Closing of Carbon Cycle by Waste Gasification for Circular Economy Implementation in Poland
Abstract
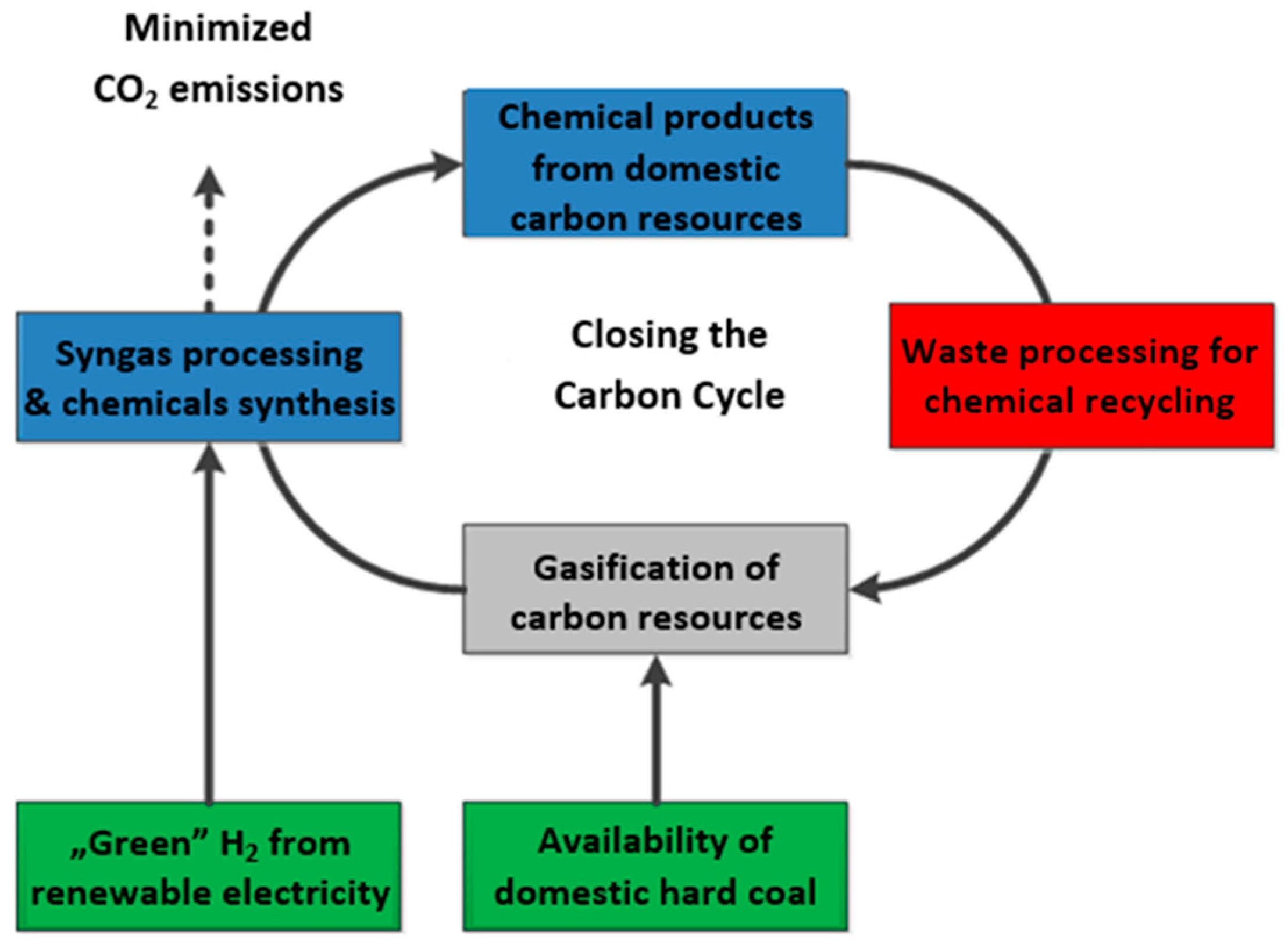
:1. Introduction
2. Materials and Methods
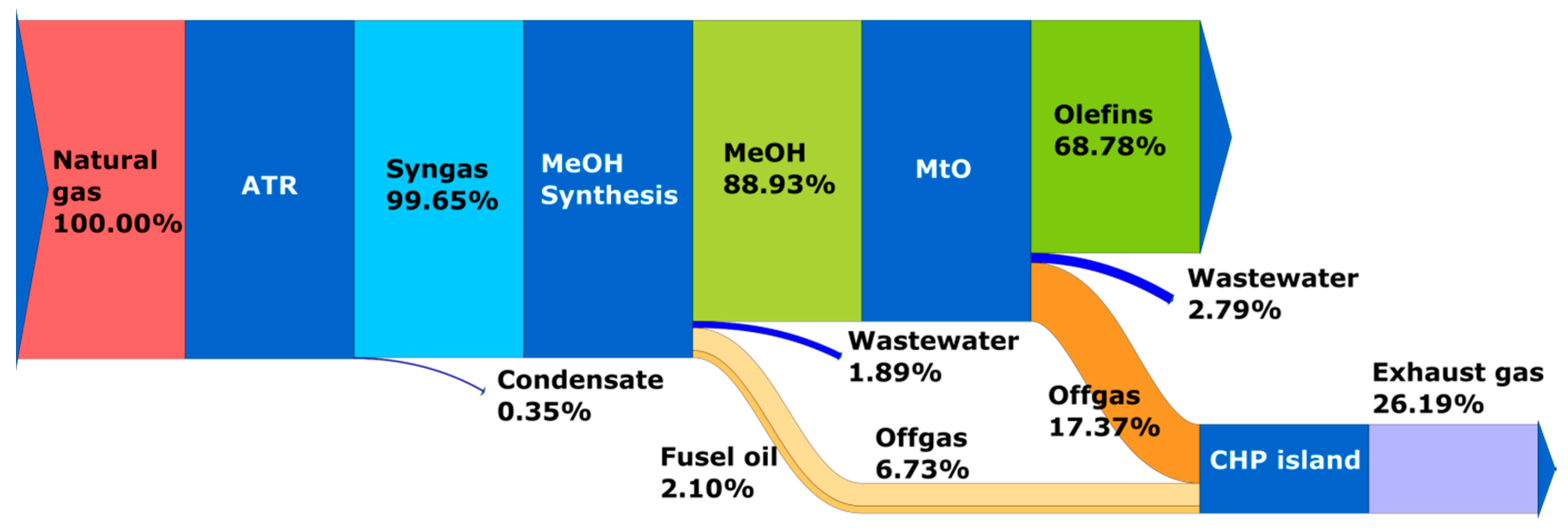
- Reference scenario 2030 time horizon—Case 1: production of olefins from natural gas (linear economy, no thermochemical recycling of waste);
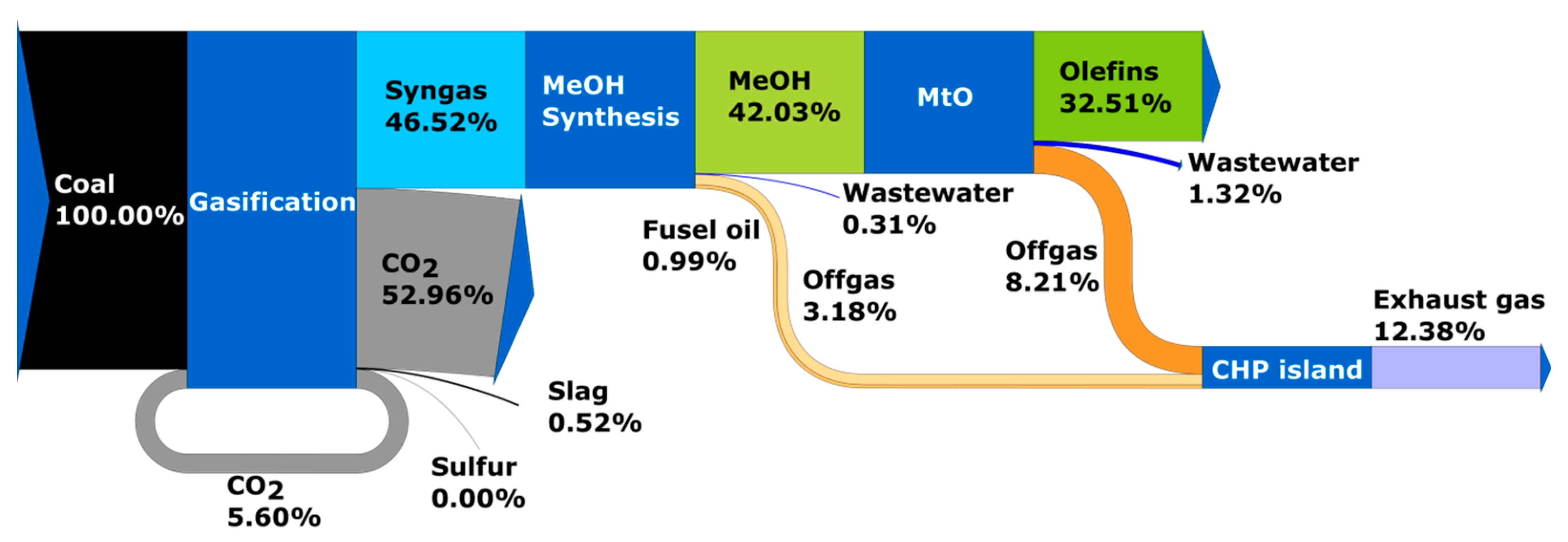
- Reference scenario 2030 time horizon—Case 2: production of olefins from coal (linear economy, no thermochemical recycling of waste);
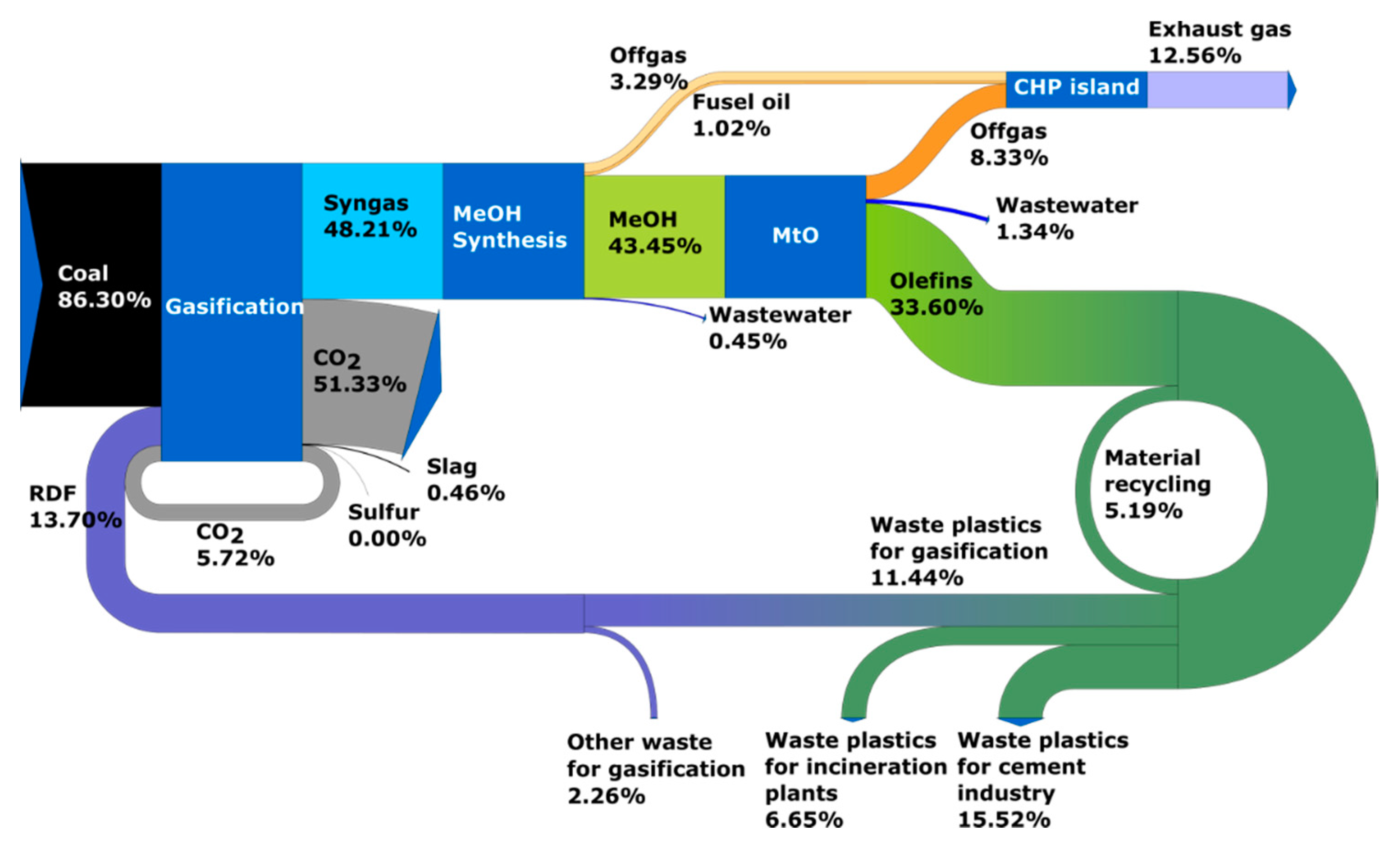
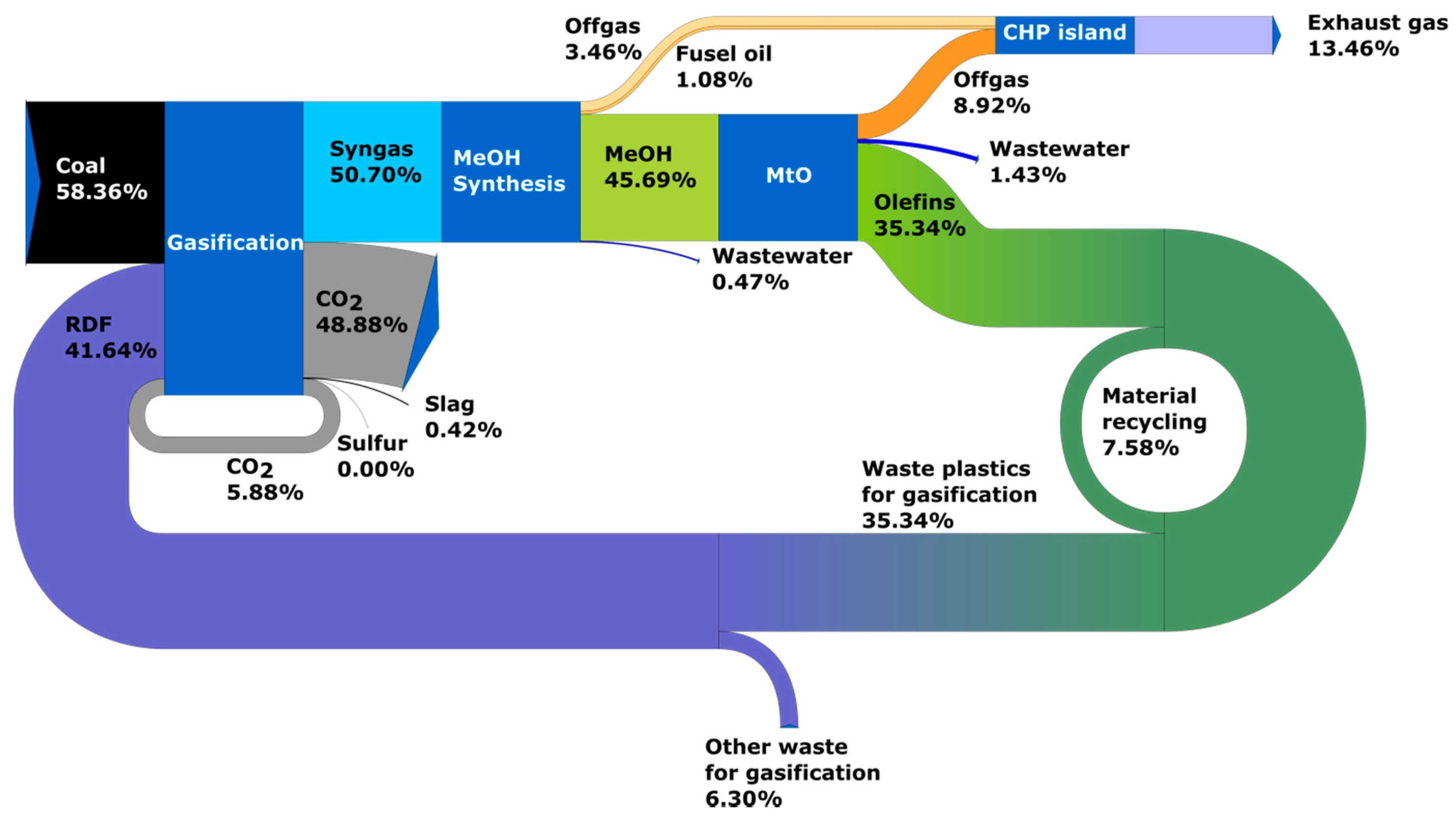
- Circular carbon economy, time horizon 2030—Case 3: olefins are produced on the basis of RDF waste and coal without ‘green hydrogen’ and the production volume covers the market demand (no production from oil). According to the adopted scenario, it is assumed that in 2030 part of the waste plastics is disposed of in cement plants and incinerators and only the unused surplus is directed to gasification;
- Circular carbon economy, time horizon 2050—Case 4: olefins are produced from RDF waste and coal without ‘green hydrogen’ and the production volume covers the market demand. In this scenario, the amount of waste plastics available increases due to the increase in waste and the reduction in use for energy production (incinerators) and in the cement industry;
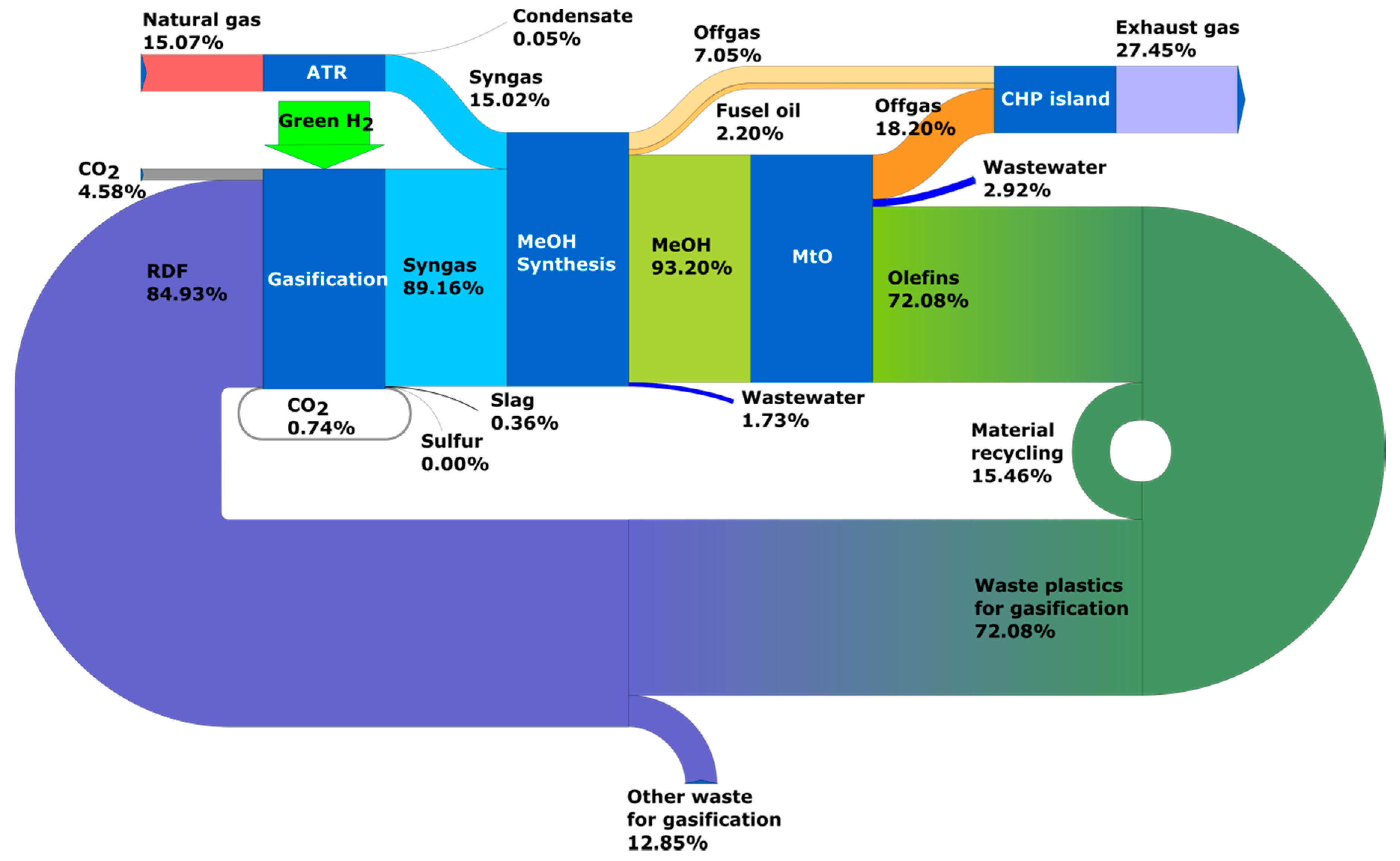
- Circular carbon economy, time horizon 2050—Case 5: olefins are produced from RDF waste and coal with ‘green hydrogen’ and production volumes covers market demand;
- Circular carbon economy, time horizon 2050—Case 6: olefins are produced from RDF waste and natural gas with ‘green hydrogen’ and the production volume covers market demand.
- Distribution of carbon element in the system;
- Direct and indirect CO2 emissions;
- Demand for hard coal to balance the raw material requirements.
2.1. Process Model of Olefins Production
2.1.1. Solid Fuel Gasification System
2.1.2. Conversion of Natural Gas
2.1.3. Methanol and Olefins Synthesis System
2.1.4. Hydrogen Production System
2.2. Methodology of Calculating CO2 Emissions in the Analyzed Systems
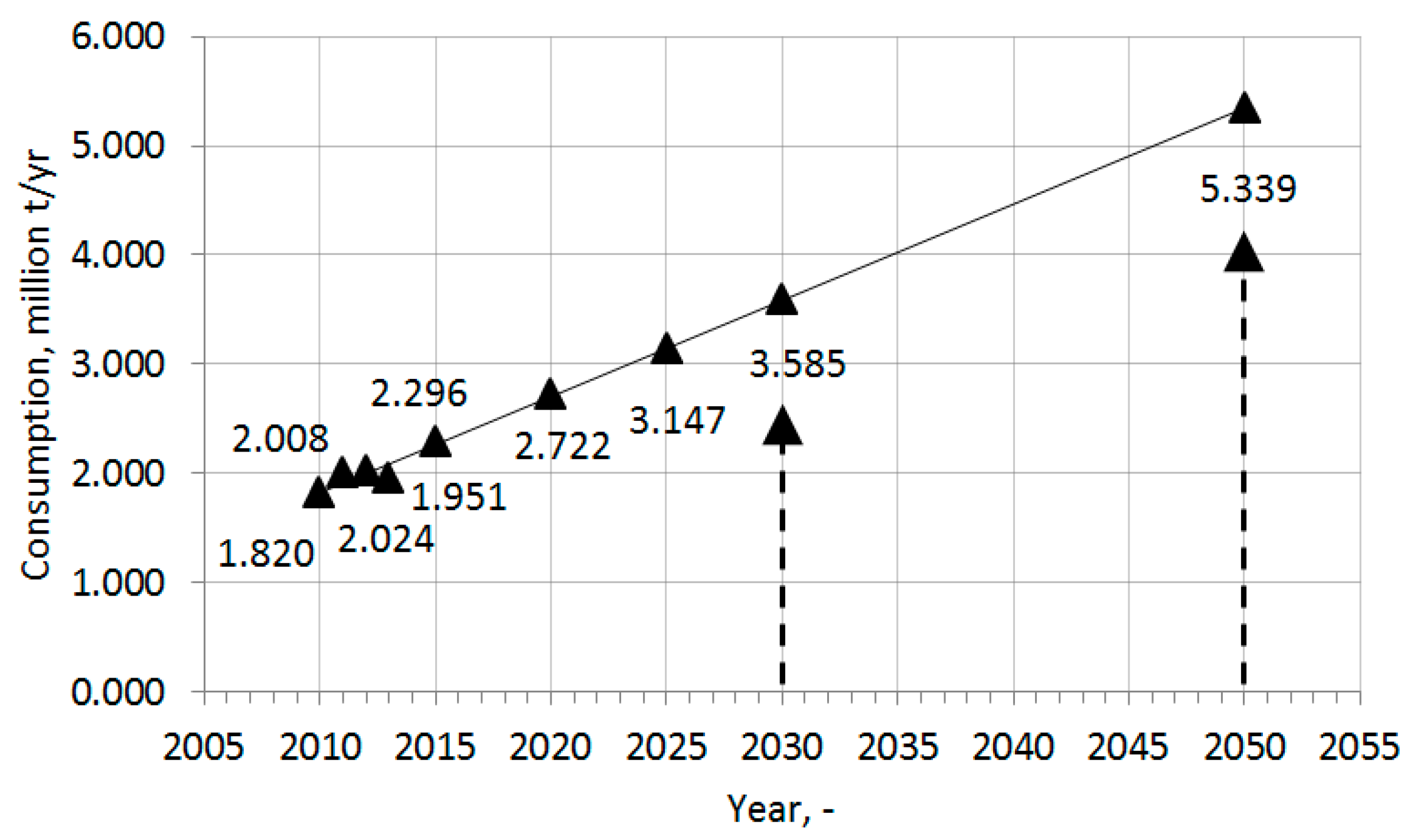
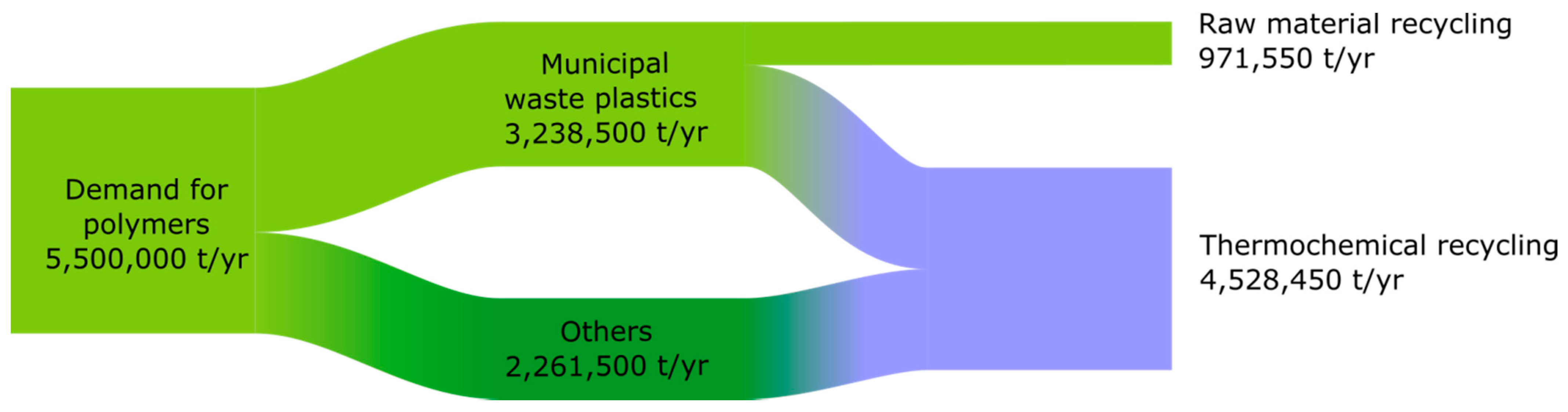
2.3. Demand for Polymers
2.4. Production of Municipal Waste in Poland
- The production of municipal waste in 2020 is approx. 12.1 million tonnes, equivalent to 315 kg/person (assuming a population of 38.5 million);
- The unit waste production in Poland will reach the current level in Germany (635 kg/person) in 2050;
- The change in waste generation between 2020 and 2050 will be linear.
- in 2030: 15.6 million tonnes;
- in 2050: 21.6 million tonnes.
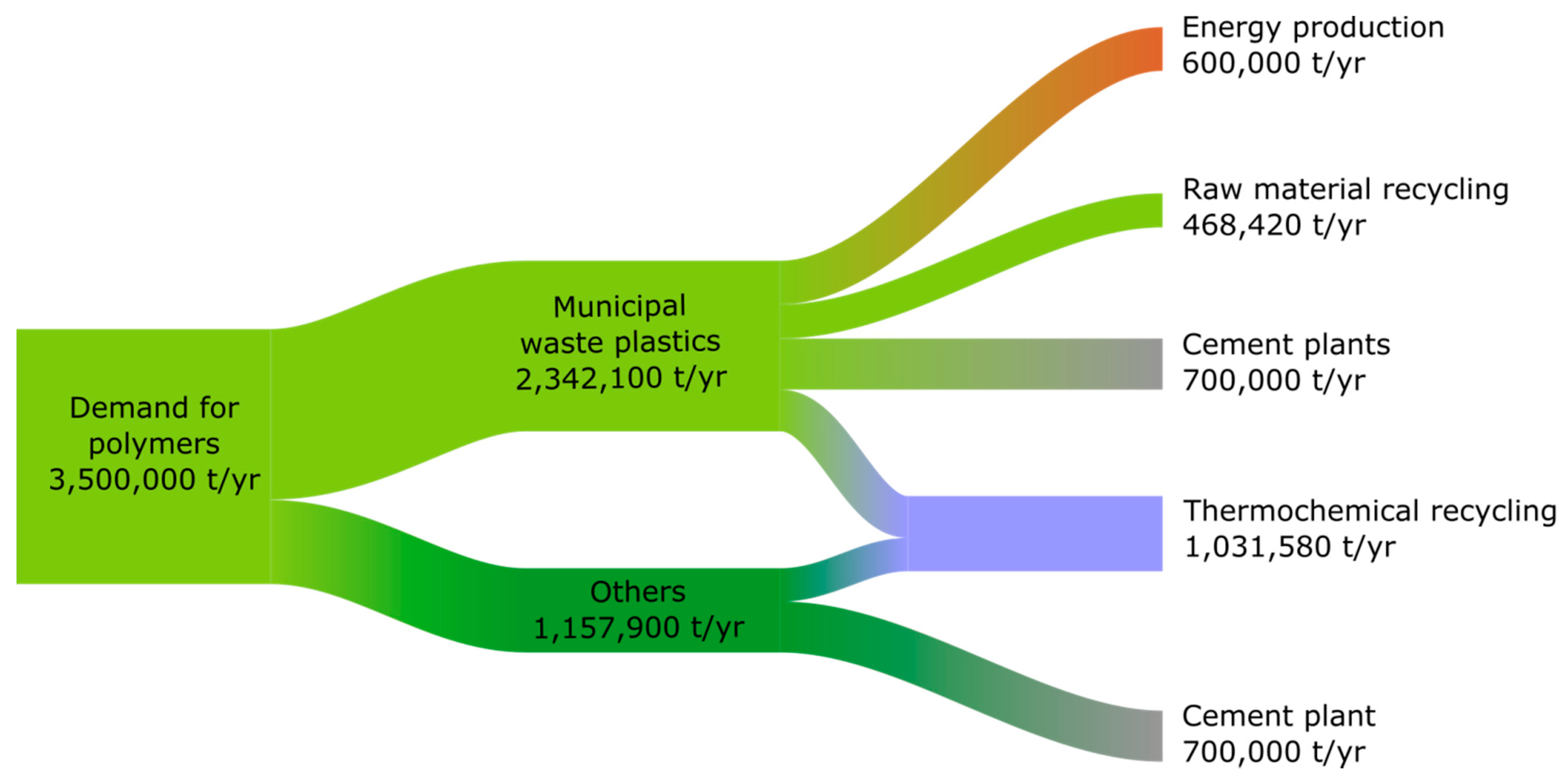
2.5. Plastics Waste/RDF Distribution Model
- The level of separate collection of plastics waste in Poland will be 60% in 2030 and 75% in 2050;
- The content of plastics waste in RDF remains constant over time at around 40%;
- About 20% of plastics waste in 2030 and 30% in 2050 are subject to raw material recycling;
- In 2030, 1.4 million plastics waste will be disposed of in cement plants, half of which will come from municipal waste;
- In 2030, 1.5 million tonnes of RDF (0.6 million tonnes of plastics waste) will be used for energy;
- In 2050, waste fuels for energy production and in cement plants will be replaced by renewable fuels (no use of alternative fuel in the form of plastics waste and RDF);
- There is no accumulation of plastics in the circulation, i.e., the amount of plastics waste is equal to the amount of demand;
- The plastics waste stream from non-municipal sectors is the result of the difference between the plastics demand stream and the amount of plastics waste in municipal waste;
- Plastics waste from non-municipal sectors will supply the other half of the plastics waste demand of cement plants in 2030;
- The surplus plastics waste stream over and above the amount used in cement plants, waste incineration plants, or recovered in raw material recycling is recycled into feedstock;
- The morphological composition of municipal waste is unchanged over time and amounts to paper—10%, metal—2.6%, plastics—15%, glass—10%, other—62.4%.
2.6. Fuel Characteristic
2.7. Estimation of Investment and Operating Costs
3. Results
3.1. Results of Process Calculations—Distribution of Carbon Element in Analyzed Systems
3.2. Economic Analysis
4. Discussion
5. Conclusions
- Verification of the current waste fuel distribution and its medium- and long-term availability;
- Detailed analysis of the waste fuels properties and their fluctuation;
- Changing the national policy in the field of chemical industry development.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, I.; Kabir, Z. Waste-to-Energy Generation Technologies and the Developing Economies: A Multi-Criteria Analysis for Sustainability Assessment. Renew. Energy 2020, 150, 320–333. [Google Scholar] [CrossRef]
- Lee, R.P.; Keller, F.; Meyer, B. A Concept to Support the Transformation from a Linear to Circular Carbon Economy: Net Zero Emissions, Resource Efficiency and Conservation through a Coupling of the Energy, Chemical and Waste Management Sectors. Clean Energy 2017, 1, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Szostak, E.; Duda, P.; Duda, A.; Górska, N.; Fenicki, A.; Molski, P. Characteristics of Plastic Waste Processing in the Modern Recycling Plant Operating in Poland. Energies 2020, 14, 35. [Google Scholar] [CrossRef]
- Wielgosiński, G.; Czerwińska, J.; Szufa, S. Municipal Solid Waste Mass Balance as a Tool for Calculation of the Possibility of Implementing the Circular Economy Concept. Energies 2021, 14, 1811. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Gnatowski, A. Recycling of Plastic Waste, with Particular Emphasis on Thermal Methods—Review. Energies 2022, 15, 2114. [Google Scholar] [CrossRef]
- da Silva, L.F.; Resnitzkyd, M.H.C.; Santibanez Gonzalez, E.D.R.; de Melo Conti, D.; da Costa, P.R. Management of Plastic Waste and a Circular Economy at the End of the Supply Chain: A Systematic Literature Review. Energies 2022, 15, 976. [Google Scholar] [CrossRef]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic Waste and Its Management Strategies for Environmental Sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Keller, F.; Voss, R.L.; Lee, R.P.; Meyer, B. Life Cycle Assessment of Global Warming Potential of Feedstock Recycling Technologies: Case Study of Waste Gasification and Pyrolysis in an Integrated Inventory Model for Waste Treatment and Chemical Production in Germany. Resour. Conserv. Recycl. 2022, 179, 106106. [Google Scholar] [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Godoi Bizarro, D.; De Wild, P.; Vreugdenhil, B.; van Harmelen, T. Plastic Recycling in a Circular Economy; Determining Environmental Performance through an LCA Matrix Model Approach. Waste Manag. 2021, 121, 331–342. [Google Scholar] [CrossRef]
- De Pascale, A.; Arbolino, R.; Szopik-Depczyńska, K.; Limosani, M.; Ioppolo, G. A Systematic Review for Measuring Circular Economy: The 61 Indicators. J. Clean. Prod. 2021, 281, 124942. [Google Scholar] [CrossRef]
- Kirchner, M.; Sommer, M.; Kratena, K.; Kletzan-Slamanig, D.; Kettner-Marx, C. CO2 Taxes, Equity and the Double Dividend—Macroeconomic Model Simulations for Austria. Energy Policy 2019, 126, 295–314. [Google Scholar] [CrossRef]
- Lee, R.P. Alternative Carbon Feedstock for the Chemical Industry?—Assessing the Challenges Posed by the Human Dimension in the Carbon Transition. J. Clean. Prod. 2019, 219, 786–796. [Google Scholar] [CrossRef]
- Fogt Jacobsen, L.; Pedersen, S.; Thøgersen, J. Drivers of and Barriers to Consumers’ Plastic Packaging Waste Avoidance and Recycling—A Systematic Literature Review. Waste Manag. 2022, 141, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.P.; Tschoepe, M.; Voss, R. Perception of Chemical Recycling and Its Role in the Transition towards a Circular Carbon Economy: A Case Study in Germany. Waste Manag. 2021, 125, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Schöggl, J.-P.; Stumpf, L.; Baumgartner, R.J. The Narrative of Sustainability and Circular Economy—A Longitudinal Review of Two Decades of Research. Resour. Conserv. Recycl. 2020, 163, 105073. [Google Scholar] [CrossRef]
- Acerbi, F.; Taisch, M. A Literature Review on Circular Economy Adoption in the Manufacturing Sector. J. Clean. Prod. 2020, 273, 123086. [Google Scholar] [CrossRef]
- Cobo, S.; Dominguez-Ramos, A.; Irabien, A. From Linear to Circular Integrated Waste Management Systems: A Review of Methodological Approaches. Resour. Conserv. Recycl. 2018, 135, 279–295. [Google Scholar] [CrossRef]
- Tsai, F.M.; Bui, T.-D.; Tseng, M.-L.; Lim, M.K.; Hu, J. Municipal Solid Waste Management in a Circular Economy: A Data-Driven Bibliometric Analysis. J. Clean. Prod. 2020, 275, 124132. [Google Scholar] [CrossRef]
- Lahane, S.; Kant, R.; Shankar, R. Circular Supply Chain Management: A State-of-Art Review and Future Opportunities. J. Clean. Prod. 2020, 258, 120859. [Google Scholar] [CrossRef]
- Mhatre, P.; Panchal, R.; Singh, A.; Bibyan, S. A Systematic Literature Review on the Circular Economy Initiatives in the European Union. Sustain. Prod. Consum. 2021, 26, 187–202. [Google Scholar] [CrossRef]
- Thunman, H.; Berdugo Vilches, T.; Seemann, M.; Maric, J.; Vela, I.C.; Pissot, S.; Nguyen, H.N.T. Circular Use of Plastics-Transformation of Existing Petrochemical Clusters into Thermochemical Recycling Plants with 100% Plastics Recovery. Sustain. Mater. Technol. 2019, 22, e00124. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A Review on Thermal and Catalytic Pyrolysis of Plastic Solid Waste (PSW). J. Environ. Manage. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Grause, G.; Eger, C.; Kaminsky, W.; Okuwaki, A. Pyrolysis of Poly(Ethylene Terephthalate) in a Fluidised Bed Plant. Polym. Degrad. Stab. 2004, 86, 499–504. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical Routes for the Valorization of Waste Polyolefinic Plastics to Produce Fuels and Chemicals. A Review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Zhuang, J.; Tang, J.; Aljerf, L. Comprehensive Review on Mechanism Analysis and Numerical Simulation of Municipal Solid Waste Incineration Process Based on Mechanical Grate. Fuel 2022, 320, 123826. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Wu, Y.; Xu, G.; Liu, W.; Liu, T. Design and Performance Evaluation of a New Waste Incineration Power System Integrated with a Supercritical CO2 Power Cycle and a Coal-Fired Power Plant. Energy Convers. Manag. 2020, 210, 112715. [Google Scholar] [CrossRef]
- Voss, R.; Lee, R.P.; Seidl, L.; Keller, F.; Fröhling, M. Global Warming Potential and Economic Performance of Gasification-Based Chemical Recycling and Incineration Pathways for Residual Municipal Solid Waste Treatment in Germany. Waste Manag. 2021, 134, 206–219. [Google Scholar] [CrossRef]
- Solis, M.; Silveira, S. Technologies for Chemical Recycling of Household Plastics—A Technical Review and TRL Assessment. Waste Manag. 2020, 105, 128–138. [Google Scholar] [CrossRef]
- Paukov, A.; Magaril, R.; Magaril, E. An Investigation of the Feasibility of the Organic Municipal Solid Waste Processing by Coking. Sustainability 2019, 11, 389. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.N.; Redhwi, H.H. Catalytic Coprocessing of Waste Plastics and Petroleum Residue into Liquid Fuel Oils. J. Anal. Appl. Pyrolysis 2009, 86, 141–147. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Liu, J.; Li, T.; Xu, G.; Liu, W. Thermodynamic and Economic Evaluation of a Novel Waste-to-Energy Design Incorporating Anaerobic Digestion and Incineration. Energy Convers. Manag. 2022, 252, 115083. [Google Scholar] [CrossRef]
- Himanen, M.; Hänninen, K. Composting of Bio-Waste, Aerobic and Anaerobic Sludges—Effect of Feedstock on the Process and Quality of Compost. Bioresour. Technol. 2011, 102, 2842–2852. [Google Scholar] [CrossRef]
- Melendez, J.R.; Mátyás, B.; Hena, S.; Lowy, D.A.; El Salous, A. Perspectives in the Production of Bioethanol: A Review of Sustainable Methods, Technologies, and Bioprocesses. Renew. Sustain. Energy Rev. 2022, 160, 112260. [Google Scholar] [CrossRef]
- El-Qelish, M.; Hassan, G.K.; Leaper, S.; Dessì, P.; Abdel-Karim, A. Membrane-Based Technologies for Biohydrogen Production: A Review. J. Environ. Manage. 2022, 316, 115239. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Q.; Zhang, Z.; Jing, Y.; Hu, J.; He, C.; Lu, C. A Review on Biological Recycling in Agricultural Waste-Based Biohydrogen Production: Recent Developments. Bioresour. Technol. 2022, 347, 126595. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Basu, S.; Shetti, N.P.; Kamali, M.; Walvekar, P.; Aminabhavi, T.M. Waste-to-Energy Nexus: A Sustainable Development. Environ. Pollut. 2020, 267, 115501. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. A Technical Review of Bioenergy and Resource Recovery from Municipal Solid Waste. J. Hazard. Mater. 2021, 403, 123970. [Google Scholar] [CrossRef]
- Ali, J.; Rasheed, T.; Afreen, M.; Anwar, M.T.; Nawaz, Z.; Anwar, H.; Rizwan, K. Modalities for Conversion of Waste to Energy—Challenges and Perspectives. Sci. Total Environ. 2020, 727, 138610. [Google Scholar] [CrossRef]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a Circular Economy for Plastic Packaging Wastes—The Environmental Potential of Chemical Recycling. Resour. Conserv. Recycl. 2020, 162, 105010. [Google Scholar] [CrossRef]
- Shonnard, D.; Tipaldo, E.; Thompson, V.; Pearce, J.; Caneba, G.; Handler, R. Systems Analysis for PET and Olefin Polymers in a Circular Economy. Procedia CIRP 2019, 80, 602–606. [Google Scholar] [CrossRef]
- Higman, C.; van der Burgt, M. (Eds.) Gasification, 2nd ed.; Gulf Professional Publishing: Burlington, VT, USA, 2008; ISBN 978-0-7506-8528-3. [Google Scholar]
- Kaur, G.; Uisan, K.; Ong, K.L.; Lin, C.S.K. Recent Trends in Green and Sustainable Chemistry & Waste Valorisation: Rethinking Plastics in a Circular Economy. Curr. Opin. Green Sustain. Chem. 2018, 9, 30–39. [Google Scholar] [CrossRef]
- Rogelj, J.; Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement Climate Proposals Need a Boost to Keep Warming Well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rissman, J.; Bataille, C.; Masanet, E.; Aden, N.; Morrow, W.R.; Zhou, N.; Elliott, N.; Dell, R.; Heeren, N.; Huckestein, B.; et al. Technologies and Policies to Decarbonize Global Industry: Review and Assessment of Mitigation Drivers through 2070. Appl. Energy 2020, 266, 114848. [Google Scholar] [CrossRef]
- Aguilar-Hernandez, G.A.; Dias Rodrigues, J.F.; Tukker, A. Macroeconomic, Social and Environmental Impacts of a Circular Economy up to 2050: A Meta-Analysis of Prospective Studies. J. Clean. Prod. 2021, 278, 123421. [Google Scholar] [CrossRef]
- Woods, M.; Kuehn, N.; Shah, V.; White lll, C.; Goellner, J. Baseline Analysis of Crude Methanol Production from Coal and Natural Gas; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA; Albany, OR, USA, 2014.
- Xiang, D.; Yang, S.; Liu, X.; Mai, Z.; Qian, Y. Techno-Economic Performance of the Coal-to-Olefins Process with CCS. Chem. Eng. J. 2014, 240, 45–54. [Google Scholar] [CrossRef]
- Johansson, E. Process Integration Study of Biomass-to-Methanol (via Gasification) and Methanol-to-Olefins (MTO) Processes in an Existing Steam Cracker Plant; Chalmers University of Technology: Göteborg, Sweden, 2013. [Google Scholar]
- Technology Roadmap—Hydrogen and Fuel Cells—Analysis. Available online: https://www.iea.org/reports/technology-roadmap-hydrogen-and-fuel-cells (accessed on 3 June 2022).
- KOBIZE. Available online: https://www.kobize.pl/ (accessed on 7 June 2022).
- Lubiewa-Wieleżyński, W. Forecasts of Chemical Industry Production in Poland until 2020. Warszawa, Poland. 2014; unpublished. [Google Scholar]
- Chmielniak, T.; Bigda, J.; Billig, T.; Popowicz, J.; Fryza, R.; Sobolewski, A. The waste distribution model in Poland in the context of introducing the circular carbon economy. The 2030 and 2050 perspectives. Przemysl Chem. 2020, 99, 1529–1533. [Google Scholar] [CrossRef]
- Waligórska, M.; Kostrzewa, Z.; Potyra, M.; Rutkowska, L. Population Projection 2014–2050; Demographic Surveys and Labour Market Department, Central Statistical Office: Warsaw, Poland, 2014.
- Riley, J.; Atallah, C.; Siriwardane, R.; Stevens, R. Technoeconomic Analysis for Hydrogen and Carbon Co-Production via Catalytic Pyrolysis of Methane. Int. J. Hydrogen Energy 2021, 46, 20338–20358. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for CO2-Free H2 Production: A Green Process to Overcome Renewable Energies Unsteadiness. Chem. Ing. Tech. 2020, 92, 1596–1609. [Google Scholar] [CrossRef]

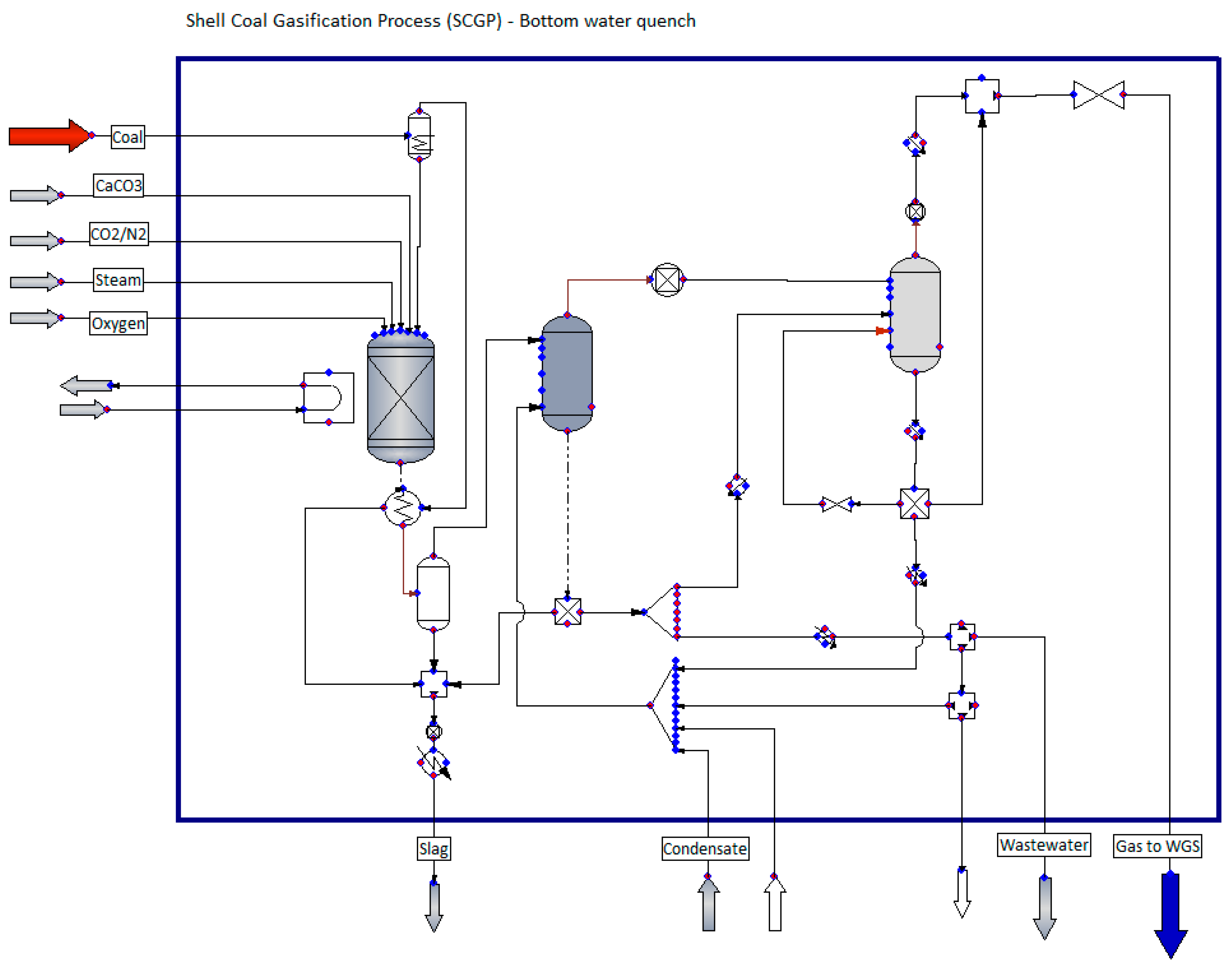


| Specification | Unit | Value |
|---|---|---|
| Gasification system | ||
| Oxygen production | – |
|
| Gasification unit | ||
| Reactor | – | Shell/Quench |
| Gasification pressure | bar | 42 |
| Ratio O2/C 1 | kg O2/kg C | 1.56–1.254 (calculated value, depending on the case under consideration) |
| Ratio steam/C 1 | kg H2O/kg C | 0.119–0.133 (calculated value, depending on the case under consideration) |
| Conversion rate of the C element | % | 95.0 |
| Calorific value of the gas 2 | kJ/STC m3 | 4806 (calculated value) |
| Gas cooling | – bar | Quench Convection exchanger, steam production: HP-60; IP-15.4; LP-5 |
| Oxidant | - | - |
| Oxygen | vol.% | 95 |
| Steam | bar | 60 |
| Fuel transport medium: nitrogen | kg/h | 7920 |
| Pre-treatment of raw gas | – | Cyclones, water scrubber |
| Gas conversion and purification system | ||
| WGS Unit | – | Yes, II-stage system |
| CO conversion rate | % | 96 |
| Desulphurization Unit | – | Yes, Selexol I-stage |
| Separation efficiency | % | 92 |
| Sulfur recovery | – | None |
| CO2 separation | – | Yes, Selexol |
| Separation efficiency | % | 92 |
| Indicator | Unit | Value |
|---|---|---|
| Consumption of natural gas | kg/kgsyngas | 0.4934 |
| Consumption of oxygen | kg/kgsyngas | 0.5769 |
| Consumption of boiler water | kg/kgsyngas | 0.1105 |
| Syngas production | kg/kgsyngas | 1.0000 |
| Condensate production | kg/kgsyngas | 0.1808 |
| Indicator | Unit | Value |
|---|---|---|
| MeOH synthesis | ||
| Consumption of synthesis gas | kg/kgMeOH | 1.18 |
| Electricity consumption | kWh/kgMeOH | 0.0254 |
| Cooling water consumption | kg/kgMeOH | 80.2 |
| Steam production (25.5 barg, 227 °C) | kg/kgMeOH | 1.00 |
| Fusel oil production | kg/kgMeOH | 0.042 |
| Off-gas production | kg/kgMeOH | 0.126 |
| Wastewater production | kg/kgMeOH | 0.0135 |
| Olefins synthesis | ||
| Cooling water consumption | kg/kgMeOH | 12.539 |
| Electricity consumption | kWh/kgMeOH | 0.149 |
| Ethylene production | kg/kgMeOH | 0.212 |
| Propylene production | kg/kgMeOH | 0.126 |
| Off-gas production | kg/kgMeOH | 0.107 |
| Steam production | kg/kgMeOH | 2.694 |
| Wastewater production | kg/kgMeOH | 0.554 |
| Parameter | Symbol | Unit | Hard Coal | RDF (40% Plastic Waste) |
|---|---|---|---|---|
| LHV, analytical | Qia | kJ/kg | 25,606 | 20,046 |
| HHV, analytical | Qsa | kJ/kg | 26,711 | 21,526 |
| Ash content, as received | Ar | wt.% | 13.0 | 18.42 |
| Total carbon content, analytical | Cta | wt.% | 64.6 | 47.75 |
| Total hydrogen content, analytical | Ha | wt.% | 4.4 | 6.34 |
| Nitrogen content, analytical | Na | wt.% | 1.01 | 0.92 |
| Total sulphur content, analytical | Sta | wt.% | 1.5 | 0.35 |
| Oxygen content, analytical | Oa | wt.% | 10.6 | 21.22 |
| Moisture content, analytical | Wa | wt.% | 5.0 | 5.00 |
| Ash content, analytical | Aa | wt.% | 13.0 | 18.42 |
| Parameter | Unit | Assumed for Calculation | Type E Natural Gas Parameter Range |
|---|---|---|---|
| Methane content | vol.% | 99.0 | >96.0 |
| Carbon dioxide content | vol.% | 1.0 | ≤3.0 |
| Minimal HHV | MJ/m3 | 38.0 | ≥38.0 |
| Wobbe index range | MJ/m3 | 49.0 | 45.0–56.9 |
| Equipment Item | Scaling Parameter | Unit | Value | Scaling Exponent Factor |
|---|---|---|---|---|
| Unloading, preparation and storage of raw material/fuel | Fuel stream | kg/h | 122,800 | 0.55 |
| Gasification | Fuel stream | kg/h | 122,800 | 0.55 |
| Gas conditioning | Gas stream after scrubber | kg/h | 301,500 | 0.60 |
| AGR | AGR acid gas stream | kg/h | 5850 | 0.57 |
| Sulfur removal | Sulfur stream | kg/h | 1350.8 | 0.58 |
| Methanol synthesis | Methanol production | kg/h | 66,700 | 0.60 |
| ASU | Oxygen stream | kg/h | 46,700 | 0.50 |
| ATR | Natural gas stream | kg/h | 81,288 | 0.60 |
| Olefins synthesis | Olefins stream | kg/h | 21,560 | 0.60 |
| Electrolysis | Electric power | MW | 1 | 1.00 |
| Component | Unit | Value |
|---|---|---|
| Apparatus and equipment | % 1 | 100 |
| Equipment installation | % 1 | 25 |
| Installation of instrumentation and control and measurement equipment | % 1 | 16 |
| Piping installation | % 1 | 35 |
| Electrical installation | % 1 | 7 |
| Construction work | % 1 | 15 |
| Temporary installations | % 1 | 10 |
| Auxiliary installations | % 1 | 2 |
| Design and supervision | % 1 | 33 |
| Other costs | % 1 | 6 |
| Delivery costs | % 1 | 12 |
| Contingencies | % 1 | 40 |
| Commissioning | % 1 | 5 |
| Fixed assets outlays | % 1 | 306 |
| Item | Unit | Value |
|---|---|---|
| Hard coal cost | €/t | 60.98 |
| Income from the utilization of RDF | €/t | 97.82 |
| RDF transportation cost | €/t | 1.62 |
| Natural gas cost | €/kg | 0.32 |
| Process water cost | €/m3 | 0.70 |
| Cooling water cost | €/m3 | 0.03 |
| Electricity cost | €/MWh | 52.27 |
| Revenue from the sale of electricity | €/MWh | 52.27 |
| The cost of chemicals, catalysts 1 | $/t MeOH | 2.09 |
| The cost of CO2 emissions 2 | €/t | 25 |
| Repairs, renovations | % from Investment expenditures | 1.0 |
| Spare parts | % from Investment expenditures | 0.50 |
| Insurance | % from Investment expenditures | 0.50 |
| Labour | person/1 installation | 40 |
| Average salary | €/person·yr | 17,200 |
| Wastewater management | €/t | 1.62 |
| Ash disposal | €/t | 34.09 |
| Profit from the sale of sulfur | €/t | 159.09 |
| Constructions tax 3 | % from expenditure on buildings | 2 |
| Building tax 4 | €/m2 | 5.08 |
| Land tax 4 | €/m2 | 0.19 |
| Parameter | Unit | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|---|
| GtO2030 | CtO2030 | RDFCtO2030 | RDFCtO2050 | RDFCtO2050H2 | RDFGtO2050H2 | ||
| Raw material | |||||||
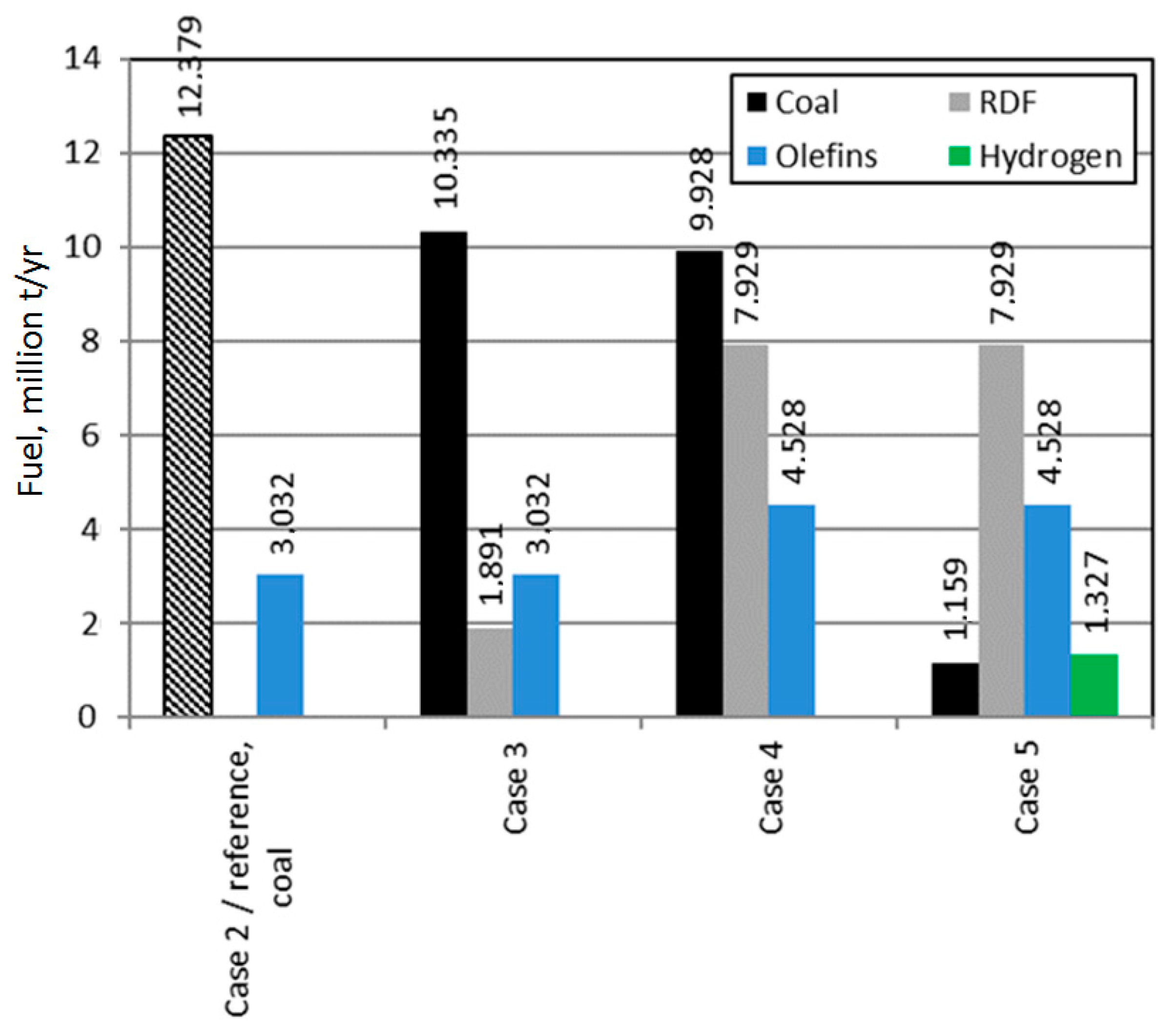
| Hard coal | million t/yr | - | 12.379 | 10.335 | 9.928 | 1.159 | - |
| RDF | million t/yr | - | - | 1.892 | 7.929 | 7.929 | 7.929 |
| Natural gas | million t/yr | 5.221 | - | - | - | - | 1.121 |
| Gasification island | |||||||
| Oxygen demand 1 | kg/kg | 1.169 | 0.747 | 0.733 | 0.744 | 0.720 | 0.779 |
| Steam demand 1 | kg/kg | 0.224 | 0.077 | 0.077 | 0.077 | 0.077 | 0.095 |
| CO2 demand 1 | kg/kg | 0.000 | 0.134 | 0.134 | 0.134 | 0.134 | 0.117 |
| H2 demand 1 | kg/kg | - | - | - | - | 0.146 | 0.122 |
| Products | |||||||
| Olefins production | million t/yr | 3.032 | 3.032 | 3.032 | 4.528 | 4.528 | 4.528 |
| Incl. ethylene | million t/yr | 1.901 | 1.901 | 1.901 | 2.840 | 2.840 | 2.840 |
| Incl. propylene | million t/yr | 1.130 | 1.130 | 1.130 | 1.688 | 1.688 | 1.688 |
| Electricity | |||||||
| Electricity demand 1 | MWh/t | 0.342 | 0.403 | 0.403 | 0.408 | 6.348 | 5.173 |
| Gross electricity production 1 | MWh/t | 1.113 | 0.695 | 0.712 | 0.729 | 1.136 | 1.027 |
| Net electricity production 1 | MWh/t | 0.771 | 0.293 | 0.310 | 0.321 | −5.212 | 4.146 |
| Net electricity production | TWh/yr | 4.023 | 3.621 | 3.784 | 5.724 | −47.367 | −37.520 |
| Carbon balance | |||||||
| Olefins | kg/kg | 0.6878 | 0.3251 | 0.3360 | 0.3534 | 0.7293 | 0.7208 |
| Direct CO2 emissions | million t/yr | 13.850 | 29.304 | 25.107 | 26.037 | 5.281 | 5.512 |
| Indirect CO2 emissions 2 | million t/yr | −2.977 | −2.680 | −2.800 | −4.235 | −4.216 | −4.881 |
| Total CO2 emissions | million t/yr | 10.873 | 26.624 | 22.307 | 21.802 | 1.065 | 0.630 |
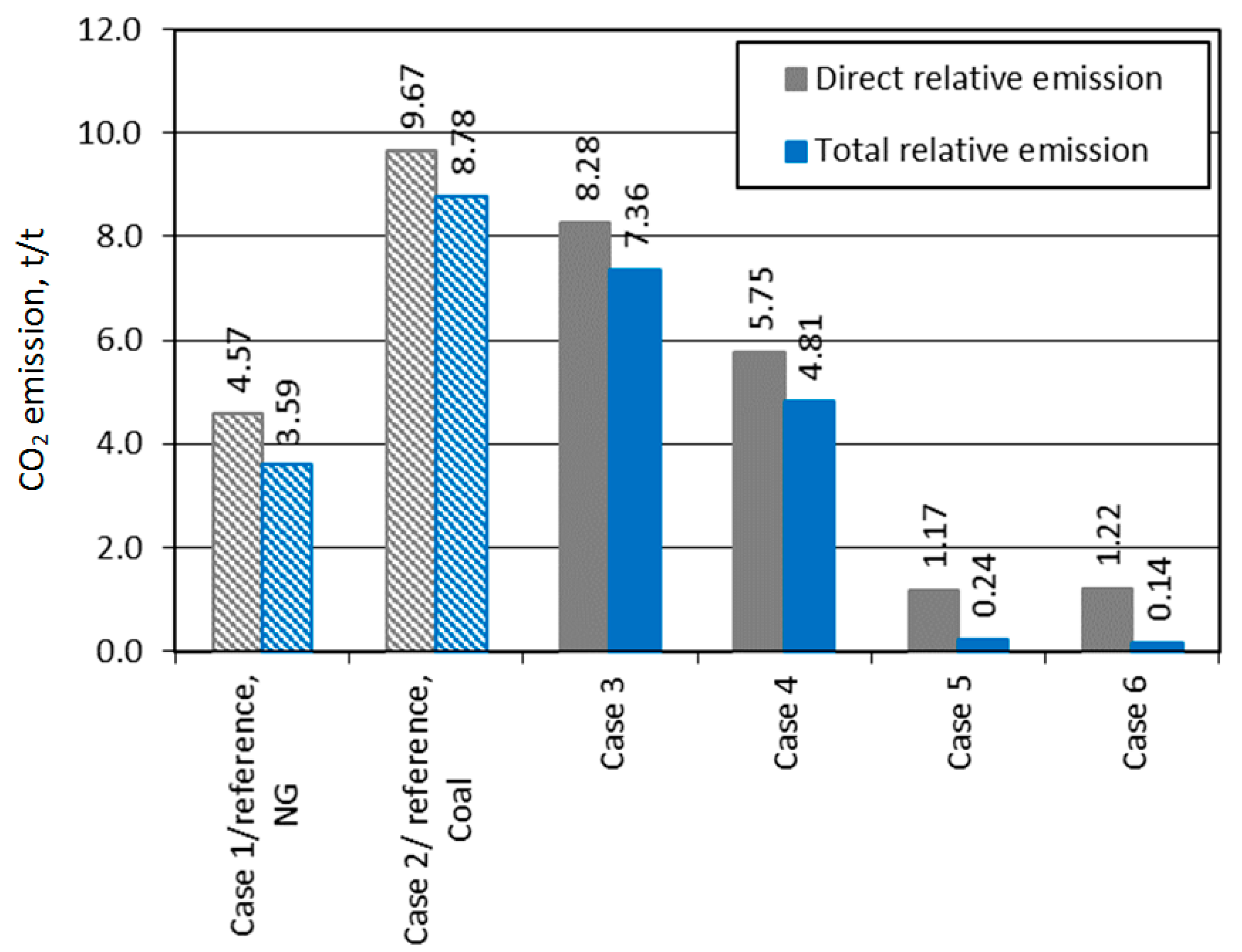
| Specific direct CO2 emissions per products | kg/t | 4569 | 9666 | 8282 | 5750 | 1166 | 1,217 |
| Specific indirect CO2 emissions per products | kg/t | 3587 | 8782 | 7358 | 4814 | 235 | 139 |
| 8906 3 | 7348 3 | ||||||
| Case | Base Year | Capital Investment | Production Cost per Year | Specific Production Cost |
|---|---|---|---|---|
| Million PLN | Million PLN | PLN/t Olefins | ||
| Linear Economy | ||||
| 1 | 2030 | 33,910.2 | 7664.5 | 2528.2 |
| 2 | 2030 | 57,381.9 | 6640.7 | 2190.5 |
| Circular Economy | ||||
| 3 | 2030 | 58,561.9 | 4645.2 | 1532.3 |
| 4 | 2050 | 84,807.6 | 2962.4 | 654.2 |
| 5 | 2050 | 100,412.2 | 10,620.9 | 2345.4 |
| 6 | 2050 | 94,980.2 | 9518.1 | 2101.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolewski, A.; Chmielniak, T.; Bigda, J.; Billig, T.; Fryza, R.; Popowicz, J. Closing of Carbon Cycle by Waste Gasification for Circular Economy Implementation in Poland. Energies 2022, 15, 4983. https://doi.org/10.3390/en15144983
Sobolewski A, Chmielniak T, Bigda J, Billig T, Fryza R, Popowicz J. Closing of Carbon Cycle by Waste Gasification for Circular Economy Implementation in Poland. Energies. 2022; 15(14):4983. https://doi.org/10.3390/en15144983
Chicago/Turabian StyleSobolewski, Aleksander, Tomasz Chmielniak, Joanna Bigda, Tomasz Billig, Rafał Fryza, and Józef Popowicz. 2022. "Closing of Carbon Cycle by Waste Gasification for Circular Economy Implementation in Poland" Energies 15, no. 14: 4983. https://doi.org/10.3390/en15144983
APA StyleSobolewski, A., Chmielniak, T., Bigda, J., Billig, T., Fryza, R., & Popowicz, J. (2022). Closing of Carbon Cycle by Waste Gasification for Circular Economy Implementation in Poland. Energies, 15(14), 4983. https://doi.org/10.3390/en15144983






