synthetic hydrocarbon fuels from CO2. Environ. Sci. Technol. 2014, 48, 7111–7121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Zhang, D. A critical review of comparative global historical energy consumption and future demand: The story told so far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Williams, J.H.; DeBenedictis, A.; Ghanadan, R.; Mahone, A.; Moore, J.; Morrow, W.R.; Price, S.; Torn, M.S. The Technology Path to Deep Greenhouse Gas Emissions Cuts by 2050: The Pivotal Role of Electricity. Science 2012, 335, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. REPowerEU Plan; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Ochedi, F.O.; Liu, D.; Yu, J.; Hussain, A.; Liu, Y. Photocatalytic, electrocatalytic and photoelectrocatalytic conversion of carbon dioxide: A review. Environ. Chem. Lett. 2021, 19, 941–967. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydin, G.; Aydiner, K. Sources and mitigation of methane emissions by sectors: A critical review. Renew. Energy 2012, 39, 40–48. [Google Scholar] [CrossRef]
- Yusuf, R.O.; Noor, Z.Z.; Abba, A.H.; Hassan, M.A.A.; Din, M.F.M. Methane emission by sectors: A comprehensive review of emission sources and mitigation methods. Renew. Sustain. Energy Rev. 2012, 16, 5059–5070. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Budzianowski, W.M. Value-added carbon management technologies for low CO2 intensive carbon-based energy vectors. Energy 2012, 41, 280–297. [Google Scholar] [CrossRef]
- Rafiee, A.; Rajab Khalilpour, K.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Gaffney, O.; Steffen, W. The Anthropocene equation. Anthr. Rev. 2017, 4, 53–61. [Google Scholar] [CrossRef]
- Olah, G.A.; Prakash, G.K.S.; Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 2011, 133, 12881–12898. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Liu, G. Carbon Dioxide Geological Storage: Monitoring Technologies Review. In Greenhouse Gases—Capturing, Utilization and Reduction; IntechOpen: London, UK, 2012. [Google Scholar]
- Wimbadi, R.W.; Djalante, R. From decarbonization to low carbon development and transition: A systematic literature review of the conceptualization of moving toward net-zero carbon dioxide emission (1995–2019). J. Clean. Prod. 2020, 256, 120307. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021—The Physical Science Basis; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Knutti, R.; Rogelj, J.; Sedlácek, J.; Fischer, E.M. A scientific critique of the two-degree climate change target. Nat. Geosci. 2016, 9, 13–18. [Google Scholar] [CrossRef] [Green Version]
- United Nations Adoption of the Paris Agreement. Available online: http://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf (accessed on 4 October 2021).
- Depledge, J.; Saldivia, M.; Peñasco, C. Glass half full or glass half empty?: The 2021 Glasgow Climate Conference. Clim. Policy 2022, 22, 147–157. [Google Scholar] [CrossRef]
- European Commission European Union Green Deal. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52021DC0550&from=EN (accessed on 4 October 2021).
- United Kingdom Government The Climate Change Act 2008 (2050 Target Amendment) Order. 2019. Available online: https://www.legislation.gov.uk/uksi/2019/1056/contents/made (accessed on 4 October 2021).
- Rogelj, J.; Den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutti, R.; Rogelj, J. The legacy of our CO2 emissions: A clash of scientific facts, politics and ethics. Clim. Change 2015, 133, 361–373. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Eisaman, M.D.; Aradóttir, E.S.P.; Passarini, F.; Wang, T.; Sheehan, S.W. The role of carbon capture, utilization and storage for economic pathways that limit global warming to below 1.5 °C. iScience 2022, 25, 104237. [Google Scholar] [CrossRef] [PubMed]
- Luderer, G.; Pietzcker, R.C.; Bertram, C.; Kriegler, E.; Meinshausen, M.; Edenhofer, O. Economic mitigation challenges: How further delay closes the door for achieving climate targets. Environ. Res. Lett. 2013, 8, 034033. [Google Scholar] [CrossRef] [Green Version]
- Rogelj, J.; McCollum, D.L.; Reisinger, A.; Meinshausen, M.; Riahi, K. Probabilistic cost estimates for climate change mitigation. Nature 2013, 493, 79–83. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency Global Energy Data and Statistics. Available online: https://www.iea.org/data-and-statistics/data-browser/?country=WORLD&fuel=Energyconsumption&indicator=TFCShareBySector (accessed on 1 October 2021).
- International Energy Agency. Renewable Energy Market Update; International Energy Agency: Paris, France, 2021. [Google Scholar]
- IRENA. Post-COVID Recovery: An Agenda for Resilience, Development and Equality; IRENA: Abu Dhabi, United Arab Emirates, 2020; ISBN 9789292602451. [Google Scholar]
- Eroğlu, H. Effects of Covid-19 outbreak on environment and renewable energy sector. Environ. Dev. Sustain. 2021, 23, 4782–4790. [Google Scholar] [CrossRef] [PubMed]
- Olabi, V.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Abdelkareem, M.A. Impact of COVID-19 on the Renewable Energy Sector and Mitigation Strategies. Chem. Eng. Technol. 2022, 45, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.T.; Nižetić, S.; Olcer, A.I.; Ong, H.C.; Chen, W.-H.; Chong, C.T.; Thomas, S.; Bandh, S.A.; Nguyen, X.P. Impacts of COVID-19 pandemic on the global energy system and the shift progress to renewable energy: Opportunities, challenges, and policy implications. Energy Policy 2021, 154, 112322. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Wang, Q. How does the EU’s COVID-19 economic recession impact the renewable energy of other countries? The spillover effect. Energy Strateg. Rev. 2022, 40, 100825. [Google Scholar] [CrossRef]
- Napp, T.A.; Gambhir, A.; Hills, T.P.; Florin, N.; Fennell, P.S. A review of the technologies, economics and policy instruments for decarbonising energy-intensive manufacturing industries. Renew. Sustain. Energy Rev. 2014, 30, 616–640. [Google Scholar] [CrossRef]
- De Kleijne, K.; Hanssen, S.V.; van Dinteren, L.; Huijbregts, M.A.J.; van Zelm, R.; de Coninck, H. Limits to Paris compatibility of CO2 capture and utilization. One Earth 2022, 5, 168–185. [Google Scholar] [CrossRef]
- Karimi, F.; Khalilpour, R. Evolution of carbon capture and storage research: Trends of international collaborations and knowledge maps. Int. J. Greenh. Gas Control 2015, 37, 362–376. [Google Scholar] [CrossRef]
- Jarvis, S.M.; Samsatli, S. Technologies and infrastructures underpinning future CO2 value chains: A comprehensive review and comparative analysis. Renew. Sustain. Energy Rev. 2018, 85, 46–68. [Google Scholar] [CrossRef]
- Chalmers, H.; Gibbins, J. Carbon capture and storage: The ten year challenge. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2010, 224, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Lemontzoglou, A.; Pantoleontos, G.; Asimakopoulou, A.G.; Tsongidis, N.I.; Konstandopoulos, A.G. Analysis of CO2 transport including impurities for the optimization of point-to-point pipeline networks for integration into future solar fuel plants. Int. J. Greenh. Gas Control 2017, 66, 10–24. [Google Scholar] [CrossRef]
- Lackner, K.S.; Grimes, P.; Ziock, H.J. Carbon dioxide extraction from air: Is it an option? In Proceedings of the 24th International Technical Conference on Coal Utilization and Fuel Systems, Clearwater, FL, USA, 8–11 March 1999.
- Mishra, U.C.; Sarsaiya, S.; Gupta, A. A systematic review on the impact of cement industries on the natural environment. Environ. Sci. Pollut. Res. 2022, 29, 18440–18451. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Shayegh, S.; Bosetti, V.; Tavoni, M. Future Prospects of Direct Air Capture Technologies: Insights From an Expert Elicitation Survey. Front. Clim. 2021, 3, 46. [Google Scholar] [CrossRef]
- McQueen, N.; Gomes, K.V.; McCormick, C.; Blumanthal, K.; Pisciotta, M.; Wilcox, J. A review of direct air capture (DAC): Scaling up commercial technologies and innovating for the future. Prog. Energy 2021, 3, 032001. [Google Scholar] [CrossRef]
- Erans, M.; Sanz-Pérez, E.S.; Hanak, D.P.; Clulow, Z.; Reiner, D.M.; Mutch, G.A. Direct air capture: Process technology, techno-economic and socio-political challenges. Energy Environ. Sci. 2022, 15, 1360–1405. [Google Scholar] [CrossRef]
- Hnolog, E.C. CCSP Carbon Capture and Storage Program; VTT Technology: Esbo, Finland, 2013. [Google Scholar]
- European Commission. A Strategy for Competitive, Sustainable and Secure Energy. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2010:0639:FIN:En:PDF (accessed on 9 October 2021).
- European Commission. Technology Map of the European Strategic Energy Technology Plan (SET-Plan)—Technology Descriptions. 2013. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC86357 (accessed on 9 October 2021).
- Koukouzas, N.; Tyrologou, P.; Karapanos, D.; Carneiro, J.; Pereira, P.; de Mesquita Lobo Veloso, F.; Koutsovitis, P.; Karkalis, C.; Manoukian, E.; Karametou, R. Carbon Capture, Utilisation and Storage as a Defense Tool Against Climate Change: Current Developments in West Macedonia (Greece). Energies 2021, 14, 3321. [Google Scholar] [CrossRef]
- Ghosh, A.; Kiran, B. Carbon Concentration in Algae: Reducing CO2 From Exhaust Gas. Trends Biotechnol. 2017, 35, 806–808. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas Control 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.H.; Tsang, D.C.W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Nicot, J.P.; Duncan, I.J. Review: Common attributes of hydraulically fractured oil and gas production and CO2 geological sequestration. Greenh. Gases Sci. Technol. 2012, 2, 352–368. [Google Scholar] [CrossRef]
- Xiang, Z.; Hu, Z.; Cao, D.; Yang, W.; Lu, J.; Han, B.; Wang, W. Metal-organic frameworks with incorporated carbon nanotubes: Improving carbon dioxide and methane storage capacities by lithium doping. Angew. Chem.—Int. Ed. 2011, 50, 491–494. [Google Scholar] [CrossRef]
- Huang, P.H.; Cheng, H.H.; Lin, S.H. Adsorption of carbon dioxide onto activated carbon prepared from coconut shells. J. Chem. 2015, 2015, 106590. [Google Scholar] [CrossRef]
- Global CCS Institute. The Global Status of CCS 2018; Global CCS Institute Publications: Melbourne, Australia, 2018. [Google Scholar]
- Global CCS Institute. CO2RE Database; Global CCS Institute Publications: Melbourne, Australia, 2018. [Google Scholar]
- Davison, J. Performance and costs of power plants with capture and storage of CO2. Energy 2007, 32, 1163–1176. [Google Scholar] [CrossRef]
- Elias, R.S.; Wahab, M.I.M.; Fang, L. Retrofitting carbon capture and storage to natural gas-fired power plants: A real-options approach. J. Clean. Prod. 2018, 192, 722–734. [Google Scholar] [CrossRef]
- Reiter, G. Power-to-Gas. In Fuel Cells: Data, Facts and Figures; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2016; pp. 357–368. [Google Scholar]
- Anwar, M.N.; Fayyaz, A.; Sohail, N.F.; Khokhar, M.F.; Baqar, M.; Khan, W.D.; Rasool, K.; Rehan, M.; Nizami, A.S. CO2 capture and storage: A way forward for sustainable environment. J. Environ. Manag. 2018, 226, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Sifat, N.S.; Haseli, Y. A critical review of CO2 capture technologies and prospects for clean power generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Damm, D.L.; Fedorov, A.G. Conceptual study of distributed CO2 capture and the sustainable carbon economy. Energy Convers. Manag. 2008, 49, 1674–1683. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Desideri, U.; Paolucci, A. Performance modelling of a carbon dioxide removal system for power plants. Energy Convers. Manag. 1999, 40, 1899–1915. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [Green Version]
- Deutch, J.M.; Moniz, E.J. Retrofitting of Coal-Fired Power Plants for CO2 Emissions Reductions; Massachusetts Institute of Technology: Cambridge, MA, USA, 2009. [Google Scholar]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Yoro, K.O.; Daramola, M.O.; Sekoai, P.T.; Armah, E.K.; Wilson, U.N. Advances and emerging techniques for energy recovery during absorptive CO2 capture: A review of process and non-process integration-based strategies. Renew. Sustain. Energy Rev. 2021, 147, 111241. [Google Scholar] [CrossRef]
- Manzolini, G.; Sanchez Fernandez, E.; Rezvani, S.; Macchi, E.; Goetheer, E.L.V.; Vlugt, T.J.H. Economic assessment of novel amine based CO2 capture technologies integrated in power plants based on European Benchmarking Task Force methodology. Appl. Energy 2015, 138, 546–558. [Google Scholar] [CrossRef]
- Budzianowski, W.M. Single solvents, solvent blends, and advanced solvent systems in CO2 capture by absorption: A review. Int. J. Glob. Warm. 2015, 7, 184. [Google Scholar] [CrossRef]
- Sanchez Fernandez, E.; Goetheer, E.L.V.; Manzolini, G.; Macchi, E.; Rezvani, S.; Vlugt, T.J.H. Thermodynamic assessment of amine based CO2 capture technologies in power plants based on European Benchmarking Task Force methodology. Fuel 2014, 129, 318–329. [Google Scholar] [CrossRef]
- Vaz, S.; Rodrigues de Souza, A.P.; Lobo Baeta, B.E. Technologies for carbon dioxide capture: A review applied to energy sectors. Clean. Eng. Technol. 2022, 8, 100456. [Google Scholar] [CrossRef]
- Van Straelen, J.; Geuzebroek, F.; Goodchild, N.; Protopapas, G.; Mahony, L. CO2 capture for refineries, a practical approach. Int. J. Greenh. Gas Control 2010, 4, 316–320. [Google Scholar] [CrossRef]
- Kunze, C.; Spliethoff, H. Assessment of oxy-fuel, pre- and post-combustion-based carbon capture for future IGCC plants. Appl. Energy 2012, 94, 109–116. [Google Scholar] [CrossRef]
- Plaza, M.G.; Martínez, S.; Rubiera, F. CO2 Capture, Use, and Storage in the Cement Industry: State of the Art and Expectations. Energies 2020, 13, 5692. [Google Scholar] [CrossRef]
- Hills, T.; Leeson, D.; Florin, N.; Fennell, P. Carbon Capture in the Cement Industry: Technologies, Progress, and Retrofitting. Environ. Sci. Technol. 2016, 50, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J.; Turner, S.A.; Napier-Moore, P.A.; Clark, M.; Davison, J.E. CO2 Capture in the Cement Industry. Energy Procedia 2009, 1, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Kuramochi, T.; Ramírez, A.; Turkenburg, W.; Faaij, A. Comparative assessment of CO2 capture technologies for carbon-intensive industrial processes. Prog. Energy Combust. Sci. 2012, 38, 87–112. [Google Scholar] [CrossRef]
- Bains, P.; Psarras, P.; Wilcox, J. CO2 capture from the industry sector. Prog. Energy Combust. Sci. 2017, 63, 146–172. [Google Scholar] [CrossRef]
- Volkart, K.; Bauer, C.; Boulet, C. Life cycle assessment of carbon capture and storage in power generation and industry in Europe. Int. J. Greenh. Gas Control 2013, 16, 91–106. [Google Scholar] [CrossRef]
- Meylan, F.D.; Moreau, V.; Erkman, S. CO2 utilization in the perspective of industrial ecology, an overview. J. CO2 Util. 2015, 12, 101–108. [Google Scholar] [CrossRef]
- de Falco, M.; Iaquaniello, G.; Centi, G. CO2: A Valuable Source of Carbon; Springer: London, UK, 2013; Volume 137, ISBN 9781447151180. [Google Scholar]
- Huang, C.H.; Tan, C.S. A review: CO2 utilization. Aerosol Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, N.; Hill, D. Carbon Dioxide Utilization: Electrochemical Conversion of CO2—Opportunities and Challenges. 2011. Available online: https://www.semanticscholar.org/paper/Carbon-Dioxide-Utilization-Electrochemical-of-CO-2/e19d9acc8c4fa86b8866dccf27b71282343bb09b (accessed on 4 October 2021).
- Chauvy, R.; Meunier, N.; Thomas, D.; De Weireld, G. Selecting emerging CO2 utilization products for short- to mid-term deployment. Appl. Energy 2019, 236, 662–680. [Google Scholar] [CrossRef]
- Alok, A.; Shrestha, R.; Ban, S.; Devkota, S.; Uprety, B.; Joshi, R. Technological advances in the transformative utilization of CO2 to value-added products. J. Environ. Chem. Eng. 2022, 10, 106922. [Google Scholar] [CrossRef]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef]
- Shih, C.F.; Zhang, T.; Li, J.; Bai, C. Powering the Future with Liquid Sunshine. Joule 2018, 2, 1925–1949. [Google Scholar] [CrossRef] [Green Version]
- Langanke, J.; Wolf, A.; Hofmann, J.; Böhm, K.; Subhani, M.A.; Müller, T.E.; Leitner, W.; Gürtler, C. Carbon dioxide (CO2) as sustainable feedstock for polyurethane production. Green Chem. 2014, 16, 1865–1870. [Google Scholar] [CrossRef]
- Keller, N.; Rebmann, G.; Keller, V. Catalysts, mechanisms and industrial processes for the dimethylcarbonate synthesis. J. Mol. Catal. A Chem. 2010, 317, 1–18. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Lei, L.; Chen, F. High temperature solid oxide H2O/CO2 co-electrolysis for syngas production. Fuel Process. Technol. 2017, 161, 248–258. [Google Scholar] [CrossRef]
- Herranz, J.; Pătru, A.; Fabbri, E.; Schmidt, T.J. Co-electrolysis of CO2 and H2O: From electrode reactions to cell-level development. Curr. Opin. Electrochem. 2020, 23, 89–95. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Electrode Build-Up of Reducible Metal Composites toward Achievable Electrochemical Conversion of Carbon Dioxide. ChemSusChem 2016, 9, 333–344. [Google Scholar] [CrossRef]
- Lu, X.; Leung, D.Y.C.; Wang, H.; Leung, M.K.H.; Xuan, J. Electrochemical Reduction of Carbon Dioxide to Formic Acid. ChemElectroChem 2014, 1, 836–849. [Google Scholar] [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef] [Green Version]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef]
- Ameta, R.; Ameta, S.C. Photocatalysis—Principles and Applications; Taylor & Francis: Milton Park, UK, 2017; ISBN 9781482254938. [Google Scholar]
- Dey, G.R.; Belapurkar, A.D.; Kishore, K. Photo-catalytic reduction of carbon dioxide to methane using TiO2 as suspension in water. J. Photochem. Photobiol. A Chem. 2004, 163, 503–508. [Google Scholar] [CrossRef]
- Richardson, R.D.; Holland, E.J.; Carpenter, B.K. A renewable amine for photochemical reduction of CO2. Nat. Chem. 2011, 3, 301–303. [Google Scholar] [CrossRef] [PubMed]
- GCCSI. 2021 Global Status of CCS Report; Global CCS Institute Publications: Melbourne, Australia, 2021. [Google Scholar]
- GCCSI. Global Status of CCS 2020; Global CCS Institute Publications: Melbourne, Australia, 2020. [Google Scholar]
- Hong, W.Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci. Technol. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Carbon Capture from Flue Gas and the Atmosphere: A Perspective. Front. Energy Res. 2020, 8, 560849. [Google Scholar] [CrossRef]
- Kheirinik, M.; Ahmed, S.; Rahmanian, N. Comparative Techno-Economic Analysis of Carbon Capture Processes: Pre-Combustion, Post-Combustion, and Oxy-Fuel Combustion Operations. Sustainability 2021, 13, 13567. [Google Scholar] [CrossRef]
- Wang, Y.; He, D.; Chen, H.; Wang, D. Catalysts in electro-, photo- and photoelectrocatalytic CO2 reduction reactions. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 117–149. [Google Scholar] [CrossRef]
- Takht Ravanchi, M.; Sahebdelfar, S. Catalytic conversions of CO2 to help mitigate climate change: Recent process developments. Process Saf. Environ. Prot. 2021, 145, 172–194. [Google Scholar] [CrossRef]
- De, S.; Dokania, A.; Ramirez, A.; Gascon, J. Advances in the Design of Heterogeneous Catalysts and Thermocatalytic Processes for CO2 Utilization. ACS Catal. 2020, 10, 14147–14185. [Google Scholar] [CrossRef]
- Bahmanpour, A.M.; Signorile, M.; Kröcher, O. Recent progress in syngas production via catalytic CO2 hydrogenation reaction. Appl. Catal. B Environ. 2021, 295, 120319. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2021, 44, 101413. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2021, 368, 2–19. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Spataru, D.; Karam, L.; Lopes, J.M.; Henriques, C. Promising catalytic systems for CO2 hydrogenation into CH4: A review of recent studies. Processes 2020, 8, 1646. [Google Scholar] [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galadima, A.; Muraza, O. Catalytic thermal conversion of CO2 into fuels: Perspective and challenges. Renew. Sustain. Energy Rev. 2019, 115, 109333. [Google Scholar] [CrossRef]
- Alper, E.; Yuksel Orhan, O. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Vu, T.T.N.; Desgagnés, A.; Iliuta, M.C. Efficient approaches to overcome challenges in material development for conventional and intensified CO2 catalytic hydrogenation to CO, methanol, and DME. Appl. Catal. A Gen. 2021, 617, 118119. [Google Scholar] [CrossRef]
- Quintino, F.M.; Nascimento, N.; Fernandes, E.C. Aspects of Hydrogen and Biomethane Introduction in Natural Gas Infrastructure and Equipment. Hydrogen 2021, 2, 301–318. [Google Scholar] [CrossRef]
- SRIA Clean Hydrogen for Europe. Strategic Research and Innovation Agenda—Final Draft; SRIA: Luxembourg, 2020. [Google Scholar]
- Nnabuife, S.G.; Ugbeh-Johnson, J.; Okeke, N.E.; Ogbonnaya, C. Present and Projected Developments in Hydrogen Production: A Technological Review. Carbon Capture Sci. Technol. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen production. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherland, 2019; pp. 45–83. [Google Scholar]
- Ingersoll, J.G. The Renewable Hydrogen–Methane (RHYME) Transportation Fuel: A Practical First Step in the Realization of the Hydrogen Economy. Hydrogen 2022, 3, 84–111. [Google Scholar] [CrossRef]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Catal. Rev. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A short review on Ni based catalysts and related engineering issues for methane steam reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Gorre, J.; Ruoss, F.; Karjunen, H.; Schaffert, J.; Tynjälä, T. Cost benefits of optimizing hydrogen storage and methanation capacities for Power-to-Gas plants in dynamic operation. Appl. Energy 2020, 257, 113967. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Mazza, A.; Bompard, E.; Chicco, G. Applications of power to gas technologies in emerging electrical systems. Renew. Sustain. Energy Rev. 2018, 92, 794–806. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Kato, T.; Kubota, M.; Kobayashi, N.; Suzuoki, Y. Effective utilization of by-product oxygen from electrolysis hydrogen production. Energy 2005, 30, 2580–2595. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef]
- Kondziella, H.; Bruckner, T. Flexibility requirements of renewable energy based electricity systems—A review of research results and methodologies. Renew. Sustain. Energy Rev. 2016, 53, 10–22. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Recent developments in photothermal reactors with understanding on the role of light/heat for CO2 hydrogenation to fuels: A review. Chem. Eng. J. 2022, 427, 131617. [Google Scholar] [CrossRef]
- Ulmer, U.; Dingle, T.; Duchesne, P.N.; Morris, R.H.; Tavasoli, A.; Wood, T.; Ozin, G.A. Fundamentals and applications of photocatalytic CO2 methanation. Nat. Commun. 2019, 10, 3169. [Google Scholar] [CrossRef] [Green Version]
- Kong, T.; Jiang, Y.; Xiong, Y. Photocatalytic CO2 conversion: What can we learn from conventional COx hydrogenation? Chem. Soc. Rev. 2020, 49, 6579–6591. [Google Scholar] [CrossRef]
- Liu, M.; Yi, Y.; Wang, L.; Guo, H.; Bogaerts, A. Hydrogenation of carbon dioxide to value-added chemicals by heterogeneous catalysis and plasma catalysis. Catalysts 2019, 9, 275. [Google Scholar] [CrossRef] [Green Version]
- Michiels, R.; Engelmann, Y.; Bogaerts, A. Plasma Catalysis for CO2 Hydrogenation: Unlocking New Pathways toward CH3OH. J. Phys. Chem. C 2020, 124, 25859–25872. [Google Scholar] [CrossRef]
- Dębek, R.; Azzolina-Jury, F.; Travert, A.; Maugé, F. A review on plasma-catalytic methanation of carbon dioxide—Looking for an efficient catalyst. Renew. Sustain. Energy Rev. 2019, 116, 109427. [Google Scholar] [CrossRef]
- Liu, A.; Gao, M.; Ren, X.; Meng, F.; Yang, Y.; Gao, L.; Yang, Q.; Ma, T. Current progress in electrocatalytic carbon dioxide reduction to fuels on heterogeneous catalysts. J. Mater. Chem. A 2020, 8, 3541–3562. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.X.; Gandionco, K.A.; Bond, A.M.; Zhang, J. Electrocatalytic carbon dioxide reduction: From fundamental principles to catalyst design. Mater. Today Adv. 2020, 7, 100074. [Google Scholar] [CrossRef]
- Poormohammadian, S.J.; Bahadoran, F.; Vakili-Nezhaad, G.R. Recent progress in homogeneous hydrogenation of carbon dioxide to methanol. Rev. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Sasaki, T. CO2 hydrogenation in ionic liquids: Recent update. Curr. Opin. Green Sustain. Chem. 2022, 36, 100633. [Google Scholar] [CrossRef]
- Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, H.; An, K.; Lee, J.S. Recycling Carbon Dioxide through Catalytic Hydrogenation: Recent Key Developments and Perspectives. ACS Catal. 2020, 10, 11318–11345. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Xi, X.; Liao, L.; Ren, J.; Xie, J.; Heeres, H.J.; Palkovits, R. Catalysts design for higher alcohols synthesis by CO2 hydrogenation: Trends and future perspectives. Appl. Catal. B Environ. 2021, 291, 120073. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Costamagna, P. Three-pipeline gas grid: A new concept for power-to-gas associated with complete carbon capture and utilization. Energy Convers. Manag. 2021, 229, 113739. [Google Scholar] [CrossRef]
- Kezibri, N.; Bouallou, C. Conceptual design and modelling of an industrial scale power to gas-oxy-combustion power plant. Int. J. Hydrogen Energy 2017, 42, 19411–19419. [Google Scholar] [CrossRef]
- Bailera, M.; Kezibri, N.; Romeo, L.M.; Espatolero, S.; Lisbona, P.; Bouallou, C. Future applications of hydrogen production and CO2 utilization for energy storage: Hybrid Power to Gas-Oxycombustion power plants. Int. J. Hydrogen Energy 2017, 42, 13625–13632. [Google Scholar] [CrossRef] [Green Version]
- Frigo, S.; Spazzafumo, G. Comparison of different system layouts to generate a substitute of natural gas from biomass and electrolytic hydrogen. Int. J. Hydrogen Energy 2020, 45, 26166–26178. [Google Scholar] [CrossRef]
- Katla, D.; Jurczyk, M.; Skorek-Osikowska, A.; Uchman, W. Analysis of the integrated system of electrolysis and methanation units for the production of synthetic natural gas (SNG). Energy 2021, 237, 121479. [Google Scholar] [CrossRef]
- Momeni, M.; Soltani, M.; Hosseinpour, M.; Nathwani, J. A comprehensive analysis of a power-to-gas energy storage unit utilizing captured carbon dioxide as a raw material in a large-scale power plant. Energy Convers. Manag. 2021, 227, 113613. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef]
- Dillman, K.J.; Heinonen, J. A ‘just’ hydrogen economy: A normative energy justice assessment of the hydrogen economy. Renew. Sustain. Energy Rev. 2022, 167, 112648. [Google Scholar] [CrossRef]
- Ashok, J.; Falbo, L.; Das, S.; Dewangan, N.; Visconti, C.G.; Kawi, S. Catalytic CO2 Conversion to Added-Value Energy Rich C1 Products. In An Economy Based on Carbon Dioxide and Water; Springer: Cham, Switzerland, 2019; pp. 155–210. ISBN 9783030158682. [Google Scholar]
- Bao, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Ding, M.; Hong, X.; Liu, G.; Tsang, S.C.E. Advances in higher alcohol synthesis from CO2 hydrogenation. Chem 2021, 7, 849–881. [Google Scholar] [CrossRef]
- Reiter, G.; Lindorfer, J. Evaluating CO2 sources for power-to-gas applications-A case study for Austria. J. CO2 Util. 2015, 10, 40–49. [Google Scholar] [CrossRef]
- Von Der Assen, N.; Müller, L.J.; Steingrube, A.; Voll, P.; Bardow, A. Selecting CO2 Sources for CO2 Utilization by Environmental-Merit-Order Curves. Environ. Sci. Technol. 2016, 50, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Mores, P.L.; Arias, A.M.; Scenna, N.J.; Caballero, J.A.; Mussati, S.F.; Mussati, M.C. Membrane-based processes: Optimization of hydrogen separation by minimization of power, membrane area, and cost. Processes 2018, 6, 221. [Google Scholar] [CrossRef] [Green Version]
- Eveloy, V.; Gebreegziabher, T. A Review of Projected Power-to-Gas Deployment Scenarios. Energies 2018, 11, 1824. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, A.; Bardow, A. Power-to-What?-Environmental assessment of energy storage systems. Energy Environ. Sci. 2015, 8, 389–400. [Google Scholar] [CrossRef]
- Böttger, D.; Götz, M.; Lehr, N.; Kondziella, H.; Bruckner, T. Potential of the Power-to-Heat technology in district heating grids in Germany. Energy Procedia 2014, 46, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Salpakari, J.; Mikkola, J.; Lund, P.D. Improved flexibility with large-scale variable renewable power in cities through optimal demand side management and power-to-heat conversion. Energy Convers. Manag. 2016, 126, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, P.; Batteiger, V.; Roth, A.; Weindorf, W.; Raksha, T. Power-to-Liquids as Renewable Fuel Option for Aviation: A Review. Chemie-Ingenieur-Technik 2018, 90, 127–140. [Google Scholar] [CrossRef]
- Buffo, G.; Marocco, P.; Ferrero, D.; Lanzini, A.; Santarelli, M. Power-to-X and Power-to-Power Routes; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128148549. [Google Scholar]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Fuel Cells & Hydrogen Joint Undertaking Overview of the Market Segmentation for Hydrogen across Potential Customer Groups, Based on Key Application Areas, Study by HINICIO. Available online: http://www.certifhy.eu/images/D1_2_Overview_of_the_market_segmentation_Final_22_June_low-res.pdf (accessed on 11 March 2022).
- Melaina, M.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; Hydrogen and Fuel Cell Technologies Office: Denver, CO, USA, 2013. [Google Scholar]
- Altfeld, K.; Pinchbeck, D. Admissible hydrogen concentrations in natural gas systems. Gas Energy 2013, 2013, 1–16. [Google Scholar]
- İnci, M.; Büyük, M.; Demir, M.H.; İlbey, G. A review and research on fuel cell electric vehicles: Topologies, power electronic converters, energy management methods, technical challenges, marketing and future aspects. Renew. Sustain. Energy Rev. 2021, 137, 110648. [Google Scholar] [CrossRef]
- Sorlei, I.-S.; Bizon, N.; Thounthong, P.; Varlam, M.; Carcadea, E.; Culcer, M.; Iliescu, M.; Raceanu, M. Fuel Cell Electric Vehicles—A Brief Review of Current Topologies and Energy Management Strategies. Energies 2021, 14, 252. [Google Scholar] [CrossRef]
- Lehner, M.; Tichler, R.; Steinmüller, H.; Koppe, M. Power-to-Gas: Technology and Business Models; Springer: Cham, Switzerland, 2014; ISBN 978-3-319-03994-7. [Google Scholar]
- Hermesmann, M.; Grübel, K.; Scherotzki, L.; Müller, T.E. Promising pathways: The geographic and energetic potential of power-to-x technologies based on regeneratively obtained hydrogen. Renew. Sustain. Energy Rev. 2021, 138, 110644. [Google Scholar] [CrossRef]
- Siebels, C. Power-to-X-Anwendungen mit Anschluss an das Stromnetz. Chemie Ing. Tech. 2021, 93, 547–552. [Google Scholar] [CrossRef]
- Farfan, J.; Fasihi, M.; Breyer, C. Trends in the global cement industry and opportunities for long-term sustainable CCU potential for Power-to-X. J. Clean. Prod. 2019, 217, 821–835. [Google Scholar] [CrossRef]
- Lonis, F.; Tola, V.; Cau, G. Assessment of integrated energy systems for the production and use of renewable methanol by water electrolysis and CO2 hydrogenation. Fuel 2021, 285, 119160. [Google Scholar] [CrossRef]
- Nyári, J.; Magdeldin, M.; Larmi, M.; Järvinen, M.; Santasalo-Aarnio, A. Techno-economic barriers of an industrial-scale methanol CCU-plant. J. CO2 Util. 2020, 39, 101166. [Google Scholar] [CrossRef]
- Elsernagawy, O.Y.H.; Hoadley, A.; Patel, J.; Bhatelia, T.; Lim, S.; Haque, N.; Li, C. Thermo-economic analysis of reverse water-gas shift process with different temperatures for green methanol production as a hydrogen carrier. J. CO2 Util. 2020, 41, 101280. [Google Scholar] [CrossRef]
- Michailos, S.; McCord, S.; Sick, V.; Stokes, G.; Styring, P. Dimethyl ether synthesis via captured CO2 hydrogenation within the power to liquids concept: A techno-economic assessment. Energy Convers. Manag. 2019, 184, 262–276. [Google Scholar] [CrossRef]
- De Falco, M.; Natrella, G.; Capocelli, M.; Popielak, P.; Sołtysik, M.; Wawrzyńczak, D.; Majchrzak-Kucęba, I. Exergetic Analysis of DME Synthesis from CO2 and Renewable Hydrogen. Energies 2022, 15, 3516. [Google Scholar] [CrossRef]
- Wu, T.-W.; Chien, I.-L. A novel energy-efficient process of converting CO2 to dimethyl ether with techno-economic and environmental evaluation. Chem. Eng. Res. Des. 2022, 177, 1–12. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, C.; Jun, K.W.; Kim, S.K.; Park, H.G.; Zhao, T.; Wang, L.; Wan, H.; Guan, G. Transformation of CO2 into liquid fuels and synthetic natural gas using green hydrogen: A comparative analysis. Fuel 2021, 291, 120111. [Google Scholar] [CrossRef]
- Rispoli, A.L.; Verdone, N.; Vilardi, G. Green fuel production by coupling plastic waste oxy-combustion and PtG technologies: Economic, energy, exergy and CO2-cycle analysis. Fuel Process. Technol. 2021, 221, 106922. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Yoo, B.; Lee, C. Techno-Economic and Environmental Analysis for Direct Catalytic Conversion of CO2 to Methanol and Liquid/High-Calorie-SNG Fuels. Catalysts 2021, 11, 687. [Google Scholar] [CrossRef]
- Adelung, S.; Maier, S.; Dietrich, R.U. Impact of the reverse water-gas shift operating conditions on the Power-to-Liquid process efficiency. Sustain. Energy Technol. Assess. 2021, 43, 100897. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, C.; Jun, K.W.; Kim, S.K.; Park, H.G.; Zhao, T.; Wang, L.; Wan, H.; Guan, G. Green liquid fuel and synthetic natural gas production via CO2 hydrogenation combined with reverse water-gas-shift and Co-based Fischer-Tropsch synthesis. J. CO2 Util. 2021, 51, 101619. [Google Scholar] [CrossRef]
- Parigi, D.; Giglio, E.; Soto, A.; Santarelli, M. Power-to-fuels through carbon dioxide Re-Utilization and high-temperature electrolysis: A technical and economical comparison between synthetic methanol and methane. J. Clean. Prod. 2019, 226, 679–691. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, R.; Jun, K.W.; Kim, S.K.; Hwang, S.M.; Park, H.G.; Guan, G. Direct conversion of carbon dioxide to liquid fuels and synthetic natural gas using renewable power: Techno-economic analysis. J. CO2 Util. 2019, 34, 293–302. [Google Scholar] [CrossRef]
- Kuusela, K.; Uusitalo, V.; Ahola, J.; Levänen, J. The transformation of plastics production from net positive greenhouse gas emissions to net negative: An environmental sustainability assessment of CO2-based polypropylene. J. CO2 Util. 2021, 52, 101672. [Google Scholar] [CrossRef]
- Do, T.N.; Kim, J. Green C2-C4 hydrocarbon production through direct CO2 hydrogenation with renewable hydrogen: Process development and techno-economic analysis. Energy Convers. Manag. 2020, 214, 112866. [Google Scholar] [CrossRef]
- Koj, J.C.; Wulf, C.; Zapp, P. Environmental impacts of power-to-X systems—A review of technological and methodological choices in Life Cycle Assessments. Renew. Sustain. Energy Rev. 2019, 112, 865–879. [Google Scholar] [CrossRef]
- Thonemann, M.A.N. Environmental impacts of CO2-based chemical production: A systematic literature review and meta-analysis. Appl. Energy 2020, 263, 114599. [Google Scholar] [CrossRef]
- Wevers, J.B.; Shen, L.; van der Spek, M. What Does It Take to Go Net-Zero-CO2? A Life Cycle Assessment on Long-Term Storage of Intermittent Renewables with Chemical Energy Carriers. Front. Energy Res. 2020, 8, 104. [Google Scholar] [CrossRef]
- Blanco, H.; Codina, V.; Laurent, A.; Nijs, W.; Maréchal, F.; Faaij, A. Life cycle assessment integration into energy system models: An application for Power-to-Methane in the EU. Appl. Energy 2020, 259, 114160. [Google Scholar] [CrossRef]
- Garcia-Garcia, G.; Fernandez, M.C.; Armstrong, K.; Woolass, S.; Styring, P. Analytical Review of Life-Cycle Environmental Impacts of Carbon Capture and Utilization Technologies. ChemSusChem 2021, 14, 995–1015. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F.Z. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Quarton, C.J.; Samsatli, S. Power-to-gas for injection into the gas grid: What can we learn from real-life projects, economic assessments and systems modelling? Renew. Sustain. Energy Rev. 2018, 98, 302–316. [Google Scholar] [CrossRef]
- Wulf, C.; Zapp, P.; Schreiber, A. Review of Power-to-X Demonstration Projects in Europe. Front. Energy Res. 2020, 8, 191. [Google Scholar] [CrossRef]
- Chehade, Z.; Mansilla, C.; Lucchese, P.; Hilliard, S.; Proost, J. Review and analysis of demonstration projects on power-to-X pathways in the world. Int. J. Hydrogen Energy 2019, 44, 27637–27655. [Google Scholar] [CrossRef]
- Sterner, M.; Specht, M. Power-to-gas and power-to-x—the history and results of developing a new storage concept. Energies 2021, 14, 6594. [Google Scholar] [CrossRef]
- Lewandowska-Bernat, A.; Desideri, U. Opportunities of power-to-gas technology in different energy systems architectures. Appl. Energy 2018, 228, 57–67. [Google Scholar] [CrossRef]
- Hashimoto, K. Metastable metals for “green” materials for global atmosphere conservation and abundant energy supply. Mater. Sci. Eng. A 1994, 179–180, 27–30. [Google Scholar] [CrossRef]
- Hao, H.; Liu, Z.; Zhao, F.; Li, W. Natural gas as vehicle fuel in China: A review. Renew. Sustain. Energy Rev. 2016, 62, 521–533. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Jaramillo, L.Y.; Henao, W.; de la Torre, U. Nanomaterials for CO2 Hydrogenation. In Emerging Nanostructured Materials for Energy and Environmental Science; Springer: Cham, Switzerland, 2019; pp. 173–214. ISBN 9783030044749. [Google Scholar]
- Jentsch, M.; Trost, T.; Sterner, M. Optimal use of Power-to-Gas energy storage systems in an 85% renewable energy scenario. Energy Procedia 2014, 46, 254–261. [Google Scholar] [CrossRef]
- Tichler, R.; Bauer, S. Power-to-Gas. In Storing Energy: With Special Reference to Renewable Energy Sources; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 373–389. ISBN 9780128034408. [Google Scholar]
- Lewandowska-Bernat, A.; Desideri, U. Opportunities of Power-to-Gas technology. Energy Procedia 2017, 105, 4569–4574. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kumagai, N.; Izumiya, K.; Takano, H.; Kato, Z. The production of renewable energy in the form of methane using electrolytic hydrogen generation. Energy. Sustain. Soc. 2014, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Sterner, M.; Jentsch, M.; Holzhammer, T. Energiewirtschaftliche und Ökologische Bewertung eines Windgas-Angebotes; Fraunhofer Institut für Windenergie und Energiesystemtechnik: Kassel, Germany, 2011. [Google Scholar]
- Meylan, F.D.; Moreau, V.; Erkman, S. Material constraints related to storage of future European renewable electricity surpluses with CO2 methanation. Energy Policy 2016, 94, 366–376. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Wang, Y.; Winter, L.R.; Chen, J.G.; Yan, B. CO2 hydrogenation over heterogeneous catalysts at atmospheric pressure: From electronic properties to product selectivity. Green Chem. 2021, 23, 249–267. [Google Scholar] [CrossRef]
- Thema, M.; Weidlich, T.; Hörl, M.; Bellack, A.; Mörs, F.; Hackl, F.; Kohlmayer, M.; Gleich, J.; Stabenau, C.; Trabold, T.; et al. Biological CO2-Methanation: An Approach to Standardization. Energies 2019, 12, 1670. [Google Scholar] [CrossRef] [Green Version]
- Karakashev, D.; Batstone, D.J.; Angelidaki, I. Influence of Environmental Conditions on Methanogenic Compositions.pdf. Appl. Environ. Microbiol. 2005, 71, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Whitman, W.B. Metabolic, Phylogenetic, and Ecological Diversity of the Methanogenic Archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Gomez, E. Kinetics of anaerobic treatment: A critical review. Crit. Rev. Environ. Control 1991, 21, 411–490. [Google Scholar]
- Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrogen Energy 2013, 38, 2039–2061. [Google Scholar] [CrossRef]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Kolb, S.; Plankenbühler, T.; Hofmann, K.; Bergerson, J.; Karl, J. Life cycle greenhouse gas emissions of renewable gas technologies: A comparative review. Renew. Sustain. Energy Rev. 2021, 146, 111147. [Google Scholar] [CrossRef]



| Sector | Gross Final Consumption by Sector in Gtoe (% of Total) | |||
|---|---|---|---|---|
| 1990 | 2000 | 2010 | 2018 | |
| Industry | 1.80 (28.8) | 1.86 (26.6) | 2.63 (29.9) | 2.83 (28.6) |
| Transport | 1.57 (25.2) | 1.96 (27.9) | 2.42 (27.5) | 2.88 (29.1) |
| Residential | 1.53 (24.4) | 1.80 (25.6) | 1.98 (22.5) | 2.10 (21.2) |
| Commercial and public services | 0.45 (7.2) | 0.56 (8.0) | 0.72 (8.1) | 0.80 (8.2) |
| Agriculture and forestry | 0.16 (2.6) | 0.15 (2.1) | 0.18 (2.0) | 0.20 (2.1) |
| Not specified | 0.26 (4.2) | 0.08 (1.1) | 0.11 (1.2) | 0.14 (1.5) |
| Non-energy use | 0.48 (7.7) | 0.60 (8.7) | 0.76 (8.7) | 0.92 (9.3) |
| Total | 6.25 | 7.01 | 8.80 | 9.87 |
| Energy Source | Total Energy Supply in Gtoe (% of Total) | |||
|---|---|---|---|---|
| 1990 | 2000 | 2010 | 2018 | |
| Coal | 2.22 (25.3) | 2.33 (23.1) | 3.65 (28.4) | 3.84 (26.9) |
| Oil | 3.23 (37.0) | 3.67 (36.4) | 4.13 (32.1) | 4.50 (31.5) |
| Natural gas | 1.66 (18.8) | 2.10 (20.9) | 2.74 (21.3) | 3.26 (22.8) |
| Nuclear | 0.53 (6.0) | 0.68 (6.8) | 0.72 (5.6) | 0.71 (5.0) |
| Hydro | 0.18 (2.1) | 0.22 (2.2) | 0.30 (2.3) | 0.36 (2.5) |
| Biofuels and waste | 0.90 (10.3) | 1.01 (10.0) | 1.21 (9.4) | 1.33 (9.3) |
| Wind, solar, etc. | 0.04 (0.5) | 0.06 (0.6) | 0.11 (0.9) | 0.29 (2.0) |
| Total | 8.76 | 10.07 | 12.86 | 14.29 |
| Electricity source | Total electricity generation in 106 GWh (% of total) | |||
| 1990 | 2000 | 2010 | 2018 | |
| Coal | 4.43 (37.4) | 5.99 (38.7) | 8.66 (40.3) | 10.16 (38.1) |
| Oil | 1.32 (11.1) | 1.18 (7.6) | 0.97 (4.5) | 0.78 (2.9) |
| Natural gas | 1.75 (14.8) | 2.77 (17.9) | 4.84 (22.5) | 6.15 (23.0) |
| Nuclear | 2.01 (17.0) | 2.59 (16.7) | 2.76 (12.8) | 2.71 (10.2) |
| Hydro | 2.19 (18.5) | 2.70 (17.5) | 3.54 (16.5) | 4.33 (16.2) |
| Biofuels and waste | 0.12 (1.0) | 0.14 (0.9) | 0.29 (1.3) | 0.65 (2.4) |
| Wind | 0.02 (0.2) | 0.04 (0.3) | 0.34 (1.6) | 1.27 (4.8) |
| Solar/Geothermal | 0.01 (0.1) | 0.06 (0.4) | 0.09 (0.4) | 0.64 (2.4) |
| Total | 11.85 | 15.47 | 21.49 | 26.69 |
| Sector | Annual Mtonnes of CO2 (% of Total) |
|---|---|
| Electricity and heat production | 13.7 (42.4) |
| Transportation | 7.4 (22.9) |
| Manufacturing industries and construction | 6.1 (19.0) |
| Residential | 1.9 (5.7) |
| Services | 0.9 (2.8) |
| Other (agriculture/forestry, energy industries other than electricity and heat generation, marine and aviation bunkers, fishing) | 2.3 (7.2) |
| Total | 32.3 |
| Technology | CO2 Removal Efficiency (% v/v) | CO2 Capture Cost (EUR/tn) |
|---|---|---|
| Industrial separation | 90 | 32.9–57.3 |
| Pre-combustion | 88 | 31.9–59.1 |
| Oxy-fuel combustion | >90 | 48.9–55.7 |
| Post-combustion | 90 | 43.2–69.5 |
| Chemical looping | 96–99 | <56.0 |
| Direct air capture | 85–93 | 131.4–319.1 |
| Process Parameter | Chemical Methanation | Biological Methanation | ||
|---|---|---|---|---|
| Fixed bed | Fluidized bed | Bubble (slurry) | ||
| Heat release | Very poor | Good | Very good | Very good |
| Heat control | Very poor | Average | Very good | Very good |
| Mass transfer | Average | Very good | Very poor | Very poor |
| Kinetics | Good | Good | Good | Average |
| Load flexibility | Average | Very poor | Average | Very poor |
| Catalyst stress | Good | Very poor | Good | Very good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varvoutis, G.; Lampropoulos, A.; Mandela, E.; Konsolakis, M.; Marnellos, G.E. Recent Advances on CO2 Mitigation Technologies: On the Role of Hydrogenation Route via Green H2. Energies 2022, 15, 4790. https://doi.org/10.3390/en15134790
Varvoutis G, Lampropoulos A, Mandela E, Konsolakis M, Marnellos GE. Recent Advances on CO2 Mitigation Technologies: On the Role of Hydrogenation Route via Green H2. Energies. 2022; 15(13):4790. https://doi.org/10.3390/en15134790
Chicago/Turabian StyleVarvoutis, Georgios, Athanasios Lampropoulos, Evridiki Mandela, Michalis Konsolakis, and George E. Marnellos. 2022. "Recent Advances on CO2 Mitigation Technologies: On the Role of Hydrogenation Route via Green H2" Energies 15, no. 13: 4790. https://doi.org/10.3390/en15134790
APA StyleVarvoutis, G., Lampropoulos, A., Mandela, E., Konsolakis, M., & Marnellos, G. E. (2022). Recent Advances on CO2 Mitigation Technologies: On the Role of Hydrogenation Route via Green H2. Energies, 15(13), 4790. https://doi.org/10.3390/en15134790







